ISSN : 0976-8505
Der Chemica Sinica
Evaluation of Treatment Strategies by Adsorption for Lead Removal from Aqueous Solution
Awachat RS* and Khope RU
Department of Chemistry, Shivaji Science College, Congress Nagar (M.S.), India
Abstract
The contamination due to heavy metals from industrial waste water is most important issues for industrialized area. In this work removal of lead from aqueous solution was investigated using granular activated carbon modified with 1,3,5-Triazine-2,4,6-triamine.The adsorption of lead using GAC filtrasorb 300 (GAC F-300) and filtrasorb 816 ( GAC F-816) were carried out at constant temperature 25 + 1oC at about 500 rpm. The adsorption data were well analysed for Freundlich, Langmuir and Temkin adsorption isotherm models.
Keywords
Adsorption, Lead, Granular activated carbon (GAC), Filtrasorb 300 (F-300), Filtrasorb 816 (F-816), 1,3,5-Triazine-2,4,6-triamine
Introduction
Now days an environment and human being facing a serious problem due to the toxicity, persistence and bioaccumulation affinities of heavy metals in water bodies. Metals and their compounds are extremely important to the industrial and technological development in developed countries due to numerous of applications for commercial uses [1,2]. Lead is one of the heavy metals found in industrial wastewater and its discharge into water bodies poses an adverse effect on aquatic and terrestrial lives. Lead poisoning causes severe damage to the nervous system, kidney, reproductive system, brain, liver cause illness or death. Severe exposure to lead has been connected with stillbirths, abortion, sterility and neonatal death [3-5].
Lead is naturally occur as an element in insoluble form in the earth crust and biologically inoffensive forms [6]. There are number of methods employed for the treatment of industrial effluents containing Lead. Some of the important methods are chemical precipitation, ion exchange, electrodialysis and carbon adsorption use for water treatment [7-19]. Many advanced techniques are available for removal of heavy metal involves high investments, which are not suitable for small scale industries, which discard comparatively low volumes of wastewater. Adsorption is an effective separation and purification technique used in industry especially in water and waste water treatments [20].Treatable amount of lead wastes are growing need in the industrial sector to try and find solution to remove this metal from waste waters using granular activated carbon [21-33]. Lead is considered as a major threat for various human diseases once it goes beyond the permissible limit recommended by the World Health Organisation (WHO) (3-10 μg/L) in drinking water [34].
Materials and Methods
The Granular Activated Carbon filtrasorb 300 (F-300) and filtrasorb 816 (F-816) gifted by M/s Calgon Carbon Corporation Ltd Pittusberg, USA were selected as adsorbent for adsorption study. These adsorbents were first sieved to obtain the carbon particles of desired size. All the particles were collected in clean petridish which used as adsorbent for overall studies. The sieved GAC particles were completely washed with hot double distilled water until the supernatant was free of dirt particles and then kept in an vacuum oven at a temperature of 105°C for 5 hours for dry. It was cooled in a desiccators containing anhydrous CaCl2 to verify complete removal of moisture from the pores on surface of GAC. A synthetic solution of lead ions was prepared by dissolving required quantity of Pb(NO3)2 (S.D. Fine Chem. Limited) in freshly prepared double distilled water and all working solutions were prepared by dilution with double distilled water.
Each solution was treated with 2 ml aqueous solution of alizarin Red (S) and 1 ml NaOH (2M). The optical densities of all experimental solutions were estimated using Chemito Spectrascan UV 2700 Double beam UV Visible spectrophotometer at λmax 485 nm. A Beer’s law standard curve was obtained by plotting optical densities versus concentration of lead. The equation computed was used to estimate residual concentration of Pb2+ ions [35]. All the reagents used in this work were of AR grade. A sample of 1,3,5-Triazine-2,4,6-triamine(S.D. Fine Chem. Limited) was purified and recrystallized by standard method. The melting point of 1,3,5-Triazine-2,4,6-triamine was found to be 344.5°C compared with literature value 345°C [36]. The sample was also characterized through determination of molecular weight by the technique of pH titration against standard NaOH. To study the adsorption isotherm of Lead ions, 200 ml of 0.001 M solution of 1,3,5-Triazine-2,4,6-triamine agitated with 0.5 g of GAC in reagent bottles of 300 ml capacity. It was then shaken for about five hours using Teflon bladed stirrer at about 500 rpm. After five hours the solution was decanted and the carbon particles were washed completely with double distilled water which recognized as loaded carbon. This loaded carbon was then transferred to same reagent bottle and then 200 ml of lead solution of pH = 6 were added to it. The contents were agitated for 5 hours in a thermostat at a constant temperature of 25 ± 1°C. The initial and final concentrations of lead ions were estimated by putting the values of absorbance in the equation obtained from Beer’s law curve. The experiments were repeated twice to test reproducible results.
Results and Discussion
The adsorption data of Pb2+ on GAC were analyzed in the light of Freundlich, Langmuir and Temkin models. The relationship between the liquid phase concentration and surface concentration of adsorbate at equilibrium was obtained to describe adsorption isotherm.
The quantity of Lead on the modified GAC was estimated using the equation
qe=(Co-Ce) × V/W (1)
Where,
qe=Concentration of Lead ion on the modified GAC (mg/millimoles)
Co=Initial concentration of Lead ion (mg/L)
Ce=Final concentration of Lead ion (mg/L)
W=Millimoles of the ligand actually present on GAC (0.5 g).
V=Volume of solution in liters,
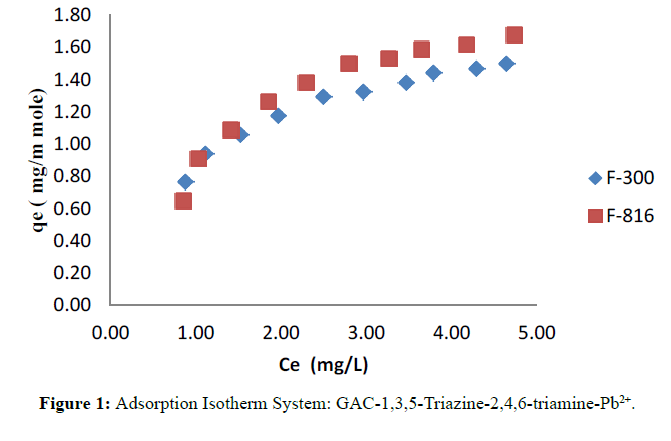
The adsorption isotherms of modified F-300 and F-816 GAC obtained by plotting qe versus Ce and shown in Figure 1.
The Langmuir equation could be expressed as
qe=Qob × Ce / (1+bCe) (2)
Where,
Qo = Amount adsorbed per unit weight of the GAC to form monolayer.
b = Langmuir constant.
Rearranging equation (2)
1/qe =1/Qob × 1/Ce+1/Qo (3)
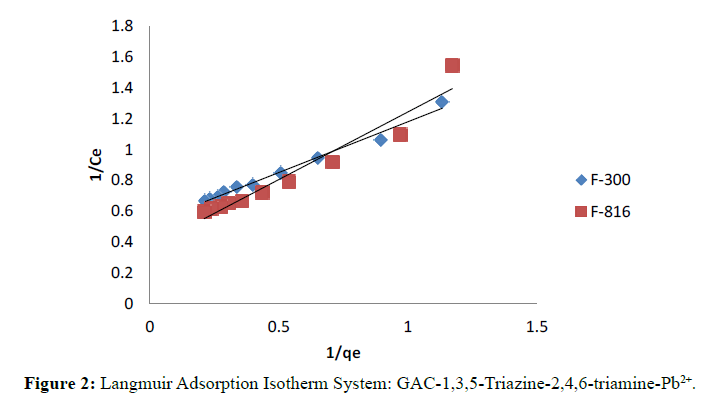
A plot of 1/qe versus 1/Ce was found to be fairly linear indicate the validity of isotherm.
The Freundlich equation express as
qe=K.Ce 1/n (4)
Where, k and l/n are Freundlich constants determined experimentally. Using equation (4)
log qe=log K+1/n log Ce (5)
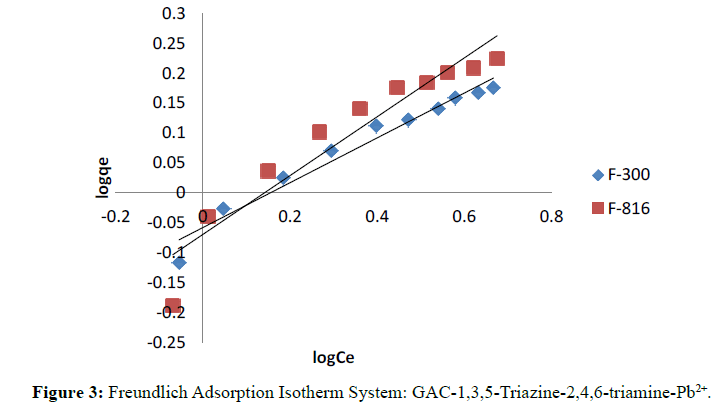
The linearity in the plot of log qe against log Ce showed validity of Freundlich equation over a range of concentrations.
Langmuir and Freundlich isotherms for F-300 and F-816 are illustrates in Figure 2 and 3. The plots of 1/qe against 1/ Ce were found to be linear indicating the validity of Langmuir model. The Langmuir constants relating to the sorption capacity (Qo) and adsorption energy (b) were determined (Table 1).
| Sr. No. | Adsorption System | LangmuirConstant | Freundlich Constant | ||||
|---|---|---|---|---|---|---|---|
| Qo | b | R2 | K | 1/n | R2 | ||
| 1 | F-300-1,3,5-Triazine-2,4,6-triamine-Pb2+ | 1.9173 | 0.7927 | 0.9882 | 0.8744 | 0.3752 | 0.9636 |
| 2 | F-816-1,3,5-Triazine-2,4,6-triamine-Pb2+ | 2.7236 | 0.4193 | 0.9374 | 0.8515 | 0.4924 | 0.9090 |
Table 1: Equilibrium Isotherm Constants For Langmuir and Freundlich model.
Qo obtained from graph was used to estimate the surface area occupied by lead ion on GAC. The surface area of the GAC through Lead adsorption can then be represented as
S'=Na.Qo.A (6)
Where,
S'=Surface area of adsorbent (cm2/g)
A=Cross-sectional area of the adsorbent molecule (cm2).
Na=Avogadro number
Determination of value of S' needed the value of A the surface area occupied by a single Lead ion. The values of A were calculated using the expression given by Brunauer and Emmet.
A=4 × 0.866[M/4√2.Na.d]2/3 (7)
Where,
M=Atomic weight of the lead
Na=The Avogadro number
d=The density of the lead, [37]
The values of S' obtained from Qo and S obtained from qe max are reported in Table 2.
| Sr. No. | System | A (cm2) | qe max (mg/m.mol.) | S (cm2/gm) | Q0 | S’(cm2/gm) |
|---|---|---|---|---|---|---|
| 1 | F-300-1,3,5-Triazine-2,4,6-triamine-Pb2+ | 5.4225 ´10-16 | 1.5000 | 1.8381 ´103 | 1.9173 | 2.3494 x103 |
| 2 | F-816-1,3,5-Triazine-2,4,6-triamine-Pb2+ | 5.4225 ´10-16 | 1.6765 | 2.0544 ´ 103 | 2.7236 | 3.3376 x103 |
Table 2: Values of S, A, QO and S' for a system GAC-1,3,5-Triazine-2,4,6-triamine-Pb2+.
Temkin model considers the effect of indirect adsorbate-adsobent interactions on adsorption and suggests that the heat of adsoption of all the layer would decrease linearly with coverage due to adsorbate-adsobent interactions. It assumes that the decrease in the heat of adsorption is linear rather than logarithmic as stated in Freundlich expression. The linearised form of Temkin equation is
qe = RT/bT ln KT + RT/bT ln Ce
Where,
KT=equilibrium binding constant (L/mg)
b=Temkin constant related to the heat of adsorption (kJ/mol)
B=RT/bT
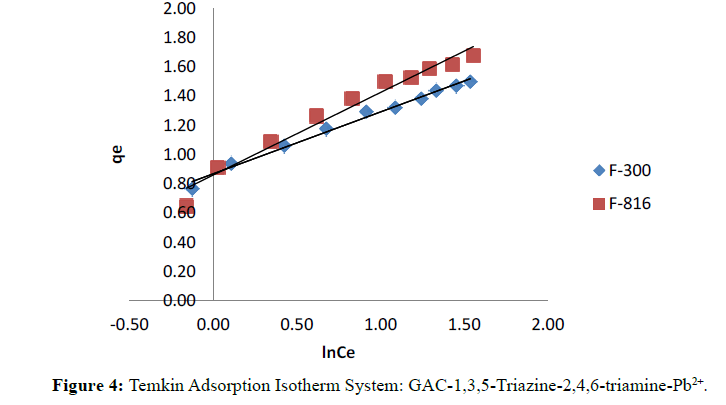
Figure 4 illustrates the plot of Temkin isotherms for F-300 and F-816. Temkin constants KT and bT are calculated from plot of qe versus ln Ce given in Table 3.
| Sr. No. | AdsorptionSystem | KT | bT | R2 |
|---|---|---|---|---|
| 1 | F-300-1,3,5-Triazine-2,4,6-triamine-Pb2+ | 7.7673 | 5849.4436 | 0.9889 |
| 2 | F-816-1,3,5-Triazine-2,4,6-triamine-Pb2+ | 4.576739 | 4382.762 | 0.9680 |
Table 3: Equilibrium Isotherm Constants For Temkin model.
Conclusion
This study examined the efficiency of adsorbent GAC in the removal of Pb2+ ions from aqueous phase in presence of 1,3,5-Triazine-2,4,6-triamine. The main advantages of the adsorption study include cost effectiveness, simplicity and offers flexibility in design. From the plot of qe against Ce, it is observed that initially Ce increases with qe but at the saturation level qe tends to be constant which indicates monolayer formation of Lead ion on the pores site of surface of adsorbent GAC. The experimental data seen to be of the favourable type and subjected for adherence to Langmuir and Temkin model better than by the Freundlich model. In adsorption study F-816 loaded with 1,3,5-Triazine-2,4,6- triamine-adsorbs lead to a remarkable extent as compared to F-300. This is probably due to presence of large active sites available on GAC surface and its porous nature.
References
- Edokpayi JN, Odiyo JO, MsagatiTAM, Popoola EO(2015) A novel approach for the removal of lead (II) ion from wastewater using Mucilaginous leaves of Diceriocaryumeriocarpum plant. Sustainability 7: 1402-1404.
- Mwangi IW, Ngila JC, Okonkwo JO (2012)A comparative study of modified and unmodified maize tassels for the removal of selected trace metals in contaminated water.Toxicol Environ Chem94: 20-39.
- Gercel O, Gerçel HF(2007) Adsorption of lead(II) ions from aqueous solutions by activated carbon prepared from biomass plant material of Euphorbia rigida.ChemEng J 132: 289-297.
- Mahmoud ME, Osman MM, Hafez OF, Hegazi AH, Elmelegy E (2010) Removal and preconcentration of lead (II) and other heavy metals from water by alumina adsorbents developed by surface-adsorbed-dithizone. Desalination 25: 123-130.
- Imamoglu M, Tekir O (2008) Removal of copper (II) and lead (II) ions from aqueous solutions by adsorption on activated carbon from a new precursor hazelnut husks. Desalination 228: 108-113.
- Ogunleye OO, Ajala MA, Agarry SE(2014) Evaluation of biosorptive capacity of banana (Musa paradisiaca) stalk for Lead (II) removal from aqueous solution.J Environ Prot5: 1451-1465.
- Juttner K, Galla U, Schmieder H(2000) Electrochemical approaches to environmental problems in the process industry. ElectrochimicaActa 45: 2575-2594.
- Bose P, Bose MM A, Kumar S(2002) Critical evaluation of treatment strategies involving adsorption and chelation for wastewater containing copper, zinc, and cyanide.Adv Environ Res 7: 179-195.
- Wingenfelder U, Hansen C,Furrer G, Schulin R(2005) Removal of heavy metals from mine water by natural zeolites. Environ SciTechnol 39: 4606-4613.
- Shamma NK, Wang LK and Hung YT(2004) Coagulation and flocculation in Physicochemical Treatment Processes.Humana Press, New Jersey, USA,pp: 103-140.
- Semerjian L, Ayoub GM (2003) High-pH-magnesium coagulation–flocculation in wastewater treatment.Adv Environ Res 7: 389-403.
- Ayoub GM,Semerjian L,Acra A,Fadel M, Koopman B (2001) Heavy metal removal by coagulation with seawater liquid bittern.JEnviron Eng 127: 196-202.
- Metcalf, Eddy (2003) Wastewater Engineering: Treatment and Reuse.McGraw Hill International Edn, New York, USA, pp: 478-483.
- Eckenfelder WW(1996) Industrial Water Pollution Control.McGraw-Hill Companies. pp:451-457.
- Jokela P, Keskitalo P(1999)Plywood mill water system closure by dissolved air flotation treatment. Water Sci. Technol 40: 33-42.
- Matis KA Zouboulis AI,Lazaridis NK and HancockI C(2003)Sorptive flotation for metal ions recovery.Int J Miner Process 70: 99-108.
- Wojtowicz A, Jarosinski A(1996) Removal of chromium Cr(III) on smectiteion exchange column, 3rd International Conference on Environment and Mineral Processing Ostrava,pp: 217-277.
- Corupeioglu MO, Huang CP (1987)The adsorption of heavy metals on to hydrous activated carbon. J Water Res 21: 1031-1044.
- Rivera Utrilla J, Garcia MAF (1987) Study of cobalt adsorption from aqueous solution on activated carbons from almond shells. Carbon 25:645.
- Guo Y, Qi J, Yang S, Yu K, Wang Z(2003)Adsorption of Cr(VI) on micro and mesoporous rice husk based active carbon. Mater ChemPhys78: 132-137.
- Khan S, Nigam SK, Dwivedi HP, Singh PK (2004)Oxidative degradation of methyl ethyl ketone by n-chlorosaccharin. Asian J Chem 16(2): 751-754.
- Olusegun KA, Otaighe JOE (2008)Adsorption Behaviour of 1-phenyl-3-methylpyrazol-5-one on Mild Steel from HCI Solution.Int J ElectrochemSci 3: 191-198.
- Islam M, Patel RK, MaterJH (2007)Evaluation of removal efficiency of fluoride from aqueous solution using quick lime.J Hazard Mater143: 303-310.
- Daifullah AAM, Yakout SM, Yang XJ, Fane AG, MacNaughton S(2001) Removal and recovery of heavy metals from wastewater by supported liquid membranes. Water SciTechnol 43: 341-348.
- Babel S, Kurniawan TA (2003)Lowcost adsorbents for heavy metals uptake from contaminated water: A review. J Hazard MaterB97: 219-243.
- Abudaia JA, Sulyman MO, Elazaby KY, Ben-Ali SM(2013) Adsorption of Pb (II) and Cu (II) from aqueous solution onto activated carbon prepared from dates stones.IJEST 4.
- Paajanen A, Lehto J, Sataapakka T, Morneau JP (1997)Sorption of cobalt on activated carbons from aqueous solutions. Sep SciTechnol 32: 813.
- Netzer A and Hughes DE (1984)Adsorption of copper leas and cobalt by activated carbon. J Water Res 18: 927-933.
- Elreefy SA (2007)Adsorption of fluoride in aqueous solutions using KMnO4-modified activated carbon derived from steam pyrolysis of rice straw. J Hazardous Mater 62: 1-32.
- Meena AK, Rajagopal C, Kiran CR, Mishra GK (2010)Removal of Heavy metal ions from aqueous solutions using chemically (Na2S) treated granular activated carbon as an adsorbent.J SciInd Res 69: 449-453.
- Meshram YK, Khati NT,KhopeRU (2014)Evaluation of Adsorptive Capacity of Bioadsorbent in Removal of Congo Red from Aqueous Solution. Der Chem Sin 5: 25-29.
- Gawande NJ, ChaudhariAR, Khope RU(2012)Influence of surface characteristics of adsorbent and adsorbate on competitive adsorption equilibrium.AdvApplSci Res 3: 1836-1841.
- GunjateJK (2016) Selective Adsorption of Cobalt In Aqueous Solution Using Chemically Modified Activated Carbon.IOSR-JESTFT 10: 161-165.
- Needleman HL(1999) History of lead poisoning in the world.The George Foundation, Bangalore, India,pp: 17-25.
- AlsamarraiKF(2011) Spectrophotometric assay of lead in human hair samples by using alizarin red (S) in samarra area. J of university of Anbar for pure science 5: 5-12.
- Lide DR (1997) CRC Handbook of Chemistry and Physics.78th Edn., Boca Raton, FL,CRC Press,pp: 3-323.
- WuanaRA,Okieimen FE (2011) Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Technology 20.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences