Fibrin: An underrated biopolymer for skin tissue engineering
Olfat Gsib1, Christophe Egles1, and Sidi A Bencherif1,2,3
1Laboratory of Biomechanics and Bioengineering (BMBI), Sorbonne Universities, University of Technology of Compiègne (UTC), Compiègne, France
2Harvard John A Paulson School of Engineering and Applied Sciences, Harvard University, Cambridge, USA
3Department of Chemical Engineering, Northeastern University, Huntington Avenue, Boston, MA, USA
- *Corresponding Author:
- Sidi A Bencherif
Department of Chemical Engineering
Northeastern University
360 Huntington Avenue, Boston
MA 02215, USA.
Tel: 617-373-4495
E-mail: s.bencherif@northeastern.edu
Received Date: April 09, 2017; Accepted Date: April 17, 2017; Published Date: April 25, 2017
Abstract
The ultimate goal in skin tissue regeneration is to develop artificial skin replacements for the restoration of damaged or missing skins in patients as well as to enhance wound healing processes. Fibrin, a naturally-occurring biopolymer involved in wound healing has seen widespread use in tissue engineering due to its bioactivity, biocompatibility, biodegradability, and facile processability. However, the versatile biopolymer should be further explored and more specifically for skin tissue engineering strategies due to its remarkable skin repair capacity: intrinsic healing properties, adaptable to biomaterial design from its fibrinogen and thrombin precursors and tunable physico-chemical features. Fibrin’s poor mechanical properties can be efficiently improved by combining the biopolymer with synthetic polymers such as polyethylene glycol. The aim of this commentary is to provide a concise insight on the key properties of fibrin for skin healing and regeneration, particularly highlighting the emerging role of fibrin-based hydrogels as skin substitutes.
Keywords
Fibrin; Wound healing; Skin tissue engineering; Hydrogel, Scaffold
Fibrin and Its Key Role in Skin Wound Healing
Formation of the fibrin network
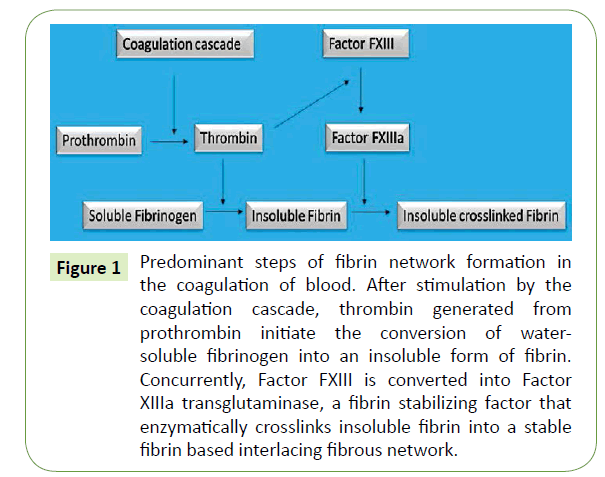
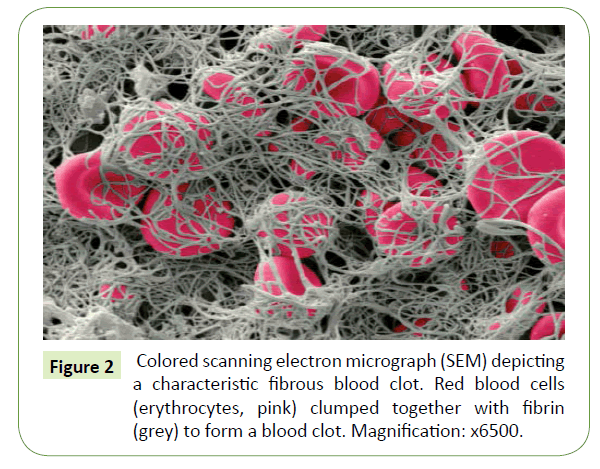
When the skin barrier is compromised, with the fibrin network as an essential component, a temporary physiological process rapidly occurs to close the wound, to stop a potential infection and to activate the restoration of the damaged skin [1]. Thrombin, a serine protease, is activated during the coagulation cascade at the beginning of the wound healing process and converts a soluble plasmatic hexameric glycoprotein of 340 kDa, fibrinogen, into insoluble fibrin monomers by cleaving two small peptides, the fibrinopeptides A and B [2-5]. The removal of fibrinopeptides exhibits “knobs” complementary to “holes” exposed at the end of the fibrinogen glycoprotein and their interactions result in the formation of protofibrils which aggregate to form fibrin fibers [2,4]. The fibrin meshwork constitutes a physiological hydrogel further cross-linked by Factor FXIIIa, a transglutaminase, in order to be stabilized and avoid early enzymatic degradation via a serine protease, plasmin [2,3,5] (Figure 1). This cross-linked fibrin embedding platelets, erythrocytes as well as other proteins (e.g. fibronectin) form the blood clot that reinforces the initial platelet plug at the vascular breach [1] (Figure 2). The fibrillar network also serves as a provisional template for promoting cell migration and proliferation. It is filled with cytokines and growth factors released in the first instance by platelets which attract at the wound bed inflammatory cells (neutrophils and then macrophages) but also activate re-epithelialization, angiogenesis, connective tissue formation and contraction [1,5,6].
Figure 1: Predominant steps of fibrin network formation in the coagulation of blood. After stimulation by the coagulation cascade, thrombin generated from prothrombin initiate the conversion of watersoluble fibrinogen into an insoluble form of fibrin. Concurrently, Factor FXIII is converted into Factor XIIIa transglutaminase, a fibrin stabilizing factor that enzymatically crosslinks insoluble fibrin into a stable fibrin based interlacing fibrous network.
Figure 2: Colored scanning electron micrograph (SEM) depicting a characteristic fibrous blood clot. Red blood cells (erythrocytes, pink) clumped together with fibrin (grey) to form a blood clot. Magnification: x6500.
Interactions of the fibrin network with skin cells
Various cell types including the main cell types in skin [5], keratinocytes and fibroblasts, are able to interact with the fibrin clot. Following injury, fibroblasts from the dermis healthy tissue are stimulated by both growth factors and fibrin itself [5] prior to migrating toward the fibrin matrix. Fibroblasts express new integrin receptors, begin to proliferate and produce newly extracellular matrix components to replace the fibrin temporary construct [1,5]. Simultaneously, keratinocytes from the margins of the wound detach themselves from each other and from the basal lamina in order to migrate toward the fibrin clot in a process called re-epithelialization [1]. Even if keratinocytes are not expressing fibrin-binding receptors, they are able to move across the fibrin mesh by up-regulating some activators of plasmin including tissue-type plasminogen activator (tPA) and urokinasetype plasminogen activator (uPA) [1]. They also secrete various metalloproteinases (MMPs) such as MMP-9, MMP-1 and MMP- 10 [1]. In combination with growth factors, fibrin stimulates the proliferation of keratinocytes which recreate a new stratified epidermis at the wound bed [7].
Fibrin Based Scaffolds and Their Uses in Skin Tissue Engineering
Advantages of using fibrin compared to other natural polymers
Displaying a set of unique features, fibrin offers several practical advantages over other biopolymers including chitosan, hyaluronic acid, collagen or gelatin [8-10]. First, its precursors, fibrinogen and thrombin, can be easily isolated from patient’s blood making autologous scaffolds possible [2]; therefore, limiting immunogenicity unlike collagen type I, the most widespread protein used in skin tissue engineering [8]. Second, the fibrin network displays versatile properties useful for customizable skin substitute development. Polymerization rate, fiber thickness, scaffold pore size and architecture can be fine-tuned by optimizing several factors including pH and precursor concentrations [2,10]. Third, several articles reported the cost effectiveness of isolating fibrin compared to other natural polymers [2,3]. Last, fibrin has been associated with well-established native wound healing properties [1,5]. Fibrin scaffolds stimulate and provide sufficient time for neo-matrix formation while gradually resorbing under the action of proteases. These healing properties are beneficial in promoting wound healing and reducing scar formation for more natural functional and aesthetic characteristics. For instance, collagen-based skin substitutes have been associated with wound contraction and poor scarring suggesting the need to develop improved skin equivalents [8,11].
Fibrin scaffolds and their limits
Fibrin is already used in several forms for skin-related biomedical applications [2,3,6,9,10]. Fibrin glues, which were initially meant to promote hemostasis in surgical procedures were extended to other clinical applications such as skin graft adhesion and wound healing [12]. Keratinocytes seeded into fibrin glues have been shown to improve the healing of refractive leg ulcers [2,3,13] and the treatment of extensive burn wounds [14]. However, the use of fibrin glues as scaffolds in skin tissue engineering remains a challenge due to their highly dense structure unfit for cell survival [15]. Fibrin hydrogels have also been used as biological matrix for skin tissue engineering. Hydrogels represent a class of high water content polymers with physical or chemical crosslinks. Their physical properties are similar to soft tissues [16]. Fibrin hydrogels formed in vitro exhibit similar structure and mechanical properties to those of the fibrin clot in vivo [2]. Different co-culture skin models (keratinocytes/fibroblasts) using fibrin hydrogels as scaffolds were reported to promote vascular growth and epidermis regeneration [2], biointegration after in vivo implantation on nude mice [17] and wound coverage of severe burned areas [18]. Co-culture models were also successfully used to study the interactions and interdependence of the two cell types [19]. Fibrin was combined with fibronectin to enhance its biologic properties and the results highlighted the increase of cellular migration into the wound site [9]. However, applications of fibrin-based scaffolds in skin tissue engineering have been limited due to their poor mechanical properties and fast biodegrability [3].
Overcoming the main limitations of fibrin scaffolds and their potential applications in skin regeneration
A number of strategies could improve the degradation rate of fibrin networks including optimizing fibrinogen precursor and calcium ion concentrations, conjugating fibrin with synthetic polymers such as polyethylene glycol (pegylation), reducing cell density or using protease inhibitors such as aprotinin [2,3]. A vascularized pegylated fibrin-collagen hydrogel incorporating stem cells was successfully developed as a dermis equivalent and showed promising results to treat large surface injured areas [20]. One approach use to reinforce the mechanical stiffness of fibrin matrix is to combine this glycoprotein with a synthetic polymer. Fibrin has been conjugated to several synthetic polymers such as polyethylene glycol, polyurethane and polycaprolactone [3]. The biopolymer has also been used for coating polycaprolactone films in order to develop an epidermal equivalent [21]. Another approach is to use an interpenetrating polymer network (IPN) system [22]. IPN is defined as a combination of polymers in a network form, where at least one polymer is synthesized and/or cross-linked in the presence of the other, either simultaneously or sequentially. The resulting networks are in part entangled on the molecular scale without covalent bonds between them. Their separations from each other are not possible unless chemical bonds are broken [22]. Different methods have been investigated to develop IPN hydrogels and combine the excellent mechanical properties of synthetic polymers with the biologic features of fibrin for skin wound dressing applications. For instance, IPN incorporating fibrin with polyethylene glycol exhibited improved mechanical properties compared to fibrin alone [23]. Fibrin was also associated to polyvinyl alcohol to develop IPN biomaterials for wound healing [24]. Recently, fibrin was combined to a biodegradable co-network of polyvinyl alcohol and serum albumin in IPN architecture. The results showed that the composite biomaterials not only prevented any shrinkage but also exhibited improved mechanical properties [25].
Conclusion and Future Perspectives
Due to its physiological role in wound healing, fibrin is one of the most attractive polymers for skin tissue engineering. Different strategies have been adopted to overcome its lack of mechanical stiffness showing that self-supported fibrin based materials could be designed for skin restoration. Although future studies are necessary to improve the physico-chemical properties of fibrin-based hydrogels, strategies to make improved biomimetic scaffolds hold promise for accelerating the bench-to-bedside translational research in skin regeneration.
Acknowledgment
This work was supported by the ANR Tecsan Fibriderm (project number ANR-13-TECS-0014) and the Picardy region.
References
- Martin P (1997) Wound healing-aiming for perfect skin regeneration. Science 276:75-81.
- Janmey PA, Winer JP, Weisel JW (2009) Fibrin gels and their clinical and bioengineering applications. J R Soc Interface 6:1-10.
- Ahmed TAE, Dare EV, Hincke M (2008) Fibrin: A versatile scaffold for tissue engineering applications. Tissue Eng Part B Rev 14: 199-215.
- Weisel JW (2005) Fibrinogen and fibrin. In: Chemistry B-A in P. Academic Press.pp: 247-299.
- Laurens N, Koolwijk P, De Maat MPM (2006) Fibrin structure and wound healing. J ThrombHaemost 4: 932-939.
- Rajangam T, An SSA (2013) Fibrinogen and fibrin based micro and nano scaffolds incorporated with drugs, proteins, cells and genes for therapeutic biomedicalapplications. Int J Nanomedicine 8: 3641-3662.
- Yamamoto M, Yanaga H, Nishina H (2005) Fibrin stimulates the proliferation of human keratinocytes through the autocrine mechanism of transforming growth factor-α and epidermal growth factor receptor. Tohoku J Exp Med 207: 33-40.
- Zhong SP, Zhang YZ, Lim CT (2010) Tissue scaffolds for skin wound healing and dermal reconstruction. Wiley Interdiscip Rev NanomedNanobiotechnol 2: 510-525.
- Priya SG, Jungvid H, Kumar A (2008) Skin tissue engineering for tissue repair and regeneration. Tissue Eng Part B Rev 14: 105-118.
- Huang S, Fu X (2010) Naturally derived materials-based cell and drug delivery systems in skin regeneration. J Control Release 142: 149-159.
- Ho G, Barbenel J, Grant MH (2009) Effect of low-level laser treatment of tissue engineered skin substitutes: Contraction of collagen lattices. J Biomed Opt 14:034002.
- Spotnitz WD (2010) Fibrin sealant: Past, present, and future: a brief review. World J Surg 34: 632-634.
- Horch RE, Bannasch H, Stark GB (2001) Transplantation of cultured autologous keratinocytes in fibrin sealant biomatrix to resurface chronic wounds. Transplant Proc 33: 642-644.
- Ronfard V, Broly H, Mitchell V (1991) Use of human keratinocytes cultured on fibrin glue in the treatment of burn wounds. Burns 17: 181-184.
- Linnes MP, Ratner BD, Giachelli CM (2007) A fibrinogen-based precision microporous scaffold for tissue engineering. Biomaterials 28: 5298-5306.
- Nguyen KT, West JL (2002) Photopolymerizable hydrogels for tissue engineering applications. Biomaterials 23: 4307-4314.
- Mazlyzam AL, Aminuddin BS, Fuzina NH (2007) Reconstruction of living bilayer human skin equivalent utilizing human fibrin as a scaffold. Burns J IntSocBurn Inj 33: 355-363.
- Meana A, Iglesias J, Del Rio M (1998) Large surface of cultured human epithelium obtained on a dermal matrix based on live fibroblast-containing fibrin gels. Burns J IntSoc Burn Inj 24: 621-630.
- Sun T, Haycock J, Macneil S (2006) In situ image analysis of interactions between normal human keratinocytes and fibroblasts cultured in three-dimensional fibrin gels. Biomaterials 27: 3459-3465.
- Natesan S, Zamora DO, Wrice NL (2013) Bilayer hydrogel with autologous stem cells derived from debrided human burn skin for improved skin regeneration. J Burn Care Res 34: 18-30.
- Khor HL, Ng KW, Htay AS (2003) Preliminary study of a polycaprolactone membrane utilized as epidermal substrate. J Mater Sci Mater Med 14: 113-120.
- Dragan, Ecaterina S (2014) Design and applications of interpenetrating polymer network hydrogels- A review. Chemical Engineering Journal 243: 572-590.
- Akpalo E, Bidault L, Boissiere M (2011) Fibrin-polyethylene oxide interpenetrating polymer networks: new self-supported biomaterials combining the properties of both protein gel and synthetic polymer. ActaBiomater 7: 2418-2427.
- Bidault L, Deneufchatel M, Vancaeyzeele C (2013) Self-supported fibrinpolyvinyl alcohol interpenetrating polymer networks: an easily handled and rehydratable biomaterial. Biomacromolecules 14: 3870-3879.
- Bidault L, Deneufchatel M, Hindie M (2015) Fibrin-based interpenetrating polymer network biomaterials with tunable biodegradability. Polymer 62: 19-27.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences