ISSN : 0976 - 8688
Der Pharmacia Sinica
Evaluation of in vitro Thrombolytic, in vitro Anti-Inflammatory and Stroke Protective Effect of Dalechampia indica Wight Extract Fractions
Sindhura S1* and Eswaraiah MC2
1Department of Pharmaceutical Science, JNTUH, Hyderabad, India
2Department of Pharmacognosy, Anurag Pharmacy College, Kodad, Telangana, India
Abstract
The present study is to investigate the in-vitro thrombolytic, anti-inflammatory and protective effect of extract fractions of Dalechampia indica Wight whole plant against thrombotic ischemia reperfusion (TIR) injury in rats. Firstly extract fractions EDI and CDI were prepared from crude extract by partition method. To observe the thrombolytic potential of extract fractions, clot lysis method with streptokinase as standard was used whereas HRBC membrane stabilization was used to study in-vitro anti-inflammatory activity. For stroke activity, rats were pretreated with different doses (200 & 400 mg/kg) of EDI and CDI for a month and stroke was induced by Bilateral common carotid artery occlusion (BCCAO) for 30 mins followed by reperfusion for 45 mins along with ferric chloride induced thrombosis in one of the common carotid arteries. We measured infarct size, brain water content, glutamate, and acetylcholine esterase levels. Pre-treatment with EDI and CDI significantly reduced the brain edema, infarct size, glutamate and acetyl cholinesterase levels this may be attributed to the anti-inflammatory and thrombolytic activity of Dalechampia indica. The extract fractions (EDI & CDI) showed dose dependent percentage protection (67.39 ± 2.11 & 52.14 ± 2.31) in HRBC Stabilization method compared with diclofenac which showed 90.18 ± 1.42% protection. EDI and CDI showed a significant percentage of clot lysis (23.96 ± 1.67 & 18.64 ± 2.09) compared to standard streptokinase (72.86 ± 1.29) while the negative control water revealed 3.34 ± 0.69% lysis of the clot. From our findings, it is observed that EDI revealed remarkable stroke protective activity. Further studies to observe in vivo clot dissolving potential and to isolate active component(s) of extract will be fruitful.
Keywords
Anti-inflammatory, BCCAO, Reperfusion, Thrombolytic
Introduction
Clotting is the healthy human response to injury that prevents the blood loss. If a clot occurs inside the blood vessel, it results in a condition called Thrombosis [1]. Type of thrombosis and the medical condition associated with it depends on the type of blood vessel affected and extent of blockage it has created in it [2].
A cerebral Ischemic stroke happens when the thrombus blocks the cerebral arteries. It is the major cause of death and disability than hemorrhagic stroke [3]. Since cerebral stroke is very complicated progressive degeneration process involving a series of mechanisms such as accumulation of reactive oxygen species, Calcium overload, Glutamate excitotoxicity, microglia aggregation and irreversible cell injury [4]. Neurodegenerative disease including stroke is always associated with chronic inflammation, which is also an important pathological process that plays a key role in the ischemic brain injury [5].
After understanding the stroke Pathobiology in detail, it is not easy to mention the particular mechanism of protection against it. So, the drug with multiple actions will be helpful. The alternate and best option of recent times are thrombolysis therapy that restores cerebral perfusion and reduces the disability caused by stroke. Till now, recombinant tissue plasminogen activator (rt-PA) specifically alteplase given within 3 h of the onset of showed better results than any other drugs. All the thrombolytic drugs available in the market are burdened with various side effects such as large dose required to produce the effect and severe bleeding etc. [6].
Recent studies have increased the focus on plant studies, which are an immense source of compounds that have medicinal values. Plant derived drugs have few side effects and great safety compared to synthetic drugs [7].
Dalechampia indica Wight (Euphorbiaceae) commonly called as dushparsa or aliparnika used in Ayurveda growing in the moist deciduous forests is used for the study. In the present study, we evaluate the in vitro thrombolytic, antiinflammatory and stroke protective effect of Dalechampia indica Wight extract fractions [8].
Materials and Methods
Plant material collection and authentication
The whole plant of Dalechampia indica was collected in the month of April 2014 from Chittoor dist. The plant material was identified and authenticated by Taxonomist Prof. K. Madhava Shetty, S.V. University, Tirupathi. A voucher was kept in the department of Pharmacognosy for reference (ANU/COG/14/02).
Chemicals
All chemical agents used were of analytical grade were purchased from Sigma Chemicals Co. (St. Louis, USA) or Himedia, Mumbai.
Preparation and fractionation of crude extract of plant material
Whole plant material was cut, washed properly, shade-dried for several days, pulverized into coarse powder and stored at room temperature (RT) for future use. The dried coarse powder (500 g) of plant extract was macerated with absolute Ethanol at room temperature for 10 days in a clean and sterilized glass container with frequent agitation. Later it was subjected to filtration. The filtrate obtained was concentrated on a water bath maintaining 40°C to dryness and designated as a crude extract of DI. The crude extract obtained subjected to fractionation by suspending in hydro alcoholic (7:3% v/v) solution and extracted successively with petroleum Ether and chloroform using separating funnel. Dried plant extracts of different solvents were weighed and stored for further use. PET Ether used as the defatting agent. In the present study, the Ethanolic (EDI) and chloroform fractions (CDI) of crude extract were screened for stroke protective effect against Thrombotic ischemic reperfusion [9].
Experimental animals
The experiments were carried out in albino Wistar rats of either sex weighing 180–200 g and were procured from Anurag Pharmacy College, Kodad, India. They were kept under standard conditions at 23-25°C 12 h light/dark cycle and fed with standard diet and water ad libitum. Before the experiment, the animals were acclimatized for 1 week under laboratory conditions. The study was conducted in accordance with CPCSEA (Committee for the Purpose of Control and Supervision of Experiment on Animals) guidelines and approved by the Institutional Animal Ethical Committee (Registration no -1712/PO/a/13/CPCSEA).
Drugs and extract administration
The Ethanolic and chloroform extract fractions of D. indica were prepared as a uniform suspension using 1% tween 80 for oral administration in experimental animals [10].
Acute toxicity studies
Acute oral toxicity test of extract fractions EDI & CDI was carried out as per Acute Toxic Class Method described in OECD 423 [11]. Before dose, the animals fasted overnight. The incidence of mortality was checked for first 24 hours and daily thereafter for 14 days. Two arbitrary doses of 200 mg/kg and 400 mg/kg were selected for the study, as the extract fractions were found safe up to 2000 mg/kg without any sign of toxicity or mortality.
In vitro thrombolytic activity
4 ml of blood was collected from each healthy human volunteer (n=3) who has not taken oral contraceptives or anticoagulants for last 10 days. Collected micro centrifuge tubes were weighed (W1). To each properly labeled tube, 0.5 ml of freshly collected blood was added and allowed to clot by incubating at 37°C for 45 mins. Serum was withdrawn completely from each tube without disturbing clot after its formation and weighed (W2). Clot weight was calculated from the difference of above weights (W2-W1). To each tube 0.1 ml of concentration (10 mg/ml) extract fractions (EDI & CDI), streptokinase (positive control) and normal saline (negative control) were added separately and incubated for 90 mins for clot lysis. After 90 mins, the liquid part was removed and clot remnants were weighed. Then, percentage clot lysis was calculated from equation mentioned below [12].
% Clot lysis = {weight of clot after lysis/weight of clot before lysis} × 100 (1)
In vitro anti-inflammatory activity
The human red blood cells (HRBC) membrane stabilization anti-inflammation method was used in the present study [13]. Chloroform and ethanolic extracts fractions of Dalechampia indica were used for evaluation. First blood was collected from a healthy human volunteer who has not taken any NSAIDS for 10-14 days before the experiment was mixed with equal volume of sterilized Alsever solution (2% dextrose, 0.8% sodium citrate, 0.5% citric acid and 0.42% sodium chloride) and centrifuged at 2500 rpm for 5 min. The cell suspension remained after supernatant removal was washed with a normal saline solution (0.9% w/v NaCl). Then centrifuged again and again until the supernatant was clear and colorless. The packed cell volume was measured and 10% suspension was made. Various concentrations of extracts fractions were prepared (100, 250, 500 and 1000 μg/ml) using distilled water and to each concentration 1 ml of phosphate buffer, 2 ml hyposaline and 0.5 ml of HRBC suspension were added and incubated at 37°C for 30 min. After that, it was centrifuged at 3,000 rpm for 20 min. The hemoglobin content of the supernatant solution was estimated spectrophotometrically at 560 nm. The standard drug was also taken in the same concentrations. Control absorbance was found from solutions omitting the extracts. The percentage of HRBC membrane stabilization or protection was calculated using the formula mentioned below:
Percentage protection=100−((OD of drug treated sample/OD of control) × 100) (2)
Experiment schedule for stroke protective activity
The animals were divided into six groups each containing 8 animals. The test group animals received different doses (200 and 400 mg/kg) of EDI and CDI orally once daily for 30 days whereas sham operated and control groups received vehicle (1% Tween 80). The treatment schedule is mentioned below [14].
Group I (SHAM): Sham operated rats treated with 1% tween 80 without occlusion/reperfusion/ferric chloride application
Group II (CONTROL): Rats treated with 1% tween 80 and BCCAO for 30 mins followed by reperfusion for 45 mins and topical application of ferric chloride
Group III (EDI-1): Rats treated with low dose (200 mg/kg p.o) of EDI and BCCAO for 30 mins followed by reperfusion for 45 mins and topical application of ferric chloride
Group IV (EDI-2): Rats treated with high dose (400 mg/kg p.o) of EDI and BCCAO for 30 mins followed by reperfusion for 45 mins and ferric chloride application
Group V (CDI-1): Rats treated with low dose (200 mg/kg p.o) of CDI and BCCAO for 30 mins followed by reperfusion for 45 mins and ferric chloride application
Group VI (CDI-2): Rats treated with high dose (400 mg/kg p.o) of CDI and BCCAO for 30 mins followed by reperfusion for 45 mins and topical application of ferric chloride
Induction of stroke
After 30 days of treatment, stroke was induced by the combination of global (BCCAO) and focal model (ferric chloride induced arterial thrombosis) followed by reperfusion. Ventral midline incision on the neck area was done on pre anesthetized rats (ketamine 80-90 mg/kg i.p. and xylazine 5-10 mg/kg i.m.) to expose common carotid arteries (CCA). Vagus nerve accompanying the CCA were carefully separated and stroke was induced by occluding both carotid arteries with vascular clips for 30 mins [15]. Then, in the process of reperfusion vascular clips were clamped and removed alternatively over a period of 45 mins [16]. Finally, the filter paper (1 × 1 mm) saturated with 25% Fecl3 was applied proximal to the surface one of the common carotid arteries for 15 minutes [17]. During the application, care must be taken such that it should not come in contact with surrounding tissue or other blood vessels. The temperature was maintained at 37 ± 0.5°C throughout the surgery. The animals were sutured and allowed to recover by housing them in individual cages. Sham operated rats received the same surgical incision without ischemic induction.
Glutamate estimation
It was estimated by the method described by Raju et al. [18]. 1.0 ml of brain supernatant was evaporated to dryness at 70°C in an oven and reconstituted in 100 ml of distilled water. 2 mM concentration of the standard solution of glutamate along with the samples was spotted on Whatman No. 1 chromatography paper using a micropipette and placed in a chamber containing butanol: acetic acid: water (12: 3: 5 v/v) as a solvent. It was removed and dried once the solvent front reached the top of the paper. A second run was performed in a similar way, after that the papers were dried and sprayed with ninhydrin reagent. Then placed in an oven at 100°C for 4 minutes. The portions which carry glutamate corresponding with the standard were cut and eluted with 0.005% CuSo4 in 75% ethanol. Their absorbance was read against blank at 515 nm in a spectrophotometer. The levels of glutamate was calculated by using the following formula;
G=OD of sample × standard in mg × 1000/OD of Standard × Volume spotted × W (3)
Where G=Amino acid content in u moles/gram wet weight tissue
1000=Conversion factor for gram wet weight tissue
W=weight of the tissue in gram
In vivo AChE assessment
AChE activity was determined by Ellman et al. method [18]. The mixture contained 0.4 ml of brain homogenate sample, 100 μl 2.7 mM DTNB and 2.6 ml 0.1 M phosphate buffer (pH 8) which was incubated at 30°C for 2 mins. Addition of 20 μl of 30 mM ATC was added to initiate the reaction. Absorbance was noted for every minute for 10 mins at 412 nm. Change in absorbance in this 10 mins gives enzyme activity. All determinations were carried out in triplicate.
Determination of brain water content
After 7 days of thrombotic ischemic reperfusion, rat brains were quickly harvested by decapitation. Cerebellum, brain stem and olfactory bulb were removed and the brain tissues were weighed immediately to obtain wet weight (WW) then subsequently dried in an oven for 48 hours at 105°C, and weighed again to get dry weight [19]. The brain water content was calculated as follows
%Brain water content=[(wet weight − dry weight)/wet weight] × 100% (4)
Determination of infarct size
The brains of rats were quickly removed after decapitation and sliced into 2 mm-thick coronal sections and incubated in 2% TTC at 37°C for 30 min. Then the brain sections were fixed with 10% formalin solution. Brain tissue stained bright red area indicates the Nonischemic area, and the unstained area or white area indicates ischemic tissue [20].
Results
In vitro thrombolytic activity
90 minutes incubation of streptokinase (positive control) with clots showed significant (P values <0.0001) clot lysis of 72.86 ± 1.09% whereas distilled water (negative control) treated-clots showed only 3.34 ± 0.69% clot lysis which is very negligible. Ethanolic fractions of D. indica showed the highly significant (P values <0.0001) clot lysis activity (23.96 ± 1.67%) compared to chloroform extract (18.64 ± 2.04). Results were shown in Table 1.
| S.No | Drug/Extract fraction | % Clot lysis |
|---|---|---|
| 1 | Distilled water (Control) | 3.34 ± 0.69 |
| 2 | Streptokinase(Standard) | 72.86 ± 1.09 |
| 3 | EDI | 23.96 ± 1.67 |
| 4 | CDI | 18.64 ± 2.04 |
| Results represented in mean ± SD (n = 3) | ||
Table 1: In vitro Thrombolytic activity (% Clot lysis) of extract fractions of Dalechampia indica
In vitro anti-inflammatory activity
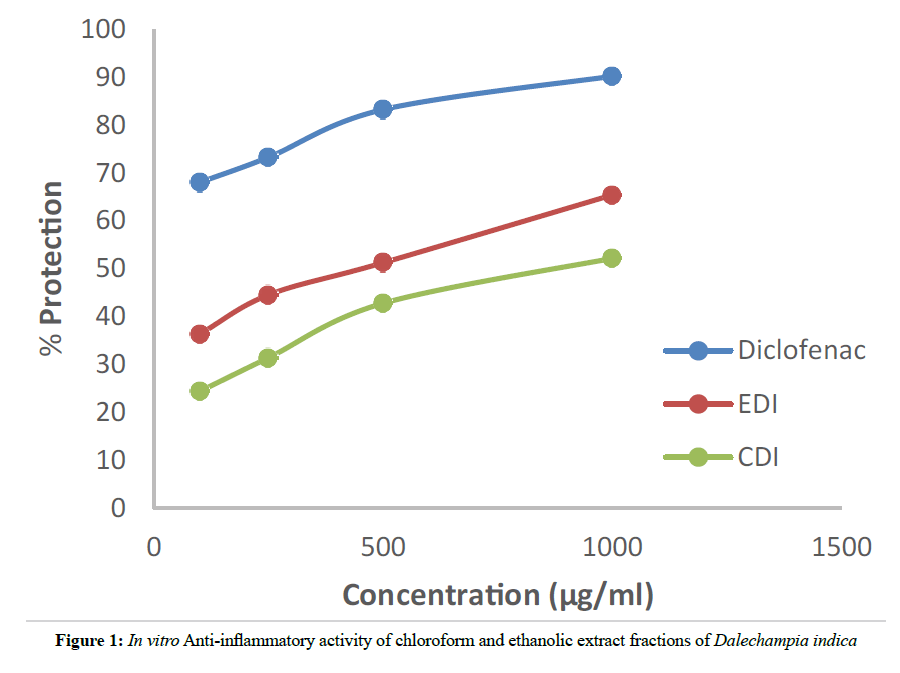
The HRBC membrane stabilization in vitro anti-inflammatory activity method was used for our study because the erythrocyte membrane is analogous to the lysosomal membrane and its stabilization indicates the anti-inflammatory activity of the extract fractions. The anti-inflammatory activity of different concentrations (100, 250, 500, 1000 μg/ml) of EDI, CDI, and Diclofenac (standard) were shown in Table 2. Both the extract fractions EDI and CDI showed dose dependent protection. At highest concentration (1000 μg/ml) EDI, CDI, and standard showed percentage protection of 67.39 ± 2.11, 52.14 ± 2.31 and 90.18 ± 1.49 respectively.
| S.No | Concentration (µg/ml) | % Protection | ||
|---|---|---|---|---|
| EDI | CDI | Diclofenac | ||
| 1 | 100 | 36.32 ± 0.74 | 24.35 ± 2.20 | 48.86 ± 2.78 |
| 2 | 250 | 44.51 ± 0.89 | 31.38 ± 2.59 | 63.26 ± 2.41 |
| 3 | 500 | 51.23 ± 0.79 | 42.76 ± 3.99 | 79.23 ± 1.52 |
| 4 | 1000 | 67.39 ± 2.11 | 52.14 ± 2.31 | 90.18 ± 1.49 |
Table 2: Effect of different doses of Ethanolic (EDI) and Chloroform (CDI) extract fractions of Dalechampia indica on stabilization of Human Red Blood Cell (HRBC) Membrane
Effect of EDI & CDI on Glutamate levels
Glutamate levels increased significantly (p<0.0001) in the ischemic control group compared to sham operated group which indicates excitotoxicity in brain tissue of ischemic control rats. The different doses of EDI and CDI significantly (p<0.0001, p<0.0001, p<0.005 & p<0.001) decreased the elevated glutamate levels seen in ischemic control group reducing excitotoxicity. The Glutamate levels of different treatment groups were mentioned in Table 3.
| GROUP | DOSE | GLUTAMATE (µmol /g) |
AChE (µmol/min/mg protein) |
|---|---|---|---|
| SHAM | 1% TWEEN 80 | 71.15 ± 0.68 | 14.50 ± 0.08 |
| Ischemic control | 1% TWEEN 80 | 82.23 ± 0.63a | 20.25 ± 0.23a |
| EDI-1 | 200 mg/kg | 76.64 ± 0.80b | 16.93 ± 0.63b*** |
| EDI-2 | 400 mg/kg | 74.36 ± 0.08b | 16.15 ± 0.19b |
| CDI-1 | 200 mg/kg | 80.26 ± 0.20b* | 29.16 ± 0.38c |
| CDI-2 | 400 mg/kg | 78.84 ± 0.24b*** | 18.48 ± 0.56b* |
| Values are reported as mean ± SEM (n=6), ap<0.0001 compared to sham, bp<0.0001, b***p<0.001, b**p, 0.01, b*p<0.05 and cp> 0.05 compared to control. AChE-Acetyl cholinesterase | |||
Table 3: Effect of different doses of Ethanolic (EDI) and Chloroform (CDI) extract fractions of Dalechampia indica on Glutamate and Acetylcholine esterase levels of ischemic reperfusion rat brain
Effect of EDI & CDI on Acetyl cholinesterase levels (AChE)
Acetyl cholinesterase enzyme level was significantly increased in the ischemic group compared to the sham operated group. EDI high dose treated group exhibited highly significant (p<0.0001) decrease in AChE levels which indicate a good improvement of memory functions associated to cholinergic system whereas CDI -1 showed less improvement compared to others with a non-significant decrease in the enzyme levels which was presented in Table 3.
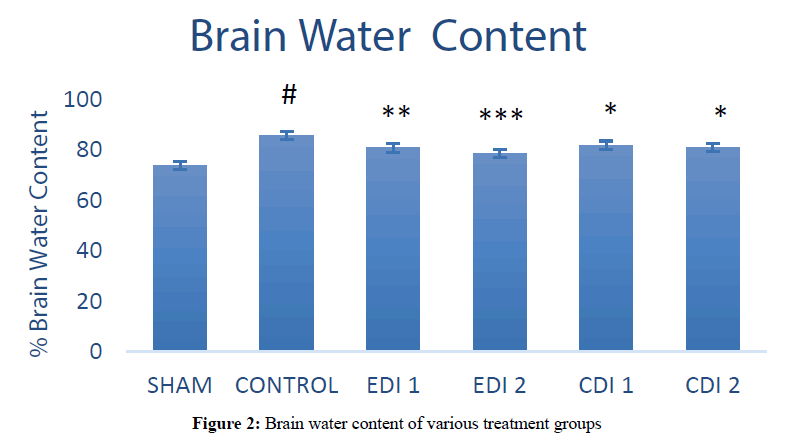
The effect of EDI and CDI on brain water content (BWC)
Thrombotic ischemia reperfusion produced significantly (P<0.0001) high brain water content (85.54 ± 1.44) in the ischemic control group compared to sham group (73.61 ± 1.24). Both doses of chloroform extract and low dose of EDI reduced the water content slightly (81.74 ± 0.24, 80.88 ± 0.49 and 80.60 ± 0.59) compared to ischemic control groups whereas high dose Ethanol extract fraction showed high significant (P<0.001) decrease in water content (78.41 ± 0.64) which indicates its protective effect against stroke induced edema. The results of BWC have been represented (Figures 1 and 2).
Figure 2: Brain water content of various treatment groups
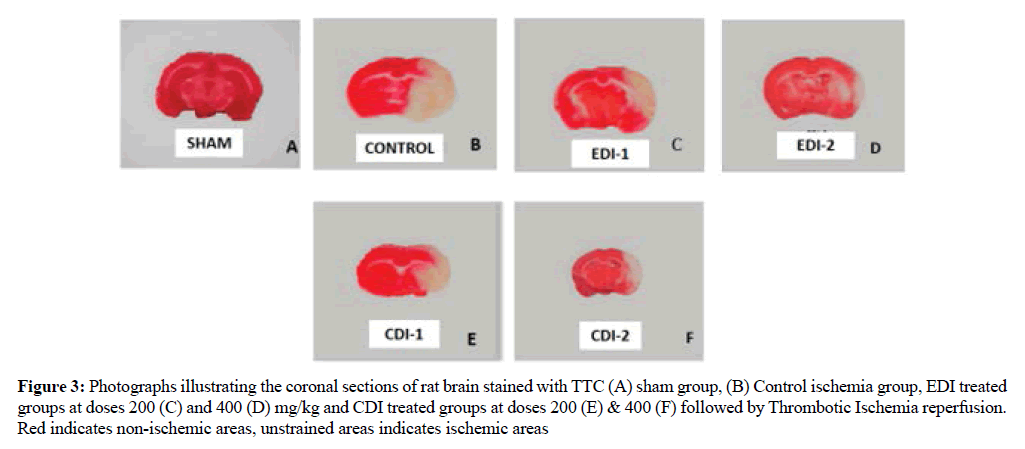
Effect of EDI and CDI on ischemic area
Ischemic damage determined by TTC staining. Compared with the ischemic control grouped EDI treated groups decreased the ischemic area or the unstained area in dose dependent manner which indicates better protection whereas CDI treated groups showed comparatively less ischemic protection than EDI which is represented (Figure 3).
Figure 3: Photographs illustrating the coronal sections of rat brain stained with TTC (A) sham group, (B) Control ischemia group, EDI treated groups at doses 200 (C) and 400 (D) mg/kg and CDI treated groups at doses 200 (E) & 400 (F) followed by Thrombotic Ischemia reperfusion. Red indicates non-ischemic areas, unstrained areas indicates ischemic areas
Discussion
Ischemic stroke is the vascular disease that occurs due to improper cerebral circulation caused by thrombosis. Despite understanding the various processes involving in the pathobiology of stroke and targeting those the successful therapeutic options are very few. One of them is recombinant tissue-plasminogen activator (rt-PA) for thrombolysis whose use is limited by its short therapeutic window and complications such as the risk of hemorrhage [21]. So there is need of extensive research for a drug which is effective with fewer side effects, targets different stages of stroke and improve the outcome. In the present study, we have chosen Dalechampia indica whole plant extract fractions for evaluating in vitro thrombolytic, anti-inflammatory and stroke protective activity.
Occlusion of both common carotid arteries followed by ferric chloride induced thrombosis in one of the carotid arteries with intermittent reperfusion produced severe ischemic damage which is very clear from increased glutamate levels of brain tissue, increased brain water content and the ischemic area in TTC stained brain sections of control group animals compared to sham animals.
Glutamate, the excitatory neurotransmitter not only maintains rapid neuron to neuron communication in the brain but also helps in neuronal growth and development by activating calcium dependent NMDA receptor [22]. In ischemic stroke, the homeostasis of glutamate is impaired causing increased accumulation of glutamate in the synaptic cleft that results in overexpression of NMDA Receptors which leads to the actions that end up with neuronal death. so, glutamate excitotoxicity created neurotoxicity plays the critical role in stroke [23]. Pre-treatment with chloroform and ethanolic extract fractions of Dalechampia indica had really decreased the excitotoxicity effect of glutamate by lowering its levels in brain tissue.
The inflammation in the brain tissue is an inevitable consequence of cerebral ischemia/reperfusion injury [24]. The ROS generated from various pathological processes during the stroke and cytotoxic factors released by dying neurons causes vaso-epithelial and blood-brain barrier damage which increases vascular permeability resulting in cerebral edema that exacerbates neuronal damage [25]. To determine this, we measured the brain water content. The results from the present study indicate that both EDI and CDI inhibited the water accumulation in the brain tissue which was increased by ischemia reperfusion injury. This may be due to the anti-inflammatory activity of the extract fractions which was revealed in the in vitro studies.
The biochemical changes such as acetylcholinesterase (AChE) levels in the brain homogenate of rats were investigated in the study as it acts as a reliable supplementary biomarker for cognitive function after stroke and plays a major role in neurological outcomes [26,27]. Pre-treatment with EDI and CDI decreased the levels of AChE and improved the neurological scores of ischemic rats.
2,3,5-triphenyl tetrazolium chloride (TTC) staining is an accurate, inexpensive and efficient technique to determine size and location of infarcts which is essential for the evaluation of cerebral ischemia [28]. The principle behind this technique is the presence of a dehydrogenase enzyme in normal tissue stains it red whereas the absence of this in ischemic tissue remains unstained or white. By this, we can clearly differentiate between ischemic and non-ischemic area and the extent of damage the stroke has created [29]. In the study, the EDI and CDI pretreated showed less ischemic damage compared to ischemic control group. This may be due to the presence of polyphenolic phytoconstituents such as phenols, flavonoids etc which has antioxidant, anti-inflammatory and thrombolytic activities.
Conclusion
In conclusion, pretreatment, especially with ethanolic extract fraction of Dalechampia indica (EDI), had significantly produced the protective effect in rats with transient focal cerebral ischemia/reperfusion injury, and the protective mechanism may be related to the inhibition of brain water content, lowering glutamate and Acetyl cholinesterase levels. This may be due to the thrombolytic and anti-inflammatory activity of Dalechampia indica which was proved in in vitro studies. Our results open up a direction for treatment of cerebrovascular disease with EDI. Further studies are pretty much necessary to isolate compounds that are responsible for this effect.
References
- Zaheer, Z., Robinson, T., Mistri, AK., Therapeutic Advances in Chronic Disease, 2011. 2(2): p. 119-131.
- Wardlaw, JM., et al., Cochrane Database of Systematic Reviews, 2003. 3(1): p. 1-12.
- Osmani, AH., Durrani, RK., Ara, J., Journal of the College of Physicians and Surgeons Pakistan, 2010. 20(1): p. 42-46.
- Xing, C., et al., International Journal of Stroke, 2012. 7(5): p. 378-385.
- Lakhan, SE., Kirchgessener, A., Hofer, M., Journal of Translational Medicine, 2009. 7(3): p. 97.
- Ringleb, PA., et al., Stroke, 2002. 33(5): p. 1437-1441.
- Firenzuoli, F., Gori, L., Evidence Based Complementary and Alternative Medicine, 2007. 4(1): p. 37-40.
- https://link.springer.com/chapter/10.1007/978-94-010-2331-3_7
- Sindhura, S., Eswaraiah, MC., IOSR Journal of Pharmacy, 2017. 7(5): p. 53-60.
- Prasad, S., et al., Thrombosis Journal, 2006.12(4): p.14.
- Kumar, V., et al., Asian Pacific Journal of Tropical Biomedicine, 2012. 12(6): p. 627-630.
- https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecd_gl423.pdf
- Shah, ZA., Vohora, SB., BMC Pharmacology Toxicology, 2002. 90(5): p. 254-259.
- Iwasaki, Y., et al., Journal of the Neurological Sciences, 1989. 90(2): p. 155-165.
- Onken, M., Berger, S., Kristian, T., Journal of Neuroscience Methods, 2012. 204(2): p. 254-261.
- Li, W., McIntyre, TM., Silverstein, LR., Redox Biology, 2013. 1(1): p. 50-55.
- Raju, TR., et al., Brain and Behavior, 2004. 3(4): p. 134-138.
- Ellman, GL., et al., Biochemical Pharmacology, 1961. 7(2): p. 88-95.
- Shamsaei, N., et al., Basic and Clinical Neuroscience, 2017. 8(1): p. 77-84.
- Moustafa, RR., Baron, JC., British Journal of Pharmacology, 2008. 153(1): p. S44-S54.
- Sattler, R., Tymianski, M., Molecular Neurobiology, 2001. 24(1): p. 107-129.
- Nicholls, DG., et al., Annals of the New York Academy of Sciences., 1999. 893(3): p. 1-12.
- Ahmad, M., et al., CNS & Neurological Disorders-Drug Targets, 2014. 13(8): p. 1378-1396.
- Ayata, C., Ropper, AH., Journal of Clinical Neuroscience, 2002. 9(2): p. 113-124.
- Cheyuo, C., et al., Journal of Cerebral Blood Flow & Metabolism, 2011. 31(5): p. 1187-1195.
- Gnatek, Y., et al., Frontiers in Molecular Neuroscience, 2012. 5(2): p. 66-69.
- https://link.springer.com/protocol/10.1007%2F978-1-61779-782-8_9
- Popp, A., et al., PLoS ONE, 2009. 4(3): p. 4764.
- Sun, AY., et al., NeuroMolecular Medicine, 2008. 10(4): p. 259-274.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences