ISSN : 2321-2748
American Journal of Phytomedicine and Clinical Therapeutics
Evaluation of Anti-inflammatory Activity of Enriched Boswellia Extract in Human Dermal Fibroblast Cells In-vitro
Sudhakar A*, Shantakumar J and Joseph MV
R&D Department, Olive Lifesciences Pvt Ltd, Nelamangala, Karnataka, India
- *Corresponding Author:
- Sudhakar A
Olive Lifesciences Pvt Ltd
No. 5805/05, Nh-4, Near Navayuga Toll Gate Office
Nelamangala Town Bangalore, Karnataka -562123, India
E-mail: sudhakar@olivelifesciences.com
Received Date: February 15, 2021; Accepted Date: March 02, 2021; Published Date: March 09, 2021
Citation: Sudhakar A, Kumar S, Joseph MV (2021) Evaluation of Anti-inflammatory Activity of Enriched Boswellia Extract in Human Dermal Fibroblast Cells In-vitro. Am J Phytomed Clin Ther Vol.9 No.3:6.
Abstract
Objective: The enriched Boswellia extract (Olibose®) and Regular Boswellic acid extract (RBAE) prepared from the resin of Boswellia serrata were evaluated for anti-inflammatory activity in Human Dermal Fibroblasts (HDF) Cells In-vitro.
Methodolgy: Anti-inflammatory activity was determined by inducing inflammation on HDF and L929 cell lines. After the induction of inflammation, cells were treated with Olibose®, RBAE extracts and standard inflammatory drug Dexamethasone. Levels of Inflammatory markers i.e., TNF-α and NO (Nitric oxide) were measured and anti-inflammatory efficacy was assessed.
Results: Results of the present study revealed that, the enriched extract (Olibose®) possess better anti-inflammatory activity in comparison with RBAE.
Conclusion: In this study, Olibose® exhibited dose dependant anti-inflammatory activity against LPS induced induction of TNF-α and NO in HDF cells. Olibose® was found to have better anti-inflammatory activity than RBAE in vitro. Since arthiritis/ osteoarthiritis are associated with inflammation, this study proves that Olibose® can be used to reduce above said complications.
Keywords
Boswellia serrata; Olibose®; Boswellic acids; Inflammation; TNF-α; Nitric oxide (NO); LPS; HDF cell lines; Dexamethasone
Introduction
Boswellia serrata belongs to Family: Burseraceae and Genus: Boswellia. It is a moderate to large sized branching tree that grows in dry mountainous regions of India, Northern Africa and the Middle East [1,2]. In folk medicines, it has been used for its anti-inflammatory, antiarthritic and antiseptic effects since ancient times [3]. For the last 2 decades, various preparations of the oleo gum resin of Boswellia serrata or Indian Frankincense, commonly known as olibanum, have gained popularity among the consumers in the management of a variety of inflammatory diseases, including arthritis, inflammatory bowel disease, allergy, and asthma [4,5]. The high-quality extract from Boswellia is available in India for more than 25 years and marketed under the name Shallaki. In Ayurveda, Boswellia serrata is one of the ancient and most valued herbs and describe the benefits of its Gum resin for treating antirheumatic (Antiarthiritis) conditions. Boswellia serrata gum is a resinous exudate of genus Boswellia. The resin is generally harvested throughout the summer and autumn. Resin is collected by making an incision on the trunk of the tree, which is semi-solid to Solid in nature. This hardens slowly into amorphous and tear-shaped product with an aromatic scent [3].
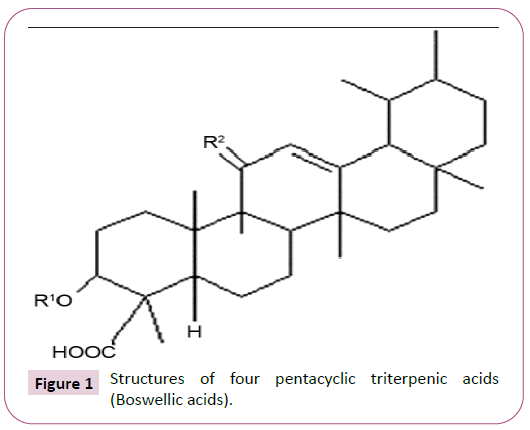
Boswellia gum resin consists of pentacyclic triterpenic acids, known as boswellic acids (BAs), which are the active phytochemicals accountable for the biological and anti-inflammatory activities of the resin [6]. The structures of four major pentacyclic triterpenic acids (boswellic acids) i.e., b-boswellic acid, Acetyl-b-boswellic acid, 11-keto-b-boswellic acid (KBA) and Acetyl-11-keto-b-boswellic acids (AKBA) are shown in Figure 1. In Boswellia gum resin extracts, the anti-inflammatory properties are mainly attributed to KBA and AKBA and are considered as most active modulators among the BAs [3,6]. Boswellic acids block 5-lipoxygenase activity, a major pro-inflammatory enzyme to reduce leukotriene synthesis. This step has been considered as a fundamental basis of anti-inflammatory properties of the Boswellia extracts [7,8]. In addition, nuclear factor kappa B regulated pro-inflammatory cytokine production is also reduced by AKBA and KBA [9-11].
β-Boswellic acid, R1=H, R2=H2; acetyl-β-Boswellic acid, R1=Ac, R2=H2; 11-keto-β-boswellic acid, R1=H, R2=O; acetyl-11-keto-β- Boswellic acid, R1=Ac, R2=O.
Tumour necrosis factor-alpha (TNF-α) is a pro-inflammatory cytokine that plays a pivotal role in regulating the inflammatory response in rheumatoid arthritis (RA). It also plays a fundamental role through the activation of cytokine and chemokine expression and induction of pain. Increased concentrations of TNF-α are found in acute and chronic inflammatory conditions (e.g., trauma, sepsis, infection, rheumatoid arthritis). The central role of this cytokine has been repeatedly confirmed by a successful therapeutic blockade of membrane and soluble TNF-α in patient with RA [12] (Figures 2-5).
Boswellic acids inhibits an inflammatory marker TNF-α, and 5-lipoxygenase which are involved in synthesis of inflammatory cytokines that induce joint inflammation and pain, asthma and bronchial asthma etc. [13].
Popularly, nonsteroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen, naproxen, are used as a pain management strategy for symptomatic relief of osteoarthritis. Although synthetic NSAIDs are effective in reducing pain and inflammation, their prolonged usage may cause serious gastrointestinal toxicity, renal toxicity, and adversely affects platelet function. Also, long-term use of these NSAIDs may lead to certain types of ulcers and other disorders [6] and hence there is a prompt need to search for an alternative to these synthetic drugs where herbal medicines can be suitable candidates. There is an escalating demand for herbal drug therapy and many plant species are being explored and are used. One Such Herbal NSAID alternative is Boswellia serrata extract.
Olibose® is made from B. serrata gum by the selective extraction and purification processes which are environmentally friendly. This extract was standardized for 65% total boswellic acids by HPLC. Also, Olibose® is lighter in shade (Figures 4 and 5) compared to the regular extracts and gives an option for the formulators to employ this product in cosmetic and dermal products. In this context, the purpose of the present study was to evaluate the anti-inflammatory activity of Olibose® in regulating pro-inflammatory makers TNF-alpha and Nitric oxide (NO) in LPS stimulated macrophages in comparison with regular boswellic acid extract (RBAE) with 40% boswellic acids.
Materials and Methods
Preparation of study materials Olibose® and Regular Boswellic acid Extract (RBAE)
Study Materials Olibose® and RBAE are a composition of fractions derived from an aqueous ethanol extract of B. serrata gum resin (Figure 2). To maintain the quality and batch-tobatch consistency, Olibose® (Figure 5) and RBAE (Figure 3) were standardized to contain at least 65% and 40% of total boswellic acids (BAs) respectively.
Dried gum is grinded until it becomes a coarse powder, extracted by maceration with aqueous ethanol. Extractions are continued for 2-3 times. The combined extracts are then filtered under vacuum and the filtrate is evaporated using a rotary evaporator at 50-60°C to obtain a viscous extract. This extract is then washed to remove the resinous portion and further the organic acids are precipitated selectively by treating with alkaline water followed by acidification. Then decolorized, filtered, washed with water to neutral pH to obtain a semi dried cake. This was further dried at 50-60°C in oven under vacuum to become powder. This product is further standardised to contain 40% (RBAE) and 65% (Olibose®) Boswellic acids by HPLC.
Standardization of Olibose®
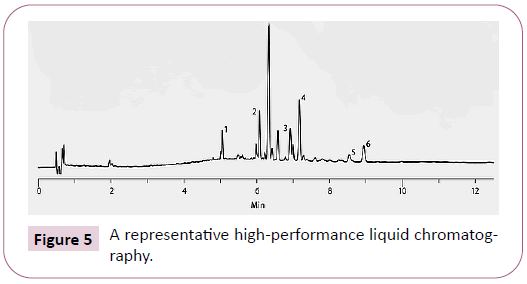
a representative high-performance liquid chromatography shows the elution profile of the major boswellic acids of olibose® at 210 nm. The peaks 1 to 6 represent 11-keto-b-boswellic acid, 3-o-acetyl-11-keto-b-boswellic acid, a-boswellic acid, b-boswellic acid, 3-o-acetyl-a-boswellic acid and 3-o-acetyl-b-boswellic acid, respectively.
Extraction and purification processes are standardized for Olibose®. Analytical method was standardized based on HPLC. Briefly, HPLC analysis was performed using a Phenomenex Luna C18, 250 × 4.6 mm, 5 μl column with a flow rate 1ml/min in a Shimadzu liquid chromatography equipped with UV/Vis Detector at a wavelength 210nm and 254nm to detect the peaks. The sample was eluted by injecting 20 μl using mobile phase (Acetonitrile: Water: Acetic acid (90:10:0.1)) with a run time of 35 min. Figure 1 shows a representative HPLC chromatogram of LI13019F1 presenting an elution profile of the major BAs monitored at 210 nm. The peaks 1 to 6 represent 11-keto-bboswellic acid (KBA), 3-O-acetyl-11-keto-b- boswellic acid, aboswellic acid, b- boswellic acid, 3-O-acetyl- a- boswellic acid, and 3-O-acetyl-b- boswellic acid, respectively (Figure 5).
Chemicals and reagents
HDF (Human dermal fibroblast) cell line was procured from ATCC, USA. Stock cells were cultured in DMEM supplemented with 10% inactivated Fetal Bovine Serum (FBS), penicillin (100 IU/ ml), streptomycin (100μg/ml) and amphotericin B (5 μg/ml) in a humidified atmosphere of 5% CO2 at 37°C until confluent. The cells were dissociated with TPVG solution (0.2% trypsin, 0.02% EDTA, 0.05% glucose in PBS). The stock cultures were grown in 25 cm2 culture flasks and all experiments were carried out in 96 well microtitre plates.
Cell lines and culture medium
HDF and L929 cell lines were cultured in Ham’s F-12/MEM media supplemented with 10% inactivated Fetal Bovine Serum (FBS), penicillin (100IU/ml), streptomycin (100 g/ml) and amphotericin B (5μg/ml) in a humidified atmosphere of 5% CO2 at 37°C until confluent. The cells were dissociated with TPVG solution (0.2% trypsin, 0.02% EDTA, 0.05% glucose in PBS). The stock cultures were grown in 25 cm2 culture flasks and all experiments will be carried out in 96 microtitre plates.
Preparation of test solution
For studies, 10 mg of Olibose® and RBAE (Boswellic acid extract (40%)) were dissolved in DMSO and volume was made up with DMEM-HG supplemented with 2% inactivated FBS to obtain a stock solution of 1mg/ml concentration and sterilized by filtration. Serial two-fold dilutions were prepared from the stock solution for carrying out cytotoxic studies.
Cytotoxicity studies
Colorimetric MTT assay was performed to assess the basal cytotoxicity of test samples. Cells at a density of 1×105/ mL were seeded in 96-well flat bottom culture plates and incubated overnight. The cells were treated with different concentration of test samples diluted in medium containing 2% FBS and incubated for 24h. About 10 μL of MTT (5mg/mL) was added to each well and incubated for 4h. The formazan dye formed was extracted with dimethyl sulfoxide and absorbance was recorded at 540nm.
Evaluation of in-vitro TNF-α inhibitory activity of Olibose® and RBAE
L929 cells were used for determining the TNF-α in the culture media [9]. In brief, HDF cells (1×106/mL) were incubated with LPS (1 μg/mL) and Olibose® and Boswellic acid extract (40%) at nontoxic doses or dexamethasone (100 μM) in culture media with 2% FBS. Dexamethasone is a member of glucocorticoid and acts as an anti-inflammatory and immunosuppressant. Treated and untreated cells were incubated at 37°C for 4 h and the culture media were collected. Collected cell supernatant was centrifuged and stored at −20°C until used. L929 cells (1 × 105/mL) were seeded in 96-well plates and incubated overnight. The culture media from L929 cells were removed and conditioned media were added along with 25 μL of actinomycin D (50 μg/mL). The L929 cells pre-treated with actinomycin D appears sensitive to TNF-mediated cytotoxicity and it has been used to measure the presence of TNF in biological fluids and cell culture supernatants. The plates were incubated for additional 24 h. The TNF-α induced toxicity in L929 cells was determined by MTT assay as described above.
Evaluation of nitric oxide inhibition assay for Olibose® and RBAE
Nitric oxide (NO) inhibitory activity was assessed as per the method described in literature. RAW 264.7 cells were treated with LPS [10] and test samples as described above and incubated for 24 h and conditioned media collected were used for nitrite determination. Determination of nitrite as a biomarker for NO was carried out. In brief, an equal volume (50 μL) of 0.1% N-1- napthylethylenediamine dihydrochloride and 1% sulfanilamide in water and 5% phosphoric acid respectively in cell culture media were mixed in flat bottom 96-well plate incubated for 10-15min. Colored end product was measured at 530nm. Different concentrations of sodium nitrite prepared in culture media were taken as standard. Concentration of the nitrite in the samples was extrapolated by comparing with the values of the sodium nitrite. The assay was carried out minimum of three times in duplicate.
Statistical analysis
The values obtained were expressed as mean ± standard deviation. Repeated measures analysis of variance was used to test significance in terms of values corresponding to the percentage of TNF-α and NO inhibition.
Results
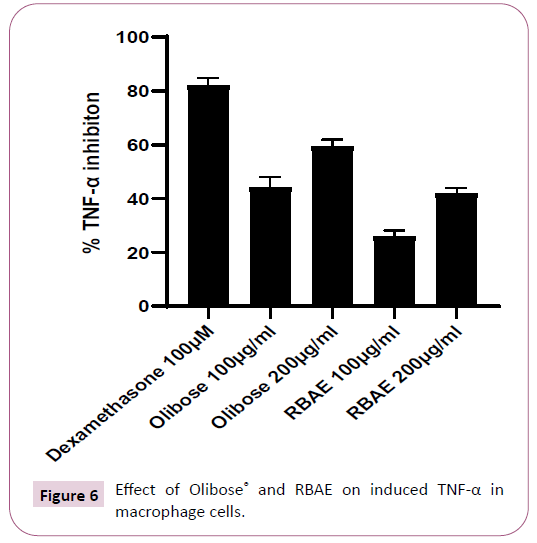
Effect of Olibose® on inhibition of TNF-α and NO production was presented in Table 1. Both enriched (65% boswellic acids) extract and Regular Boswellic acid extract (RBAE) with 40% boswellic acids showed dose dependent cytotoxicity in HDF and L929 cells. The non-toxic concentration of 200 and 100 μg/ml was chosen for anti-inflammatory study against LPS induced TNF-α and Nitric oxide. Olibose® inhibited the LPS induced TNF-α production by 59.31 ± 2.12 and 44.06 ± 3.26 percent at 200 and 100 μg/ml respectively in HDF cells over LPS control group. Treatment with RBAE inhibited TNF-α production by 41.96 ± 1.63 and 25.77 ± 1.95 percent at 200 and 100 μg/ml over LPS control respectively (Table 1 and Figure 6). Standard Drug, dexamethsone at 100 μM offered significant protection by inhibiting TNF-α production by 82.04 ± 2.35 percent over untreated LPS control. Further we analyzed the inhibitory role of Olibose® on NO generation. As NO is a shortlived molecule, nitrite was measured in the supernatants of cells treated with LPS and test products immediately after collection.
| S. No | Samples | Concentration tested (µg/ml) | % TNF inhibition | No inhibition |
|---|---|---|---|---|
| 1 | RBAE | 200 100 |
41.96 ± 1.63 25.77 ± 1.95 |
45.76 ± 2.39 25.05 ± 4.41 |
| 2 | Olibose® | 200 100 |
59.31 ± 2.12 44.06 ± 3.26 |
58.93 ± 1.79 39.33 ± 2.50 |
| 3 | Dexamethasone | 100 µM | 82.04 ± 2.35 | 73.08 ± 2.81 |
Table 1: Anti-inflammatory effect of test substance in HDF cells.
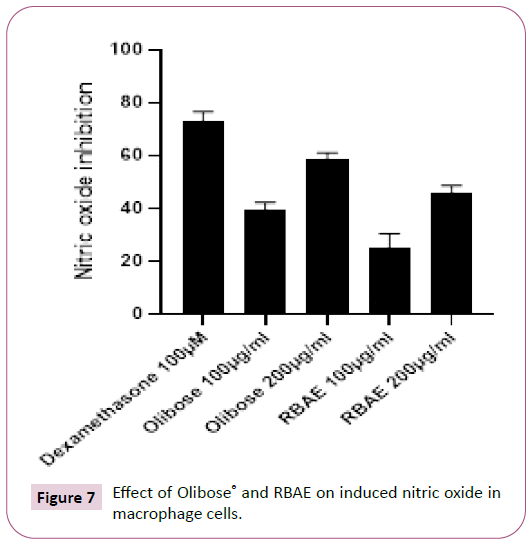
Large quantity of nitrite is produced by macrophage when stimulated with LPS. On treatment with Olibose® inhibited LPS stimulated production of nitrite better over RBAE (40%) in dose dependent manner (Table 1 and Figure 7). Olibose® inhibited the LPS induced NO generation by 58.93 ± 1.79 and 39.33 ± 2.50 percent in HDF cells at 200 and 100 μg/ml respectively over LPS control group. Treatment with RBAE inhibited NO production by 45.76 ± 2.39 and 25.05 ± 4.41 percent at 200 and 100 μg/ml, respectively over LPS control. Standard Drug, dexamethsone at 100 μM offered significant protection by inhibiting NO production by 73.08 ± 2.81 percent over untreated LPS control.
Discussion
For the management of various inflammatory diseases including osteoarthritis and chronic bowl diseases, Boswellia serrata gum resin extract is used as a popular alternative strategy [6]. Here, we present an enriched anti-inflammatory composition of Boswellia serrata gum resin extract Olibose®, which contains 65% Boswellic acids. Purpose of the present study is to evaluate the anti-inflammatory activity of Olibose® in regulating proinflammatory makers TNF-alpha and Nitric oxide (NO) in LPS stimulated macrophages in comparison with regular boswellic acid extract (RBAE) with 40% boswellic acids. 3-acetyl-11 keto boswellic acid (AKBA) is the potent active among all the boswellic acids which helps to preserve the structural integrity of joint cartilage and maintain a healthy immune mediator cascade at a cellular level. AKBA is active against pain and inflammation by inhibiting leukotriene synthesis followed by inhibition of TNF-α expression. The intention of the study was to find out the logical evidence to prove that this enriched composition effectively reduces pain and inflammation both in terms of symptomatic disease manifestations and also through inhibition of biomarker associated with above said disease conditions. As it is already a proven medicament ingredient in many Ayurveda vata-kaphahara remedies, the present study re-establishes the ayureveda concept through scientifically validated in vitro protocol for anti-inflammatory activity of this enriched composition. In vitro experiments revealed that Olibose® has better inhibition of TNF-α and NO than Regular Boswellic acid extract (RBAE). These observations prove Olibose® with 65% boswellic acids exhibits better anti-inflammatory potential compared to that of RBAE with 40% boswellic acids, by controlling the endotoxin induced inflammation. TNF-α and NO are the proinflammatory cytokines which play a crucial role in the onset and progression of inflammation. These cytokines induce apoptosis and block the synthesis of Extracellular Matrix (ECM) components in the chondrocytes [14]. Proinflammatory cytokines down regulate SOX-9 protein expression via an NF-κB-regulated signalling pathway [15,16]. The present in vitro data on TNF-α inhibition indicates the anti-inflammatory potential of our enriched composition. The pupose of the present study to conduct on HDF cells is to understand the efficacy of this unique product in the management of cutaneous inflammatory dermatoses. Olibose® is a fine colourless powder prepared using a unique process to improve its acceptance as an active ingredient in various dermal formulations. With reference to the topical use of Boswellic acids in formulations for treatment of various dermatological disorders [17-19], the current study also supports the use of Olibose® in the management of skin health i.e., various dermatological disorders like psoriasis, eczema, skin wrinkling, itching, and improvement of collagen building underneath the skin through its anti-inflammatory action.
Conclusion
The present article provides scientific evidence for the effectiveness of Olibose® against inflammation, as an alternative to NSAID which can be used as a natural ingredient in formulations treating against arthritis, osteoarthritis, bone degenerative disease and associated inflammatory complications. The activity can be attributed to its higher percentage of actives (65% boswellic acids) when compared to the regular boswellic acid extracts. This enriched composition can also be used in topical and dermatological preparations viz. spray, liniments and other topical preparations. The results from our present study support the hypothesis that the anti-inï¬?ammatory effect of boswellia extract benefits can be utilised both orally and topically without any side effects, considering its long use in ayurveda and preceding research study publications globally.
Acknowledgement
We acknowledge Radiant Research Services Pvt Ltd, Bangalore, India for providing the facility for conducting the trials.
References
- Maupetit P (1984) New constituents in olibanum resinoid and essential oils. Perfumer Flavorist 9: 19-37.
- Leung AY, Foster S (1996) Encyclopedia of common natural ingredients used in food, drugs and cosmetics. (2nd edn). New York, John Wiley and Sons, NY, USA. p. 389-391.
- Siddiqu MZ (2011) Boswellia serrata a potential anti-inflammatory agent: An overview. Ind J of Pharma Sci 73: 255-261.
- Poeckel D, Werz O (2006) Boswellic acids: Biological actions and molecular targets. Curr Med Chem. 13: 3359-3369.
- Iram F, Khan SA, Husain A (2017) Phytochemistry and potential therapeutic actions of Boswellic acids: A mini-review. Asian Pac J Trop Biomed 7: 513-523.
- Alluri VS, Dodda S, Kilari EK, Golakoti T, Sengupta K (2019) Toxicological assessment of standardized Boswellia serrata gum resin extract. Int J Toxicol 8: 423-435.
- Ammon HP (2006) Boswellic acids in chronic inflammatory diseases. Planta Med 2: 1100-1116.
- Safayhi H, Sailer ER, Ammon HP (1995) Mechanism of 5-lipoxygenase inhibition by acetyl-11-keto-boswellic acid. Mol Pharmacol 47: 1212-1216.
- Syrovets T, Buchele B, Krauss C, Laumonnier Y, Simmet T (2005) Acetyl boswellic acids inhibit lipopolysaccharide-mediated TNF-a induction in monocytes by direct interaction with IkB kinases. J Immunol 174: 498-506.
- Takada Y, Ichikawa H, Badmaev V, Aggarwal BB (2006) Acetyl-11- keto-b-boswellic acid potentiates apoptosis, inhibits invasion, and abolishes osteoclastogenesis by suppressing NF-kB and NF-kB regulated gene expression. J Immunol 176: 3127-3140.
- Umar S, Umar K, Sarwar AH, Khan A, Ahmad N, et al. (2014) Boswellia serrata extract attenuates inflammatory mediators and oxidative stress in collagen induced arthritis. Phytomedicine 21: 847-856.
- Matsuno H, Yudoh K, Katayama R, Nakazawa F, Uzuki M, et al. (2002) The role of TNF- a in the pathogenesis of inflammation and joint destruction in rheumatoid arthritis (RA): A study using a human RA/SCID mouse chimera. Rheumatology 41: 329-337.
- Alam M, Khan H, Samiullah L, Siddique KM (2012)A review on Phytochemical and Pharmacological studies of Kundur (Boswellia serrata Roxb ex Colebr.) J of Applied Pharma Sci 2: 148-156.
- Meszaros E, Malemud CJ (2012) Prospects for treating osteoarthritis: enzyme-protein Interactions regulating matrix metalloproteinase activity. Ther Adv in Chronic Dis 3: 219–229.
- Murakami S, Lefebvre V, Crombrugghe BD (2000) Potent inhibition of the master chondrogenic factor sox9 gene by interleukin-1 and tumor necrosis factor-α. J Biol Chem 275: 3687–3692.
- Rockel JS, Kudirka JC, Guzi AJ, Bernier SM (2008) Regulation of sox9 activity by crosstalk with nuclear factor-κB and retinoic acid receptors. Arthritis Res Ther 10: 1-12.
- Togni S, Maramaldi G, Di Pierro F, Biondi M (2014) A cosmeceutical formulation based on boswellic acids for the treatment of erythematous eczema and psoriasis. Clin Cosmet Investig Dermatology 7: 321-327.
- Pedretti A, Capezzera R, Zane C, Facchinetti E, Calzavara-Pinton P (2010) Effects of Topical Boswellic acid on photo and age- damaged skin: Clinical, biophysical, and echographic evaluations in a double-blind, randomized, split-face study. Planta Med 76: 555-560.
- Calzavara-Pinton P, Zane C, Facchinetti E, Capezzera R, Pedretti A (2010) Topical boswellic acids for treatment of photo aged skin. Dermatologic Therapy 23: S28–S32.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences