Exploring the Potent Nematicidal Effects of Potassium Aluminum Sulfate on Root Knot Nematode (Meloidogyne spp.) Infestation in Tomato Plants
Ambreen Maqsood* and Huma Khaliq
Department of Plant Pathology, The Islamia University of Bahawalpur, Bahawalpur, Pakistan
- *Corresponding Author:
- Ambreen Maqsood
Department of Plant Pathology,
The Islamia University of Bahawalpur,
Bahawalpur,
Pakistan,
Tel: 00923065222239;
E-mail: ambreen.maqsood@iub.edu.pk
Received date: June 18, 2023, Manuscript No. IPRJPP-23-16983; Editor assigned date: June 21, 2023, PreQC No. IPRJPP-23-16983 (PQ); Reviewed date: July 06, 2023, QC No. IPRJPP-23-16983; Revised date: August 21, 2023, Manuscript No. IPRJPP-23-16983 (R); Published date: August 29, 2023, DOI: 10.36648/ IPRJPP.6.5.186
Citation: Maqsood A, Khaliq H (2023) Exploring the Potent Nematicidal Effects of Potassium Aluminum Sulfate on Root-Knot Nematode (Meloidogyne spp.) Infestation in Tomato Plants. J Res Plant Pathol Vol:6 No:5
Abstract
Root knot nematode (Meloidogyne incognita) is one of the major plant parasitic nematodes of various vegetable crops across the globe. Nematodes are usually controlled through chemicals (nematicides). However, the negative effects on human health and environmental concerns are associated with their continuous uses. Hence, the search for a safe and effective managing approach is more relevant and effective. In the current study, the effect of different concentrations of potash alum (potassium aluminum sulfate) for different time durations on mortality and locomotion of M. incognita was studied in vitro. Potash alum showed strong nematicidal effect against the root-knot nematode at a low concentration (50 μg/mL). The median Lethal Concentration (LC50) of 7.381, 5.654, 2.091 and 1.735 μg/mL was applied at 12, 24, 48 and 72 h, respectively. Mortality rate of M. incognita after treatment with various concentrations of potash alum for 8, 24, 48 and 72 h were significantly different compared with control exposed to distilled water (P<0.01). Loco motor activity of the root-knot nematodes decreased with increased duration of exposure and was completely lost after 72 h of exposure to 50 μg/mL potash alum. Our results indicate that potash alum is effective against M. incognita and restricts their harmful effects on plants at least in part by inhibiting their movement.
Keywords
Juvenile mortality; Hatching inhibition; Overall mortality of juveniles; Plants; Nematode; Mortality; Locomotion
Introduction
Root Knot Nematodes (RKN) parasitism is a serious problem in the fields and greenhouses as it causes severe yield losses across the globe [1]. RKN (M. incognita) is an obligate plant parasite that has a broad host range of horticultural crops, including tomatoes, carrots, potatoes, cucumber and soybean [2,3]. The typical symptoms of M. incognita are galling on roots, bulbs, and tubers [4]. The above ground symptoms are wilting, stunting and yellowing due to reduced nutrients and water uptake efficiency of plants from soil and predisposition of roots for secondary pathogen invasion [5]. Generally, various strategies have been used to manage RKN, such as resistant cultivars, biological agents and the use of chemicals in various cropping systems. However, these conventional strategies become insufficient due to the following two reasons. Firstly, the resistant cultivars against M. incognita mediated with Mi genes lose their potential at high temperature climates [6,7]. Secondly, the high amounts of multiplication and spread obligate biological control agents, which is challenging due to its narrow host range [8].
Presently, the main approach for controlling root knot nematode on a large scale is use of synthetic chemical pesticides such as fumigants and non-fumigants in soil [9]. With the development in modern agricultural science and technology, new chemicals are continuously synthesized, which are hazardous for the environment and human health [10]. Although treatment with methyl bromide and aldicarb chemicals is the most effective and efficient to manage root knot nematode in fields, they are banned due to being highly toxic and their damaging effects on stratospheric ozone [11]. Therefore, efforts are being made for identifying and developing alternative strategies to control the nematodes. Hence, there is dire need to use low toxic and environmentally safe chemicals in the agricultural fields and greenhouses. Natural products have been applied for centuries in the treatment of plant, animal and human diseases caused by microbial agents due to their therapeutic properties [12]. Potassium aluminum sulfate (potash alum) is a natural chemical compound of double sulfate of potassium and aluminum with chemical formulae KAl (SO4)2 being utilized in many industrial processes. It is an odorless, colorless octahedral crystalline solid. Potash alum is a salt of potassium and aluminum with sulfate. When Al3+ ions are solvated by water molecules, their high charge pulls electron density toward them, making the electron density between O−H in solvating water molecules less. Potash alum is an active ingredient of many medicines, mainly antiseptic and astringent, due to its antibacterial and antifungal properties [13]. Potash alum is also used in culinary, flame retardant, tanning, dyeing, chemical flocculent, lake pigments and dissolving iron and steel in industries [14].
The current study was planned to investigate the followings: i) Nematicidal activity of potash alum on second stage juveniles (J2s) of M. incognita, ii) Hatching inhibition effect on free eggs in vitro and iii) Effect of potash alum on M. incognita reproduction in a pot experiment in the presence of F. oxysporum.
Materials and Methods
Potash alum (99.8%) was obtained from the Zhengbang biochemical Co., Ltd. (Nanchang, China). In bioassay, five different concentrations of potash alum (10, 20, 30, 40 and 50 μg/mL) were used in this experiment. The used concentrations were based on the relationship of potash alum and M. incognita mortality assessment in a preliminary experiment. When the concentrations were increased 10 percent, the M. incognita mortality rate ranged from 10% to 90 %. Distilled water was used as a solvent and control in the whole experiments.
Nematode culture
Nematode culture was prepared on the roots of tomato cv. Money Maker (China vegetable seed technology Co., Ltd. Beijing, China). Thirty days old tomato seedlings were transplanted in pots, each containing 1 kg autoclaved peat moss purchased from Nanning Guiyuxin agricultural technology Co., Ltd. Nanning, China and maintained at 28°C ± 2°C with a 14 hrs light (22000 Lux) and 10 hrs dark photoperiod within a GXZ-28°C incubator (Jiangnan instrument factory, Ningbo, China). After 40 days, tomato roots were uprooted and rinsed with regular tap water gently. M. incognita eggs were extracted with the sodium hypochlorite (NaOCl) method and placed to hatch in petri dishes containing distilled water at room temperature (25°C ± 2°C) by Baermann funnel method [15,16]. The freshly hatched eggs and second stage juveniles of M. incognita were used in this experiment.
Effect of potash alum on the ovicidal activity of M. incognita
The ovicidal activity was evaluated following the method of Ridzuan, et al. Fresh eggs of M. incognita were placed in a 24 well micro plate that already contained different concentrations of potash alum (0, 10, 20, 30, 40 and 50 μg/mL) [17]. One hundred fresh eggs were dispensed in each well with 200 μL of each concentration of potash alum with five replicates. Furthermore, 200 μL of sterilized distilled water was used as a control. For the accuracy of the results, the experiment was repeated three times. The micro plates were covered with the lid and incubated at room temperature of 28°C ± 2°C for 3 days. The eggs hatching was microscopically (Ti-S, Nikon Instruments Inc., Tokyo, Japan) observed after 0, 3, 6, 9, 12 and 15 days of exposure. To ascertain whether potash alum had an ovistatic effect, unhatched eggs were transferred to sterilized water to examine the resumption of motility. The cumulative hatching rate was determined by the following formula.
Cumulative hatching rate=(The number of hatched J2s)/ (The initial number of eggs) × 100.
Effect of potash alum on the nematicidal activity of M. incognita
Sixty fresh second stage juveniles (J2s) of M. incognita were shifted in 24 well micro plate containing different concentrations (0, 10, 20, 30, 40 and 50 μg/mL) of potash alum and sterilized distilled water was used as a control. Each well contained 200 μL of potash alum of required concentration. The covered micro plates were incubated at room temperature of 28°C ± 2°C for 72 hrs in humid conditions. The egg hatching was microscopically (Ti-S, Nikon instruments Inc., Tokyo, Japan) monitored after 0, 3, 6, 9, 12, and 15 hrs of exposure. To ascertain whether larvistatic or larvicidal activity, the immotile J2s were rinsed with sterilized water to examine resumption of motility. These Juveniles were considered dead when they remained immotile on probing with fine hair needle. All treatments have five replicates and the experiment was repeated thrice for the accurateness of results. The mortality percentage was calculated as follows [18].
Mortality=(The number of dead J2s ÷ total J2s) ×100
Pot experiment
M. incognita reproduction solely or in the presence of F. oxysporum on tomato cultivar at different concentrations of potash alum was investigated in the greenhouse of Guangxi university China. Thirty day old tomato seedlings were transplanted in pots (7 × 7 × 8) inches containing 1 kg autoclaved peat moss. The experiment was investigated under a complete randomized design with four replicates. Three holes were created across the rhizospheric area of every plant. A total of 1500 second stage Juveniles (J2s) were inoculated in each pot with the help of a pipette in these holes after two days of transplanting and the holes were covered with surface soil. While, in co-inoculation F. oxysporum (1 × 105 CFU mL−1) and M. incognita (1500 J2s) were applied in each pot with five replicates. Subsequently, 80 mL of each concentration (30, 50, 200 and 250 μg/mL) was added to the respective pot in single inoculation. The seedlings were grown in the greenhouse at a temperature of 25℃ ± 2℃ under a 14 hrs light and 10 hrs dark period. After thirty five days of inoculation, all tomato roots were uprooted gently and rinsed with running regular tap water to get rid of peat moss. The nematode reproduction factors (the number of eggs, egg masses, juveniles, females), plant height, weight and total number of galls per treatment were determined. This experiment was repeated twice for the accuracy of the results. The eggs were separated from roots with the NaOCl method as previously described. The roots were stained with acid fuchsin solution (0.35 g of acid fuchsin, 25 mL of acetic acid, and 75 mL of distilled water) to count the number of females. The J2s population of M. incognita in soil was determined by centrifugation and floating method and juveniles were counted and expressed as J2s per 250 cm3 of soil.
Model validations
The quantitative model was constructed between different concentrations of potash alum and exposure time and the regression evaluation of the coefficient determination R2 with the Root Mean Square Error (RMSE). The R2 and RMSE are two key goodnesses to fit measure regression coefficient. Prediction errors showed how many values are far away and around the regression lines. We used the RMSE model to assess the relationship of different concentrations and exposure time. RMSE was calculated by the given formula.
Data analysis
Data on ovicidal and larvicidal activity were analyzed using two way variance analysis to measure the relationship between ovicidal and nematicidal activity, concentration and posttreatment time (exposure time), while the differences between treatment means were determined by Duncan's multiple range test at P=0.05 for their significance. For this study statistical analysis was performed on IBM-SPSS statistics (version 25.0) software. The graphical presentation was done on prism graph pad. Different statistical packages such as EPA probit analysis program (version 1.5) software were used to measure (LC50) with 95% confidence interval of ovicidal activity and nematicidal activity.
Results and Discussion
The elimination of root knot nematodes from vegetable crops is essential for food security. The management of root knot nematode is a challenging progression and current control strategies depend on the application of pesticides. However, many synthetic chemicals are banned in the market due to hazardous and toxic effects on human health and the environment. Many natural compounds are valuable source of drugs and have been used since ancient times to treat diseases. Potash alum is natural compound, used in many drugs for humans, plants and animals. However, its nematicidal effect is still unknown. Therefore, the present study was planned to investigate the appropriate concentration of potash alum and time exposure against M. incognita.
Ovicidal activity
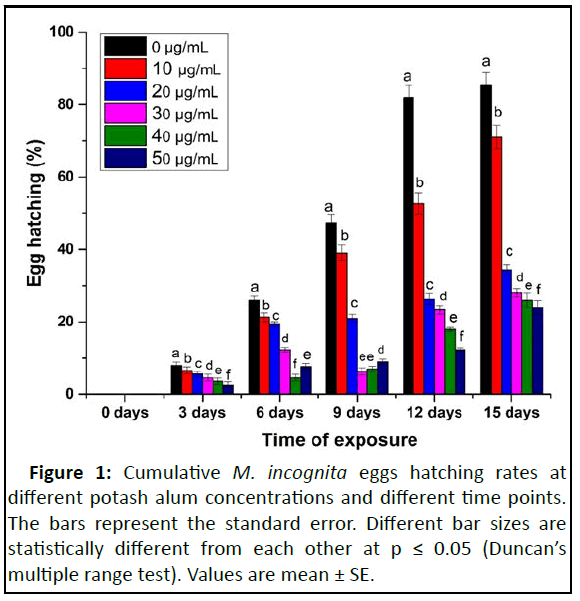
The nematicidal potential of potash alum at different time points was determined on the cumulative egg hatching rate of M. incognita (Figure 1). Matured egg masses were chosen and also assured that all the eggs had the same embryonic stage. The egg hatching rate decreased significantly on increasing exposure time up to 15 days in low to high concentrations. In comparison to sterilized distilled water (control), the maximum inhibition in cumulative egg hatching (76%) was recorded at 50 μg/mL potash alum concentration, followed by 40 μg/mL (74%), 30 μg/mL (72%), 20 μg/mL (66%) and 10 μg/mL (29%) after 15 days. The un hatched eggs were shifted to sterilized water to monitor their hatching activity without potash alum. Further, the ovicidal activity of potash alum concentrations revealed that it has a hostile effect on egg hatching. The inhibition of hatching percentage was directly proportional to the amount of potash alum and exposure time. The statistical outcome showed a highly significant analysis of variance (p ≥ 0.0001) with 856.79, 1788.41 and 130.66 intercept between concentration and time. Shahriari, et al., found white alum to be potent against Escherichia coli O157:H7 at a concentration above 1% (p ≤ 0.05) after 4 hrs. These findings were further strengthened by Habash and Al-Banna, et al. who observed the nematicidal property of potassium phosphonate fertilizer against M. incognita and M. javanica [19]. Potash alum aqueous solution is acidic due to cationic hydrolysis with pH 3. Protons are therefore more likely to be abstracted by water. The optimum pH (6.5) is suitable for both egg hatching and juvenile survival of M. incognita [20]. Karajeh and Al-Nasir, et al. stated that calcium chloride and sodium chloride at 8 mmohs/cm significantly suppressed the egg masses hatching rate and infectivity of juveniles of M. incognita [21]. Consequently, in our findings, all concentrations of potash alum (pH 3.2) showed ovicidal and larvicidal effects on M. incognita.
Nematicidal activity
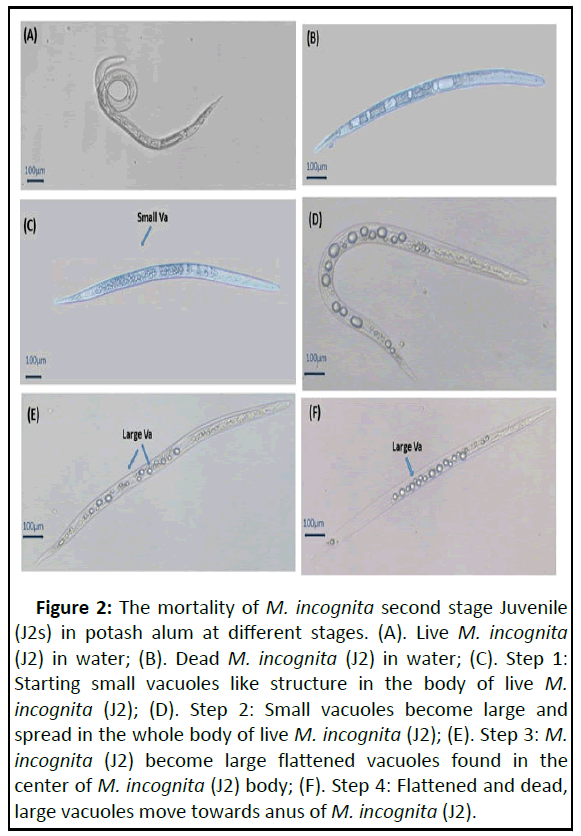
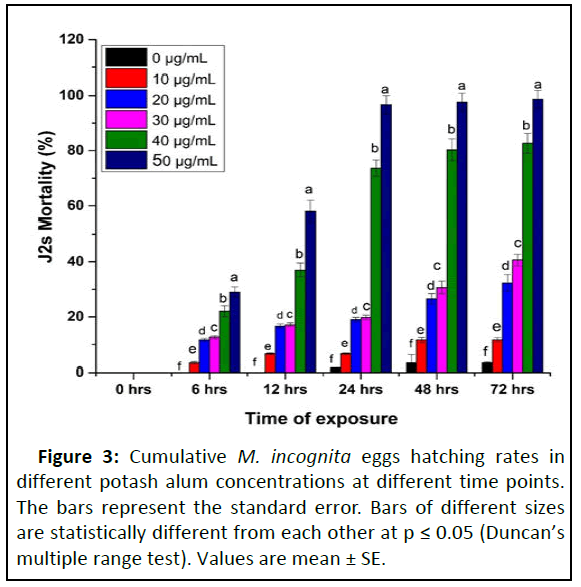
Potash alum exhibited a strong toxic effect on second stage Juveniles (J2s) of M. incognita at various concentrations (10 μg/mL to 50 μg/mL). The overall mortality of juveniles increased with time exposure and increase in concentration (Figures 2 and 3). In comparison to sterilized distilled water (control), the maximum juvenile mortality of 98% was recorded at 25 μg/mL potash alum concentration followed by 20 μg/mL (95%), 15 μg/mL (32.4%) and 10 μg/mL after 15 days. At 10 μg/mL concentration, the larvicidal activity was less than 5.2% and no significant differences were recorded for 3 to 15 hrs time exposure. The mortality rates recorded at 20 μg/mL, and 30 μg/mL after 6, 12, 24, 48 and 72 hrs were 37.0 and 11.6%, 46.0 and 16.6%, 62.0 and 19.0%, (73.9 and 26.6%, and 52.0 and 32.3%, respectively. After exposure to potash alum, juveniles were shifted to sterilized water but they did not show any resumption of vital activity. The percent of larvicidal activity was directly proportional to concentration and exposure time. The statistical outcome showed a highly significant fitness model (p>0.0001) with 7613.48, 2267.17 and 409.06 corrected model intercept between concentration and time. Table 2 aluminum and potash ions in water have increased the rate of juveniles' metabolism compared to control. This would have ensued in reducing the energy sources of juveniles to search and invade host roots and eventually lead to death [22]. Previously, He, et al., after application of Aspergillus japonicas ZW1 fermentation filtrate, observed large size bubbles in the intestine of J2s of M. incognita. Ntalli, et al. research exhibited acidoid (acetic acid) damage to the nuclei of cells and led to intensive cytoplasmic vacuolization areas in the intestine of J2s of M. incognita. Although the presence and role of these vacuoles or bubbles are not anatomically understood and hence need to be further investigated [23]. These vacuoles or bubbles were possibly filled with lipids before the chemical application, which led to lipid depletion.
Figure 2: The mortality of M. incognita second stage Juvenile (J2s) in potash alum at different stages. (A). Live M. incognita (J2) in water; (B). Dead M. incognita (J2) in water; (C). Step 1: Starting small vacuoles like structure in the body of live M. incognita (J2); (D). Step 2: Small vacuoles become large and spread in the whole body of live M. incognita (J2); (E). Step 3: M. incognita (J2) become large flattened vacuoles found in the center of M. incognita (J2) body; (F). Step 4: Flattened and dead, large vacuoles move towards anus of M. incognita (J2).
Probit analyses and model validation
The probit analyses LC50, and LC90 values, at 95% Confidence Interval (CI) of egg hatching rate and J2s mortality of M. incognita are presented in Table 4. In the ovicidal activity, the lowest LC50 was recorded at 11.800 at 15 days of exposure, followed by 13.425, 14.196, 18.432 and 25.564 at 6, 9, 12 and 3 days, respectively. The lowest LC90, 3.558, was measured at 9 days of exposure. Whereas in larvicidal activity, the lowest LC50 (1.735) was analyzed after 72 hrs of exposure, followed by 2.091, 5.654, 7.381 and 12.67 after 48, 24, 12 and 6 hrs. The lowest LC90 0.3871 was demonstrated after 24 hrs. In the nematicidal activity the LC50 value decreased with exposure of time. Under in vitro conditions, potash alum was displayed to contain more potential in larvicidal activity than ovicidal activity. Analysis of variance for exposure time and concentration towards ovicidal and nematicidal activities are presented in Tables 1 and 2. The exposure of time demonstrated that R2 values of the ovicidal assay at 6, 12, 24, 48 and 72 days were 0.454, 0.832, 0.852, 0.976 and 0.766, respectively revealing better performance as compared to RMSE (Table 1). Furthermore, R2 values of time exposure of nematicidal activity were 0.887, 0.712, 0.989, 0.899 and 0.743 at 9, 12, 24, 48 and 72 hrs, respectively, showing the best curve and best model to the attained data (Table 2).
| Assay | Exposure time | Slope | RMSE | R2 | LC50 (95% CI) | LC90 (95% CI) |
|---|---|---|---|---|---|---|
| Ovicidal | 3 d | 1.45 | 4 | 0.456 | 25.564 (20.765-30.651) | 11.643 (5.653-6.321) |
| 6 d | 1.8 | 5.66 | 0.832 | 13.425 (11.976-15.033) | 4.973 (2.559-9.667) | |
| 9 d | 0.54 | 2.64 | 0.852 | 14.196 (12.851-15.68) | 3.558 (4.04-5.267) | |
| 12 d | 1.98 | 10.76 | 0.976 | 18.432 (13.654-23.877) | 7.245 (3.99-13.67) | |
| 15 d | 0.63 | 2.43 | 0.766 | 11.800 (8.556-16.276) | 4.65 (2.76-6.92) | |
| Nematicidal | 6 hrs | 2.76 | 6.76 | 0.887 | 12.67 (5.43-17.63) | 2.214 (1.58-3.39) |
| 12 hrs | 1.87 | 5.43 | 0.712 | 7.318 (3.654-11.532) | 0.2455 (0.0538-1.121) | |
| 24 hrs | 2.54 | 6.72 | 0.989 | 5.654 (3.77-7.543 | 0.3871 (0.103-1.447) | |
| 48 hrs | 0.65 | 6.31 | 0.899 | 2.091 (1.432-3.653) | 1.5905 (0.724-3.493) | |
| 72 hrs | 1.37 | 9.75 | 0.743 | 1.7351 (1.0919-2.753) | 1.6332 (0.8955-2.978) |
Note: RMSE: Root Mean Squared Error; R2: Coefficient of determination; LC: Lethal Concentration; CI: Confidence Interval
Table 1: Regression model for exposure time in response to ovicidal and nematicidal activity.
| Assay | Conc. | Slope | RMSE | R2 | LC50 (95% CI) | LC90 (95% CI) |
|---|---|---|---|---|---|---|
| Ovicidal | 10 µg/mL | 1.15 | 4 | 0.968 | 2.2171 (1.076-4.611) | 0.676 (0.213-2.147) |
| 20 µg/mL | 1.5 | 5.66 | 0.983 | 6.7313 (5.280-8.580) | 3.287 (2.164-4.981) | |
| 30 µg/mL | 1.84 | 2.64 | 0.966 | 9.57 (7.529-12.163) | 5.511 (3.594-8.450) | |
| 40 µg/mL | 1.98 | 10.76 | 0.966 | 10.96 (9.010-13.337) | 6.754 (4.755-9.592) | |
| 50 µg/mL | 1.97 | 9.65 | 0.954 | 12.281 (11.051-13.648) | 8.193 (6.831-9.825) | |
| Nematicidal | 10 µg/mL | 2.76 | 6.76 | 0.978 | 14.196 (12.851-15.681) | 3.558 (2.404-5.267) |
| 20 µg/mL | 1.87 | 5.43 | 0.979 | 13.425 (11.989-15.033) | 4.094 (2.730-6.140) | |
| 30 µg/mL | 2.54 | 6.72 | 0.989 | 11.683 (9.202-15.290) | 4.973 (2.559-9.664) | |
| 40 µg/mL | 0.65 | 6.31 | 0.899 | 11.800 (8.55-16.274)) | 7.249 (3.991-13.166) | |
| 50 µg/mL | 1.32 | 5.78 | 0.934 | 11.927 (7.734-18.392) | 9.190 (4.933-17.119) |
Note: RMSE: Root Mean Squared Error; R2: Coefficient of determination; LC: Lethal Concentration; CI: Confidence Interval; Conc: Concentration.
Table 2: Regression model for concentrations in response to ovicidal and nematicidal activity.
Pot experiment
The application of potash alum in soil signi icantly reduced the galling index and nematode population in root and soil of tomato (Table 3). The number of root galls per gram weight, number of eggs per gram, number of females and number of second stage juveniles per 250 cm3 of soil were 10.4 and 10.1, 12.0 and 8.0, 1874 and 1534 and 118 and 108 at the 250 μg/mL potash alum concentration in a single application of M. incognita and in the presence of F. oxysporum, respectively; whereas 13.4 and 12.4, 3452 and 2672, 20 and 17 and 179 and 159 were recorded per plant at 200 μg/mL application, respectively. In both treatments, the number of galls, number of females, number of eggs per gram, and number of juveniles per 250 cm3 of soil were signi icantly lowered than measured in control plants. The 250 μg/mL application of potash alum decreased the number of root galls by 75%, the number of second stage juveniles 83%, number of eggs 83%, and number of females 82% per gram of roots as compared to control (tap water with 1500 J2s) in single M. incognita application. All potash alum concentrations did not affect the plant growth parameters in both treatments compared to control (Table 4). Similar results were observed by Kamel, et al. inn organic banana against crown rot. Aluminum sulfate salt has a strong inhibitory effect against potato dry rot and carrot spot disease at 5 mM [24-28].
| Treatment | No. of juveniles/250 cm3 of soil | Number of eggs/gram of roots | Number of females/gram of roots |
|---|---|---|---|
| Water (control) | 640 ± 16.4a | 11124 ± 145a | 83 ± 2.7a |
| 30 µg/ mL | 573 ± 17b | 9574 ± 145b | 65 ± 2.4b |
| 50 µg/ mL | 564 ± 18c | 9543 ± 148c | 62 ± 2.2bc |
| 200 µg/ mL | 179 ± 19.2d | 3452 ± 89d | 20 ± 1.2d |
| 250 µg/ mL | 118 ± 16.5e | 1874 ± 74e | 12 ± 1.7e |
Note: All the data presented are mean ± SE of three replications. The different lower case letters within a column indicate significant differences at (P ≤ 0.05, Duncan’s multiple range test).
Table 3: Effect of potash alum on M. incognita reproduction of tomato.
| Treatment | Plant height (cm) | Plant weight (g) | Root length (cm) | Galling number/gram of roots |
|---|---|---|---|---|
| Water (control) | 30.0 ± 0.7a | 15.0 ± 0.2a | 53.0 ± 2.7c | 42.3 ± 1.7a |
| 30 µg/ mL | 30.6 ± 0.6a | 14.6 ± 0.3a | 65.5 ± 3.5b | 38.2 ± 1.9b |
| 50 µg/ mL | 31.0 ± 0.6a | 14.7 ± 0.3a | 64.6 ± 2.4b | 28.5 ± 1.7c |
| 200 µg/ mL | 30.2 ± 0.6a | 14.8 ± 0.2a | 79.5 ± 3.6a | 13.4 ± 1.5d |
| 250 µg/ mL | 30.4 ± 0.7a | 14.5 ±0.3a | 80.2 ± 3.2a | 10.4 ± 1.8e |
Note: All the data presented are mean ± SE of three replications. The different lower case letters within a column indicate significant differences at (P ≤ 0.05, Duncan’s multiple range test).
Table 4: Effect of potash alum on plant growth of tomato after application of M. incognita.
Conclusion
The potash alum exhibited a strong toxic effect on second stage juveniles of M. incognita at various concentrations (10 μg/ mL to 50 μg/ mL). The overall mortality of juveniles increased with increase in concentration and exposure time. In comparison to sterilized distilled water (control), the maximum juvenile mortality of 98% was recorded at 25 μg/mL of potash alum concentration. The maximum inhibition in cumulative egg hatching (76%) was recorded at 50 μg/mL concentration. However, potash alum have a strong inhibitory effect on nematode population.
Author Contributions
M.A., conceived and designed the experiments; H.K performed the experiments; M.A analyzed the data and wrote the manuscript; H.K. and M.A reviewed the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Thanks to higher education commission, Pakistan for funding to conduct the research
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- Subedi P, Gattoni K, Liu W, Lawrence KS, Park SW ( 2020) Current utility of plant growth-promoting rhizobacteria as biological control agents towards plant parasitic nematodes. Plants 9:1167
[Crossref] [Google Scholar] [PubMed]
- Tzortzakakis EA, Gowen SR (1994) Resistance of a population of Meloidogyne spp. to parasitism by the obligate parasite pasteuria penetrans. Nematol 40:258-266
- Hussain M, Zouhar M, Rysanek P (2017) Effect of some nematophagous fungi on reproduction of a nematode pest, heterodera schachtii, and growth of sugar beet. Pak J Zool 49:189-196
- Ralmi NH, Khandaker MM, Mat N (2016) Occurrence and control of root knot nematode in crops: A review. Aust J Crop Sci 11:1649-1654
- Mai WF, Abawi GS (1987) Interactions among root-knot nematodes and fusarium wilt fungi on host plants. Annu Rev Phytopathol 25:317-338
- Cooper WR, Jia L, Goggin L (2005) Effects of jasmonate induced defenses on root-knot nematode infection of resistant and susceptible tomato cultivars. J Chem Ecol 31:1953-1967
[Crossref] [Google Scholar] [PubMed]
- Secgin Z, Kavas M, Yildirim K (2021) Optimization of agrobacterium mediated transformation and regeneration for CRISPR/Cas9 genome editing of commercial tomato cultivars. Turk J Agric For 45:704-716
- Withers TM, Allen GR, Reid CA (2015) Selecting potential nontarget species for host range testing of Eadya paropsidis. N Z Plant Prot 68:179-186
- Ji X, Li J, Dong B, Zhang H, Zhang S, et al. (2019) Evaluation of fluopyram for southern root-knot nematode management in tomato production in China. Crop Prot 122:84-89
- Carvalho FP (2017) Pesticides, environment, and food safety. Food Energy Secur 6:48-60
- Lu H, Xu S, Zhang W, Xu C, Li B, et al. (2017) Nematicidal activity of trans-2-hexenal against Southern root-knot nematode (Meloidogyne incognita) on tomato plants. J Agric Food Chem 65:544-550
[Crossref] [Google Scholar] [PubMed]
- Jamshidi-Kia F, Lorigooini Z, Amini-Khoei H (2017) Medicinal plants: Past history and future perspective. J Herb Med Pharmacol 7:1-7
- Ali ZM (2018) Synergistic antibacterial interaction between an alum and antibiotics on some microorganism. Sci J Med Res 2:47-51
- Mukherjee S, Mukherjee S (2011) Industrial mineralogy: Mineral processing, beneficiations and other related mineral usage. Appl Ind Env 2011:428-489
- Barker KR, Hussey RS (1976) Histopathology of nodular tissues of legumes infected with certain nematodes. Phytopathology 66:851-855
- Wu HY, Zhang LY, Zhou XB (2020) Effects of Myrothecium verrucaria ZW-2 fermentation filtrates on various plant-parasitic nematodes. J Plant Dis Prot 127:545-552
- Ridzuan PM, Alaina HY, Atika AR, Nazira CM, Afrina A, et al. (2019) The efficacy of Piper aduncum extract as natural larvicides against Aedes aegypti larvae. Int J Med Toxicol Leg Med 22:154-159
- Wu HY, de Oliveira Silva J, Becker JS, Becker JO (2021) Fluazaindolizine mitigates plant-parasitic nematode activity at sublethal dosages. J Pest Sci 94:573-583
- Habash S, Al-Banna L (2011) Phosphonate fertilizers suppressed root knot nematodes Meloidogyne javanica and M. incognita. J Nematol 43:95-100
[Google Scholar] [PubMed]
- Hutangura P, Jones MG, Heinrich T (1998) Optimisation of culture conditions for in vitro infection of tomato with the root knot nematode Meloidogyne javanica. Australas Plant Pathol 27:84-89
- Karajeh MR, Al-Nasir FM (2008) Salt suppression of Meloidogyne javanica on tomato. Nematol Mediterr 36:185-190
- Berg RH, Fester T, Taylor CG (2009) Development of the root knot nematode feeding cell. Cell biology of plant nematode parasitism 2009:115-152
[Google Scholar] [PubMed]
- Ntalli N, Ratajczak M, Oplos C, Menkissoglu-Spiroudi U, Adamski Z (2016) Acetic acid, 2-undecanone, and (E)-2-decenal ultrastructural malformations on Meloidogyne incognita. J Nematol 48:248-260
[Crossref] [Google Scholar] [PubMed]
- Kamel M, Cortesi P, Saracchi M (2016) The influence of potassium alum, sodium bicarbonate, and chlorine treatments on banana's crown rot disease progress. InX International Symposium on Banana: ISHS-ProMusa Symposium on Agroecological Approaches to Promote Innovative Banana. Acta Horticulturae 247-254
- Kolaei EA, Cenatus C, Tweddell RJ, Avis TJ (2013) Antifungal activity of aluminium containing salts against the development of carrot cavity spot and potato dry rot. Ann Appl Biol 163:311-317
- He Q, Wang D, Li B, Maqsood A, Wu H (2020) Nematicidal evaluation and active compounds isolation of Aspergillus japonicus ZW1 against root-knot nematodes Meloidogyne incognita. Agron 10:1222
- Shahriari R, Salari S, Shahriari S (2017) In vitro study of concentration effect and time course pattern of white alum on Escherichia coli O157: H7 growth. Afr J Tradit Complement Altern Med 14:311-318
- Türkkan M (2013) Antifungal effect of various salts against Fusarium oxysporum f. sp. cepae, the causal agent of Fusarium basal rot of onion. J Agric Sci 19:178-187
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences