Effect of Intrauterine Infusions of E. coli Endotoxin on Luteinizing Hormone Receptor Binding Capacity in Ovarian Follicles of Heifers: Exploratory Study
MC Lopez Diaz1 and Bosu WTK2
1Department of Animal Reproduction, INIA, 28040 Madrid, Spain
2Department of Medical Sciences, School of Veterinary Medicine University of Wisconsin, Madison, USA
- *Corresponding Author:
- MC Lopez Diaz
Department of Animal Reproduction
INIA, 28040 Madrid, Spain
Tel: 34 913473757
E-mail: lopez.maria@inia.es
Received date: November 09, 2016; Accepted date: January 12, 2017; Published date: January 20, 2017
Citation: Diaz MCL, Bosu WTK. Effect of Intrauterine Infusions of E. coli Endotoxin on Luteinizing Hormone Receptor Binding Capacity in Ovarian Follicles of Heifers: Exploratory Study. Insights Reprod Med. 2017, 1:1
Copyright: © 2017 Diaz MCL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
A study was conducted to determine whether follicles recovered from heifers treated with intrauterine infusions of E. coli endotoxin had altered LH binding capacities. Four Holstein heifers were synchronized (two PGF2 injections PG-1 and PG-2) and then super ovulated (with twice daily injections of FSH in decreasing doses for four days). On the third day luteolysis was induced with a third PGF2 injection (PG-3). Starting 24 h after PG-3, heifers received intrauterine infusions of either 5 cc sterile pyrogen-free water (CTR; n=2) or E. coli endotoxin at a dose of 5 μg/Kg bw (TRT; n=2), every 6 h for 7 treatments. An intensive blood sampling was carried out and plasma concentrations of LH, P4 and cortisol were determined by RIA's. All heifers were ovariectomized 64 h after PG-3 injection. Follicles were hystologicaly evaluated and classified as atretic or non-atretic. The concentrations of LH receptors in follicular wall were determined by one-point saturation analysis. A third of the follicles in TRT were classified as atretic versus a quarter in CTR heifers. Intrauterine infusions of endotoxin triggered only transitory, inconsistent rises in plasma cortisol. Treated animals had larger areas under the cortisol curve (5.9 and 7.71 v/s 4.66 and 4.13), but not significantly different from the controls (P>0.05). The concentrations of LH receptors were significantly lower (P<0.05) in TRT (1.9 ± 0.58 and 2.24 ± 0.2 fmol/mg) compared to CTR heifers (3.51 ± 0.53 and 3.72 ± 0.44 fmol/mg). In conclusion, E. coli endotoxin induced a significant reduction in the follicular concentrations of LH receptors in heifers. The mechanism whereby endotoxin affects follicular LH receptors is unknown.
Keywords
Heifers; Endotoxin; Lh receptor.
Introduction
Few studies have been devoted to determine the role of uterine infection in ovarian cyst formation since Garm [1] suggested in 1949 that infections of the genital organs do not play any part as etiologic factors in ovarian disorders. However, in post-partum dairy cows with a 40% incidence of metritis [2,3] there is an increasing evidence of the relationship between intrauterine infection and ovarian cysts degeneration [4-6]. Kaneene et al. [7] found that intrauterine infusion of Haemophilus sommus (1.5 × 109 C.F.U/ml) resulted in a 44.4% incidence of cystic follicles compared with 0% in control cows. Previous work has shown that in cows with postpartum uterine infections caused by gram negative bacteria, high plasma cortisol concentrations and endotoxemia were detected several days before ovarian cyst detection [5]. Later, follicular cysts were experimentally induced in heifers by intrauterine infusions of E. coli endotoxin during follicular phase [6]. In that study, increases in serum cortisol concentrations occurred after each endotoxin infusion and the pre-ovulatory LH surge was suppressed leading to ovarian cyst formation. It was hypothesized that E. coli endotoxin triggered increases in cortisol concentration which in turn suppressed LH surge.
High cortisol levels during follicular development could also act locally at the ovary. In ewes treated with ACTH a reduction of LH receptor concentration compromised ovulation and corpora lutea function [8]. Also, Bambino and Hsueh [9] found a direct inhibitory effect of glucocorticoids upon the concentration of testicular LH receptors in vivo and in vitro [9]. In fact, Brown et al. [10] has shown that cows diagnosed with chronic cystic ovarian disease had decreased LH receptor concentration in follicular walls. However, they could not elucidate the cause of decreased LH receptor concentrations because of the chronic condition of the cysts [10]. It is possible that the reduction in LH receptor could be the result of high cortisol levels during follicular development.
The objective of the present exploratory study was to determine whether follicles that develop in heifers during treatment with intrauterine infusions of E. coli endotoxin had altered LH binding capacities.
Materials and Methods
Four Holstein heifers aged 13-15 months, weighing between 304-362 Kg were used. They were housed in stanchions at the Charmany Instructional Facility of the School of Veterinary Medicine, fed three times daily with alfalfa hay and had free access to water. After a 30 day adaptation period, each heifer was subjected to a detailed reproductive system examination in the facility: this included both rectal palpation and ultrasonography of the reproductive tract followed by collection of endometrial swabs for bacteriologic culture. All heifers were free of uterine infections and metabolic disorders at the start of the study. Regarding the "reproductive status" of the females, all heifers were virgins and cycling before the hormonal and endotoxin treatments begun.
Synchronization of Estrus Cycle and Superovulation
Estrus cycles in the heifers were synchronized with two intramuscular injections of 0.5 mg of Cloprostenol (PGF2; Estrumate, Harver-Lockhart, Mobay Corp, Shawnee, KS USA) given ten days apart. On the ninth day after the second PGF2 injection (PG-2), a superovulatory treatment was carried out to ensure the harvest of a large number of preovulatory follicles from each heifer; follicles >8 mm were considered the "experimental unit". Superovulation treatment consisted of twice daily intramuscular injections of FSH (FSH-P, Schering Co., Kenilworth NJ USA) in decreasing doses for four days, as follows: 5/5 mg, 4/4 mg, 3/3 mg, 2/2 mg. The heifers were randomly assigned to either control group (CTR=2) or treated group (TRT=2). On the third day of superovulation treatment, luteolysis was induced in two animals (TRT=1 and CTR=1) and six hours later in the other two heifers with an injection of 0.5 mg of Cloprostenol (PG-3). On the same day, each animal was fitted with a jugular catheter for frequent blood sampling.
Treatments, Ovariectomies and Sampling
Every 6 h, starting from 24 h after third prostaglandin injection (PG-3), the TRT group heifers received intrauterine infusions of 5 μg/Kg bw E. coli endotoxin (LPS; E. coli serotype 055:B5; Lipopolysaccharide, Sigma Chemical Co., St Louis MO USA) in 5 cc sterile pyrogen-free water (total of seven infusions). Control heifers received intrauterine infusions of 5 ml of sterile pyrogen-free water using the same infusion regimens as above. Follicular development in the heifers was monitored by rectal palpation and ultrasonography, conducted every 12 h starting 24 h post PG-3 until 60 h post PG-3 when the last intrauterine infusion was given. Ultrasonographic technique previously described by Pierson and Ginther [11] was used to confirm the success of superovulatory treatment and to detect any premature ovulation in CTR animals. Blood samples were collected into heparinized tubes at 30 min intervals for 3 h, between 1 h before and 2 h after each endotoxin infusion followed by hourly sampling until 1 h before the next infusion. Blood samples were centrifuged and plasma collected and stored at -20°C until assayed for LH, cortisol and progesterone. Ovariectomies were performed through colpotomy incisions in all heifers 4 h after the last endotoxin infusion (64 h after PG-3 injection). Appropriate sedation (Xylazine 0.01 mg/lb i.m.) and caudal epidural anesthesia (2 cc of 1% Lidocaine) and postoperative antibiotic treatment were administered to the experimental animals [12,13].
Hormone Assays
Plasma samples were analyzed for LH concentrations using a double-antibody radioimmunoassay (RIA) previously validated in our laboratory [14]. Briefly, purified bovine LH (LER-1072-21) was iodinated using the chloramine-T method and an LH antibody was used at 1:600,000. All samples from one animal were analyzed in duplicate in one assay. The intraassay coefficient of variation of duplicate measurements was less than 9.3% and the interassay coefficient of variation was 4%.
Cortisol concentrations in plasma were determined by a RIA method (Coat-A-Count, Diagnostic Products Co., Los Angeles, CA USA) previously validated in our laboratory [5]. In brief, 50 μl of each specimen was added to a polypropylene test tube coated with antibody to cortisol. 1 ml of radio iodinated cortisol was then added to each tube and the tubes incubated for 25 min at 37°C. Following the incubation, all tubes, except the total count tubes, were decanted and then counted for one min in a gamma counter. The intraassay coefficient of variation of duplicate measurements was less than 9.5% and the interassay coefficient of variation was 12%.
Progesterone plasma concentrations were determined by RIA using a commercial kit previously validated in our laboratory [14]. All samples from one heifer were pressed in one assay. The intraassay coefficient of variation of duplicate measurements was less than 6.9% and the interassay coefficient of variation was 4%.
Pressing of the Ovaries
Following ovariectomies the ovaries were placed in ice-cold assay buffer (0.01 M TRIS-HCl, 0.1% BSA, 0.1% NaN3, 5 mM MgCl2; pH 7.4, 20°C) and transported to the laboratory. Ovaries were examined grossly for follicles and corpora lutea. All follicles >8 mm in diameter were dissected free of stroma and measured, weighed and the data recorded. Follicular fluid from each follicle was collected through a scalpel incision in the follicular wall, not aspirated, to prevent the granulosa cell dragging reported in numerous studies [15]. Follicular fluid was then centrifuged to remove any granulosa cells present. None of the samples had a visible pellet, indicating that the follicular fluid collection Procedure did not damage the inner layers of the follicular wall. The gross appearance of corpora lutea was classified according to Ireland et al. [16] classification into four stages:
Stage I
Red point of rupture, corresponding to ovulation point (days 1-4 of the estrous cycle).
Stage II
When CL is bisected the apex is red and the remainder of CL is orange (days 5-10 of the estrous cycle).
Stage III
CL is orange (11-17 of the estrous cycle).
Stage IV
Entire CL is yellow (days 18-21 of the estrous cycle).
Histological Classification of Follicle Health
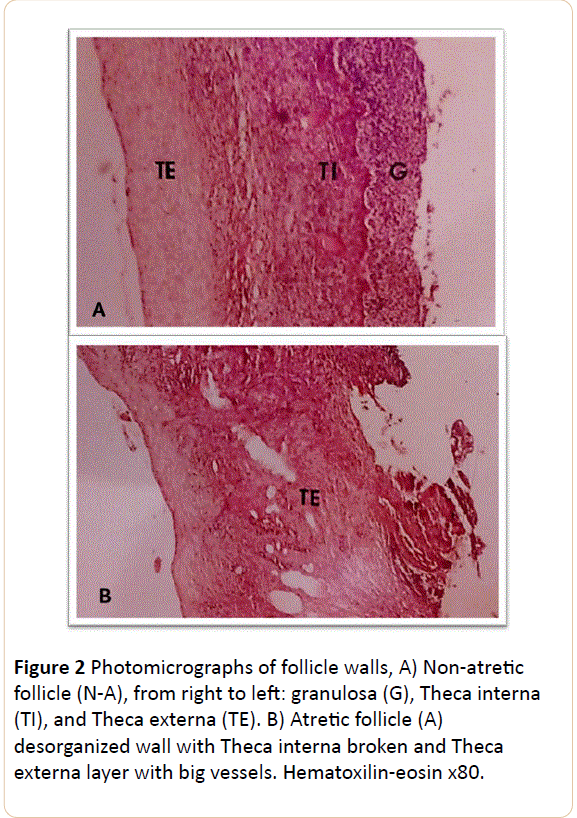
A piece of follicular wall (4 mm) was cut, fixed in Bouin's fixative, embedded in paraffin, sectioned (10 μm), stained with H-E, examined and the results used to rate the follicles. The follicles were classified according to the method of Grimes et al. [17] with some variations. First, pycknosis in granulosa cells was not considered as an atretic sign, because it is a physiological feature in preovulatory follicles [18]. Secondly, as the intermediary and non-atretic categories described by Grimes et al. [17] do not differ either in progesterone, androstenodione and testosterone concentration or in LH binding capacity we included both of them in a single, non-atretic class. Therefore, each follicle was placed into one of two categories, defined as follows: Non-atretic with 2-7 layers of granulosa cells, basal membrane usually present, characteristic preovulatory folding of granulosa and theca layers [18,19], theca layer varied in thickness but not disorganized; Atretic with granulosa layer almost absent; basal membrane absent and theca disorganized.
Measurement of Lh receptor binding capacity in follicular wall
After a histological sample was taken, the remainder of follicular wall was placed in separate vials (stored in assay buffer) and frozen at -70°C for LH radio-receptor-assay (RRA). Although degradation of LH receptors during storage was not examined, it has been shown that the loss of binding capacity of the LH receptors stored at -70°C for 3 months using our buffer is negligible [8,9]. Follicular wall was homogenized in buffer (0.01 M TRIS-HCl, 0.1% BSA, 5 mm MgCl2, 0.1% NaN3 , pH 7.4, 20°C) following the Procedure described by Barden et al. [20]. Purified human chorionic gonadotropin (CR-125; National Institute of Diabetes and Digestive, and Kidney Diseases (NIDDK) National Hormone and Pituitary Program (NHDDK) University of Maryland, School of Medicine, USA; 11,900 IU/mg) iodinated by the lactoperoxidase method was used as labeled ligand. Specific activity was calculated based on the percentage of radioactive iodine incorporated into the total mass of hormone and assuming a 95% recovery of the hormone after gel filtration chromatography of the iodination mixture. The specific activity was 23 μCi/μg. Purified hCG (CR-125; 11,900 IU/mg) was used as unlabeled ligand. Because of the limited amount of tissue available from each follicle, binding was determined by onepoint saturation analysis. Saturating concentration of labeled hCG was determined on broken follicles from experimental animals. Saturation of 10 mg of tissue was obtained with 300,000 cpm of labeled hormone. LH binding assays were performed according the procedure described by Brown et al. [10]. Briefly, aliquots of follicle wall homogenates (100 μl) were incubated with 100 μl of a saturating concentration of labeled hormone with or without 100 μl of excess unlabeled hormone (1 μg). After 16 h of incubation at room temperature the reaction was stopped with 3 ml of cold buffer (0.01 M TRIS-HCl, 0.14 M NaCl, 0.1% NaN3, pH 7.4, 4°C) and the tubes were centrifuged at 8,000x g for 30 min at 4°C. Following the aspiration of the supernatant, containing the unbound hormone, the radioactivity of bound hormone in the pellet was determined in a gamma counter. The specific binding was calculated as the difference of total binding minus nonspecific binding. All samples were run in one assay. The intraassay coefficient of variation was less than 3.8%. Protein concentration in homogenates of follicular wall were determined by the procedure of Lowry et al. [21] using a commercial Kit (Protein assay kit, Sigma Diagnostics Co., St Louis MO USA.
Statistical Analysis
Cortisol release was estimated as the area under the curve of plasma cortisol concentrations versus time. A paired t-test was used to determine if significant differences exist between treated and controls in the number of LH receptors and the amount of cortisol released. The initiation of the preovulatory LH surge was defined as a peak with a magnitude of at least 10 ng/ml for two or more 1 h interval samples [22]. Fluctuations in plasma levels were defined as pulses if: 1) it was preceded by at least 3 successive LH values that showed a progressive decline or represented basal levels and 2) the increase in LH was at least 32% above the mean of two preceding values [23].
Results
After the first intrauterine infusion, treated heifers exhibited a dramatic purulent metritis characterized by rectal palpation (increased uterine volume and tense uterine wall) and external genitalia examination findings (purulent exudate flow). However, none of the heifers displayed general symptoms of endotoxemia including depression, respiratory distress, anorexia or paresia [24,25]. The ultrasonographic exploration and rectal palpation confirmed that all animals responded to the superovulatory treatment as indicated by the presence of numerous big follicles in each ovary.
| CTR | TRT | |||
|---|---|---|---|---|
| Cow # | 1285 | 42 | ||
| Number of follicles | 7 | 8 | 12 | 6 |
| Corpus Luteum | Stage IV | None | None | None |
| Follicular diameter (mm) | 16.14 ± 1.17 | 11.5 ± 0.68 | 16.08 ± 1.27 | 13.42 ± 1.58 |
| Number of LH receptorsa (fmol/mg prot) | 3.5 ± 0.53 | 3.72 ± 0.44 | 2.24 ± 0.2 | 1.9 ± 0.58 |
| Histological classification | (3) N-A (1) A | (2) N-A (1) A | (3) N-A (2) A | (3) N-A (1) A |
Table 1: Ovarian findings in CTR and TRT animals (mean ± SE), a treatment effect (P<0.05), histological categories of the follicular wall: N-A (non-atretic) and A (atretic).
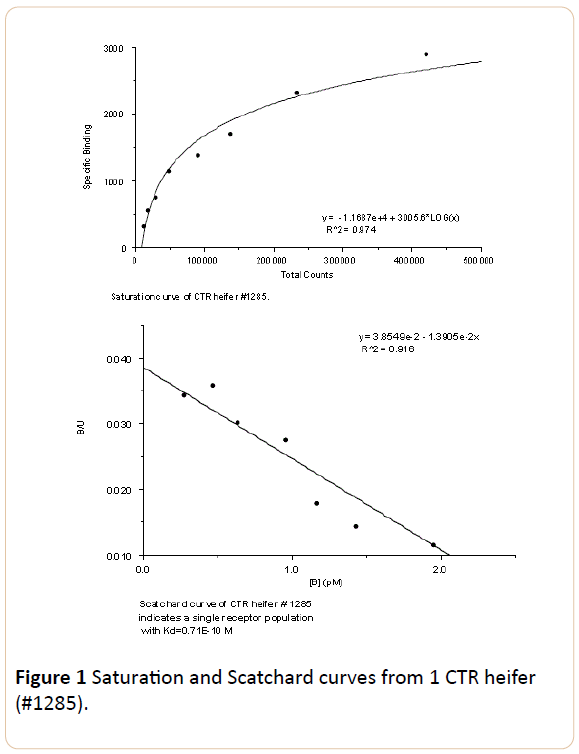
The number of follicles >8 mm collected was 7 and 8 for CTR and 12 and 6 for TRT heifers. No significant differences in the number of follicles or follicular diameter were found (Table 1). The concentrations of LH receptors were significantly lower in TRT compared to CTR heifers (P<0.05). Concentration of LH receptors in TRT heifers was approximately 58% of those in CTR heifers (Table 1). The Scatchard analysis of the saturation curve revealed a single receptor population with high affinity constant. The overall mean (x ± s.e) of Kd's from standard curves was 0.63 × 10-10 ± 0.04 × 10-10 M (Figure 1).
A total of 7 follicles from CTR and 9 from TRT heifers were histologicaly studied and ranked into two categories (A and N-A, Figure 2).
A third of the follicles in TRT were classified as atretic (A) versus a quarter in CTR heifers (Table 2). The concentrations of LH receptors (fmol/mg of protein) in A follicles from TRT and CTR heifers were smaller than those in N-A. That is, the RRA was able to detect that in A follicles (granulosa layer absent) there were lower numbers of cells than in N-A follicles. Similarly, there were decreases in the concentration of LH receptors in both N-A and A categories in TRT heifers compared with tissues from CTR heifers, indicating that the endotoxin treatment decreased the concentration of LH receptors per cell.
| CTR | TRT | |||
|---|---|---|---|---|
| Histological classification | N-A (n=5) | A (n=2) | N-A (n=6 | A (n=3) |
| Number of LH receptors (fmol/mg prot) | 3.6 ± 0.6 | 2.7 ± 0.33 | 2.4 ± 0.46 | 1.5 ± 0.56 |
Table 2: Number of LH receptors corresponding to both histological categories N-A (non-atretic) and A (atretic) of the follicular wall in CTR and TRT animals (mean ± SE).
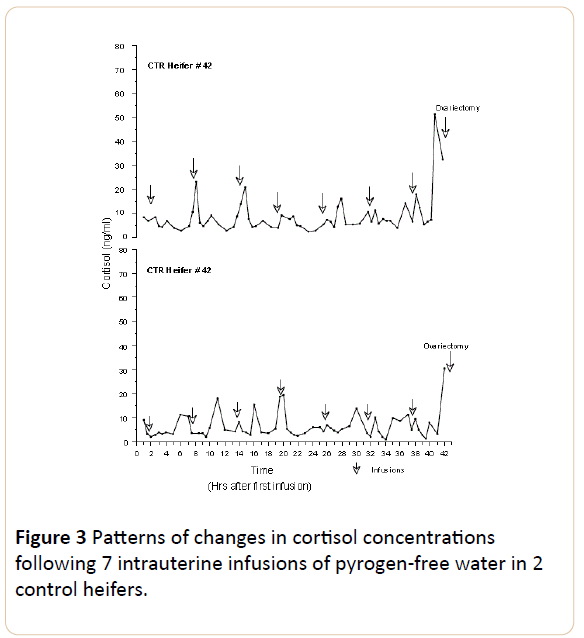
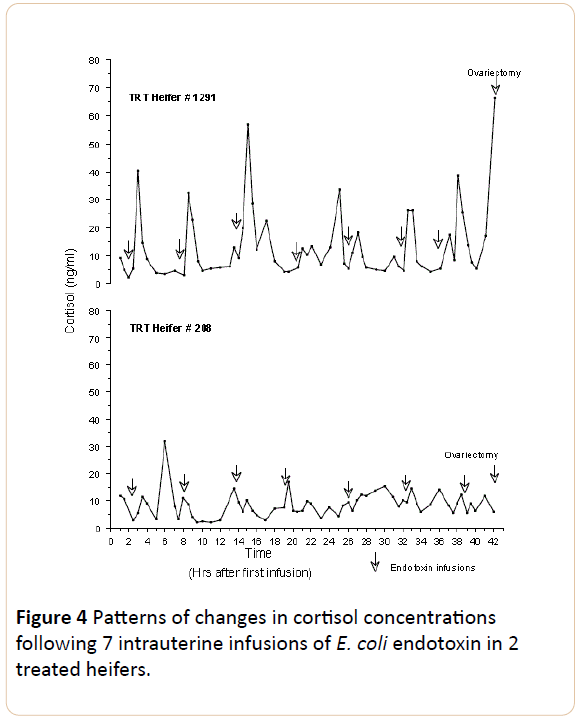
The cortisol released, estimated as the area under the curve, was greater in TRT animals (5.9 for # 208 and 7.71 for # 1291) than in CTR (4.66 for # 42 and 4.13 for # 1285), although the difference was not significant (P>0.05). In TRT heifers, # 208 and 1291, cortisol concentrations ranged between 2 and 56.73 ng/ml. In contrast, in CTR animals the highest values of cortisol concentration were lower (23.19 and 19.53 ng/ml for heifers # 42 and 1285). In treated heifer # 1291 six out of seven of the intrauterine endotoxin infusions were followed by surges in cortisol (>25 ng/ml) which lasted no more than two hours. In contrast, no clearly defined pattern was evident in any of the other three heifers (#208 TRT, # 1285 CTR and # 42 CTR).
Two different patterns of LH release were observed. Three heifers # 1285, 208, and 1291 exhibited small surges in LH (<4 ng/ml) between 49-58 h, 48.5-55 h and 35-44 h after PG-3 injection. However, no rise was detected in one CTR heifer # 42. Progesterone concentrations remained at basal levels (<1 ng/ml) from 24 h post PG-3 until the end of the experiment in both control and treated animals. Therefore, none of the animals had functional corpora lutea indicating that the PG-3 injection of the preovulatory treatment was effective.
Discussion
The main objective of the study was to determine whether follicles which developed in heifers treated with intrauterine infusions of E. coli endotoxin during follicular phase have altered LH binding capacity. The results show that average LH receptor concentration (fmol/mg of protein) was reduced in treated heifers. The significance of this reduction of LH receptor density on the fate of the follicles is not readily evident. While it is likely to be a contributing factor to ovulation failure and, possibly, to cyst development, it is not clear what degree of LH receptor reduction is necessary for the ovulation capacity to be compromised. It has been reported that cows which had borne chronic cysts for more than 10 months and had a 75% reduction in LH receptor concentrations, compared to controls; and did not ovulate in response to LH surges elicited after repeated GnRH treatments [10]. It was suggested in that study, that the reduction in concentration of LH receptors could explain the unresponsiveness of these cysts to GnRH treatment, but the question was raised as to when and why the reduction takes place. The percentage of reduced LH receptors in our study is similar to Rajameini et al.’s findings in women with polycystic ovarian degeneration (POD) [26], they found a 50% reduction in LH receptor concentration and hypothesized that an unusually high and/or mistimed preovulatory LH surge could down regulate LH receptors rendering follicles unable to undergo subsequent ovulation. In our opinion, an inadequately low basal secretion of LH can also compromise the appropriate acquisition of LH receptors by preovulatory follicles [27-29]. Our results show that intrauterine LPS infusions produce a reduction in the follicular concentration of LH receptors which can abrogate ovulation. In fact, supporting our results Williams et al. demonstrated in vivo and in vitro intrauterine infusions of LPS altered ovarian function and prevented ovulation in heifers [30,31].
In the present experiment, heifers were ovariectomized before the expected preovulatory LH surge in order to prevent.
Down regulation of LH receptors (26-29) and ovulation
Super ovulated cows frequently exhibit abnormal patterns of LH secretion: absent, early or moderate amplitude of preovulatory LH surge [32-34]. Three out of the four animals in the present study, showed a rise in LH at times similar to those reported in other studies on super ovulated heifers [33]. We do not consider these LH patterns to be preovulatory surges since: a) they failed to induce ovulation, in one case ovulation had not cured 26 h after the surge, and b) the amplitude (<4 ng/ml) was in all cases far below the generally reported levels (>10 ng/ml) [22,35]. Had larger preovulatory surges cured, it would have been detected, given the intensive blood sampling carried out. Additionally, one of the control animals had an LH rise, and yet the LH receptor numbers were similar for both controls, that is, no down-regulation of LH receptors cured. Coincident with Williams et al. results, no endotoxin effect on the pattern of LH was detected during our sampling period (up to 64 h of PG-3) [30].
From the results of this exploratory study it is not possible to identify which factor was responsible for the decrease in follicular LH receptor concentration. We had anticipated that intrauterine infusions of endotoxin would be followed by consistent, sustained increases in plasma cortisol, as described by Peter et al. [6] in a similar experimental set-up and, since glucorticoids have been proven to lower testicular LH receptors in vivo and in vitro [9], a reduction in LH receptors would cur. In the present study, however, endotoxin triggered only transitory, inconsistent rises in plasma cortisol, despite the fact that similar animals (virgin heifers) and endotoxin from the same strain of E. coli as in Peter's study were used [6]. Only one of the treated heifers showed cortisol increases, lasting no more than 2 h, after 6 out of 7 endotoxin infusions. Treated animals had a wider range of plasma cortisol concentrations and the areas under the curves were larger, but not significantly different from the controls. This mild cortisol response indicates that some degree of adrenal stimulation curred, but that absorption of endotoxin from the uterus was not substantial. In fact, Peter et al. [36] reported that endotoxin was not absorbed from the uteri of cows on day 20 postpartum. Elmore et al. [37] detected endotoxin absorption from the uteri of postpartum sows, but not from uteri of cycling ewes [38] or pony mares [39] and concluded that, given its high molecular weight, endotoxin is only absorbed from the uterus in significant quantities when endometrial damage exists. Taken together we cannot rule out the implication of pituitary-adrenal-ovarian axis in the formation of ovarian cysts in stressed animals with endotoxemia. In fact, it has been proven that challenges with of ACTH induced ovarian follicular cysts in ewes and heifers [8,40,41].
The possibility exists that lal mediators of inflammation are responsible for the observed changes in LH receptor concentrations. Presence of endotoxin in the uterine lumen attracted leukytes as was evident by the cloudy mucous discharge from the uterus seen in treated animals. Leukytes under endotoxin action release factors such as tumor necrosis factor-TNF) and interleukins (IL's), which after gaining access to the circulation act like hormones and induce a broad spectrum of systemic changes [30,40,42]. IL-is a polypeptide with molecular weight (17,500 Da) smaller than that of glycoprotein hormones like LH (30,000) which are able to reach granulosa cells in the ovarian follicle from systemic circulation [43]. In fact Khan et al. [44] identified IL-1-like in ovarian follicular fluid in human. In cultured FSH-stimulated rat granulosa cells, ILdecreased the number of LH receptors [45,46], but a direct effect of IL-on ovarian physiology has not been demonstrated in vivo.
In the last two days preceding ovulation, normally cycling cows have two big follicles [44]. Only one is estrogen-active, non-atretic and will ovulate following the preovulatory LH surge [29]. In our control animals, 3/4 of the follicles were non-atretic, in agreement with Busch et al. [47] who found a higher proportion of estrogen-active follicles in super ovulated heifers. In treated animals, the proportion of non-atretic follicles was 2/3. The histological classification of follicles into atretic or nonatretic agrees very well with the bimodal distribution of LH receptors: 2.7 and 3.6 fmol/mg of protein for atretic and nonatretic follicles, respectively, in control heifers. Similarly, in treated animals, LH receptor concentration was lower in atretic than in non-atretic follicles (1.5 vs. 2.4 fmol/mg of protein). This agrees with previous findings by Ireland and Rhe [29].
The present study clearly indicates that intrauterine E. coli endotoxin induces a significant reduction in the follicular concentrations of LH receptors. This reduction 'per se' might be sufficient to compromise ovulation and lead to cyst formation, although a direct action of LPS on LH receptor has not been demonstrated. The mechanism whereby endotoxin affects LH receptors remains to be elucidated. Our results indicate, however, that cortisol might not be involved. Rather, mediators of inflammation lally produced at the uterine level might be responsible for the effect.
Acknowledgement
We thank Dr. NL First, Department of Animal Sciences, for the provision of ovaries. Thanks are also due to Dr. RL Matteri and F Wegner, Regional Primate Research Center, Madison, for their help with the RRA. Thanks to C Cadórniga, R Rodriguez and MJ Decimavilla for their help during the sampling period. Research was supported by funds from USDA project #3234.
References
- Garm O (1949) A study of bovine nymphomania: With special reference to etiology and pathogenesis. Acta Endocrinol 3: 1-144.
- Kesler DJ, Garverick HA (1982) Ovarian cysts in dairy cattle: A review. J Anim Sci 55: 1147-1159.
- Sheldon IM, Lewis GS, LeBlanc S, Gilbert RO (2006) Defining postpartum uterine disease in cattle. Theriogenology 65: 1516-1530.
- Lopez-Diaz MC, Bosu WTK (1992) A review and an update of cystic ovarian degeneration in ruminants. Theriogenology 6: 1163–1183.
- Bosu WT, Peter AT (1987) Evidence for a role of intrauterine infections in the pathogenesis of cystic ovaries in postpartum dairy cows. Theriogenology 28: 725-736.
- Peter AT, Bosu WTK, De Decker RJ (1989) Suppression of preovulatory luteinizing hormone surges in heifers after intrauterine infusions of Escherichia coli endotoxin. Am J Vet Res 50: 368-373.
- Kaneene, JB Coe, PH, Gibson CD, Yamini, B, et al. (1986) The role of Haemophilus somnus in early embryonic death. I. The effect of the organism on embryos by Day 8 post breeding. Theriogenology 26: 189-198.
- López Díaz MC, Bosu WT (1997) Effects of ACTH on luteinizing hormone receptors in ovine follicular wall and corpus luteum. Reprod Nutr Dev 37: 599-612.
- Bambino TH, AJW Hsueh (1981) Direct inhibitory effect of glucocorticoids upon testicular luteinizing hormone receptor and steroidogenesis in vivo and in vitro. Endocrinology 108: 2142-2148.
- Brown JL, Schoenemann HM, Reeves JJ (1986) Effect of FSH treatment on LH and FSH receptors in chronic cystic-ovarian-diseased dairy cows. J Anim Sci 62: 1063-1071.
- Pierson RA, O J Ginther (1984) Ultrasonography of the bovine ovary. Theriogenology 17: 237-245.
- Cox JE (1987) Surgery of the reproductive tract in large animals. University Press, Liverpool.
- Lumb WV (1984) Veterinary anesthesia. Philadelphia: Lea & Febiger.
- Peter AT, Bosu WTK (1987) Effects of intrauterine infection on the function of the corpora lutea formed after first postpartum ovulations in dairy cows. Theriogenology 27: 593-609.
- Bellin ME, Ax RL (1984) Chondroitin sulfate: an indicator of atresia in bovine follicles. Endocrinology 114: 428-434.
- Ireland JJ, Murphee RL, Coulson PB (1980) Accuracy of predicting stages of bovine estrous cycle by gross appearance of the corpus luteum. J Dairy Sci 63: 155-160.
- Grimes RW, Matton P, Ireland JJ (1987) A comparison of histological and non-histological indices of atresia and follicular function. Biol Reprod 37: 82-88.
- Priedkalns J, Weber AF, Zemjanis R (1968) Qualitative and quantitative morphological studies of the cells of the membrane granulosa, theca interna and corpus luteum. Zeitschrift fur Zellforschung 85: 501-520.
- Dellman HD (1987). Textbook of veterinary histology. Philadelphia: Lea & Febiger.
- Braden TD, Manns JG, Cermak LD, Nett TM, Niswender GD (1986) Follicular development following parturition and during the estrous cycle in beef cows. Theriogenology 25: 833-843.
- Lowry OH, Rosebrough NJ, Farr A, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265-275.
- Refsal KR, Maldonado JHJ, Nachreiner RF (1987) Endocrine profiles in cows with ovarian cysts experimentally induced by treatment with exogenous estradiol or adrenocorticotropic hormone. Theriogenology 28: 871-889.
- D'occhio MJ, Schanbacher BD, Kinder JE (1982) Relationship between testosterone concentration and patterns of luteinizing hormone secretion in male sheep. Endocrinology 110: 1547-1554.
- Lohuis JA, Verheijden JH, Burvenich C, van Miert AS (1988) Pathophysiological effects of endotoxins in ruminants: Metabolic aspects. Vet Q 10: 117-125.
- Lohuis JACM, Verheijden JHM, Burvenich C, Miert ASJPAM (1988) Patholophysiological effects of endotoxins in ruminants. 1. Changes in body temperature and reticulo-rumen motility and the effect of repeated administration. Vet Q 10: 109- 116.
- Rajaniemi HJ, Rönnberg L, Kauppila A, Ylöstalo P, Vihko R (1980) Luteinizing hormone receptors in ovarian follicles of patients with polycystic ovarian disease. J Clin Endocrinol Metab 51: 1054-1057.
- Gore-Langton RE, Armstrong DT (1994) Follicular steroidogenesis and its control. In: The Physiology of Reproduction. Eds E Knobil & JD Neill. New York: Raven Press 1: 571–628.
- Webb RB (1982) Identification of the ovulatory follicle in the ewe: associated changes in follicular size, thecal and granulosa cell luteinizing hormone receptors, antral fluid steroids and circulating hormones during the preovulatory period. Endocrinology 110: 873-881.
- Ireland JJ, Roche JF (1983) Growth and differentiation of large antral follicles after spontaneous luteolysis in heifers: Changes in concentration of hormones in follicular fluid and specific binding of gonadotropins to follicles. J Anim Sci 57: 157-167.
- Williams EJ, Sibley K, Miller AN, Lane EA, Fishwick J, et al. (2008) The effect of Escherichia coli lipopolysaccharide and tumour necrosis factor alpha on ovarian function. Am J Reprod Immunol 60: 462-473.
- Callesen H, Greve T, Hyttel P (1986) Preovulatory endocrinology and oocyte maturation in superovulated cattle. Theriogenology 25: 71-86.
- Ben Jebara MK, Carrière PD, Price CA (1994) Decreased pulsatile LH secretion in heifers superovulated with eCG or FSH. Theriogenology 42: 685-694.
- Lindsell CE, Murphy BD, Mapletoft RJ (1986) Superovulatory and endocrine responses in heifers treated with FSH-P at different stages of the estrous cycle. Theriogenology 26: 209-219.
- Mapletoft RJ, Steward KB, Adams GP (2002) Recent advances in the superovulation in cattle. Reprod Nutr Dev 42: 601-611.
- Cook DL, Parfet JR, Smith CA, Moss GE, Youngquist RS, et al. (1991) Secretory patterns of LH and FSH during development and hypothalamic and hypophysial characteristics following development of steroid-induced ovarian follicular cysts in dairy cattle. J Reprod Fert 91: 19-28.
- Peter AT, Bosu WT, Gilbert RO (1990) Absorption of Escherichia coli endotoxin (lipopolysaccharide) from the uteri of postpartum dairy cows. Theriogenology 33: 1011-1014.
- Elmore RG, Martin CE, Berg JN (1978) Absorption of Escherichia coli endotoxin from the mammary glands and uteri of early postpartum sows and gilts. Theriogenology 10: 439-445.
- Elmore RG, Mollett TA, Berg JN, Schmidt DA (1983) Escherichia coli endotoxin absorption from the ewe's uterus and peritoneal cavity. Theriogenology 19: 841-847.
- Mollett TA, Elmore RG, Blanchard TL, Berg JN (1985) Effects of intrauterine infusion of Escherichia coli endotoxin in anestrous and steroid treated pony mares. Theriogenology 23: 597-606.
- Ribadu AY, Nakada K, Tanaka Y, Moriyoshi M, Zhang WC, et al. (1999) Lack of LH response to exogenous estradiol in heifers with ACTH-induced ovarian follicular cysts. J Vet Med Sci 61: 979-981.
- Williams EJ, Fischer DP, Noakes DE, England GC, Rycroft A, et al. (2007) The relationship between uterine pathogen growth density and ovarian function in the postpartum dairy cow. Theriogenology 68: 549-559.
- Dinarello CA (1988) Biology of interleukin. FASEB J 2: 108-115.
- Cooke BA, Rommerts FFG (1988) The mechanism of action of luteinizing hormone. I. Luteinizing hormone-receptor interactions. In: Cooke BA, King RJB and van der Molen HJ, Hormones and their actions. Elsevier Science Publishers BV (Biomedical Division), pp: 155-162.
- Khan SA, Schmidt K, Hallin P, Di Pauili R, De Geyter C, et al. (1988) Human testis cytosol and ovarian follicular fluid contain high amounts of interleukin-1-like factor(s). Mol Cell Endocrinol 58: 221-230.
- Gottschall PE, Katsuura G, Hoffmann ST, Arimura A (1988) Interleukin 1: An inhibitor of luteinizing hormone receptor formation in cultured rat granulosa cells. FASEB J 2: 2492-2496.
- Dufour J, Whitmore HL, Ginther OJ, Casida LE (1972) Identification of the ovulating follicle by its size on different days of the estrous cycle in heifers. J Anim Sci 34: 85-87.
- Busch W, Woitow G, Smollich (1987) Histologische untersuchungen an ovar und emdometrium des rindes nach induktion begrenzter polyovulationen. Zuschthyg 22: 53-63.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences