Development of Inactivated Newcastle Disease Vaccine in Sudan
Manan A AA, Kheir M.A.S, Ballal A,Nour TAM, Fatima AT
Central Veterinary Research Laboratory (CVRL) Sudan, Research Corporation (ARRC), P.O. Box 8067, El Amarat, Khartoum, Sudan.
*Corresponding author: Manan Abdelmhmoud Atalmanan , Central Veterinary Research Laboratory (CVRL) Sudan, Research Corporation (ARRC), P.O. Box 8067, El Amarat, Khartoum, Sudan; Tel:96630312408; E-mail: mhmoud_vaccines@outlook.com
Received date: September 22, 2020; Accepted date: August 21, 2021; Published date: August 31, 2021
Citation: Manan A AA (2021) Development of Inactivated Newcastle Disease Vaccine in Sudan 1:1
Abstract
Using I-2 and La-Sota strains two inactivated Newcastle Disease (ND) vaccines were produced. The vaccine viruses were inactivated by treatment with 0.05% laboratory grade formaldehyde,then each inactivated vaccine prepared as a water in oil (W/O) emulsion. For each emulsion, the aqueous phase ratio was (2.4 : 1) (allantoic fluid : tween 80 ) respectively. While the oil phase contains (9: 1) (paraffin oil : manidmonoleate (span 80)) as an oil emulsifier. The prepared vaccines were subjected to physical tests including stability, viscosity and quality of emulsification completeness . The two vaccines were confirmed to be sterile, stable for 30 days at 37â° C and for 6 months at 4co. the viscosity was 4ml/ 8 second. Tests for safety,immunogenicity, and efficacy (challenge test) as well as cross protection evidence for the two vaccines were performed in -one day- old broiler chicks. For phase I trial the vaccines were found to be safe, immunogenic ,and effective with 80% and 40% protection level for oil emulsion vaccines derived from ND I-2 and ND La-Sota strains respectively. Because of relatively higher efficacy( 80%) obtained From I-2 strain in phase I trial, this result validate further investigation for I-2 strain in phase II clinical trial.
In phase II trial , the protection reached 93% in a group vaccinated only with inactivated ND I-2 vaccine, compared with 100% protection against very virulent ND for the group vaccinated simultaneously with live and inactivated ND I-2 vaccines. Independent sample t.test was used to compare the GMT Abs titer for the post vaccination sera with statistically insignificant outcome (P>.05). The results obtained in this study confirmed that, the killed ND I-2 vaccine produced locally was safe and efficient, and could be used with high efficiency against the very virulent ND strains , and has the potential to replace the imported ND oil vaccines.
Introduction
The presence of ND in Sudan was first reported in 1951(Osman, H. 2006). Since then ND has been periodically reported from all regions of Sudan, where heavy losses have been reported (Khalafalla et al., 2001). Diagnosis was based on clinical signs and post-mortem lesions as well as the general pictures of the disease.The first attempt to characterize the local isolates was done in 1979 and the isolates were found to be virulent (Eisa,1979).
Khalafalla et al (1992) studied the pathogenic properties of ND virus isolates in Sudan and they found that all isolates were viscerotropic velogenic ND viruses. In 2006 Wegdan et al performed a phylogenetic analysis for the fusion protein gene of isolates obtained from outbreaks of ND in Sudan, and found that, all contemporary strains isolated between 2003 and 2006 were of genotype 5d.
In the commercial sector, losses due to virulent ND were 70%, 98 % and 62% in chicks, growers and adults, respectively. In intensive poultry production, inactivated vaccines are usually applied after an initial priming vaccination with a live vaccine. To combat the disease a wide variety and types of vaccine have been developed including live lentogenic, live mesogenic and inactivated vaccines (Alexander etal., 2004).
Despite the use of different vaccines including live and inactivated, (ND) is yet to be controlled in both village chickens and commercial flocks. However, given the upsurge in Newcastle Disease and the importance of inactivated vaccines, many poultry producers have been obliged for a number of years now to turn to the combined use of live and inactivated vaccines in young birds (Vincent turblin, 2009) Inactivated vaccine is more capable of eliciting an immune response in the face of existing maternal immunity (Marangons and Busani, 2006) and can be used in day-old chicks because the maternal antibodies do not affect the vaccine efficiency (Nichol et al., 2012)as well as
inactivated vaccines produce very high level of antibodies against ND virus, and provide good protection against the virulent virus. Drawing on these facts we In the present study in order to potentiate the chickens immune responses against the very virulent (vv ) NDV we utilized the thermostable seed virus of I-2 and Lasota Strain to develop inactivated ND vaccines, (oil vaccines) and investigating these candidate vaccines in day-old broiler chicks with live I-2 ND vaccine via eye drop route or without live I-2 vaccine to obtain solid immunity until marketing of broilers at 6 to 7 weeks.
The I-2 virus was originally isolated in Australia with funding from the Australian Centre for International Agricultural Research (ACIAR) (Alders et al, 2005).
This strain was identified after testing of forty-five isolates of avirulent ND virus. It was chosen for its antigenicity and thermostability. The master seed stock of virus was derived from parent stock that had survived at 56oC for thirty minutes.
The master seed was then tested for safety and freedom from bacterial contamination. (Bensink and Spradbrow, 1999) .The I-2 thermostable ND vaccine is similar to NDV4-HR but is free of commercial ownership, and the master seed virus is available to laboratories in developing countries wishing to produce the vaccine locally (Young, et al., 2002).for this study the I-2 master seed virus was first supplied by the Department of Veterinary Pathology of the University of Queensland, Australia, which then handed over by the Department of Veterinary Virology of the Veterinary Research Institute,Sudan.
For our knowledge this is first time to produce an inactivated ND vaccine derived from a thermostable ND strain.
Inactivated vaccines are produced by growing ND virus in eggs, and then treating the infective allantoic fluid with an inactivating agent, such as formalin or betaproiolactone. An adjuvant, such as mineral oil, is usually added to make the inactivated virus more immunogenic.
Also in this study we end up with a simple and more robust vaccination program which include killed vaccine and intraocular administration of live vaccine at day old which is proved to be more efficient and if it is followed by spray at the end of the second week of life it will be more immunogenic, and economically beneficiary to the broiler farmers than the conventional vaccination program (Vincent turblin, 2009), therefore it would be of good value if the local authority could recommend this vaccination program to be launched in the commercial flocks from the very beginning as a hatchery vaccine ,Such a synergistic hatchery vaccination program ( live and inactivated) has been able to demonstrate its efficacy in building an efficient “wall of protection” in front of different wild pressure levels, from subclinical to clinical infection. (Vincent turblin, 2009).Then the hatchery companies in Sudan would accept it as anew norms, and this will be helpful in our battle against the disease.
Material and methods
Development of Inactivated Newcastle disease vaccine
Pre-Clinical phase
Establishment of vaccine seed lots system
Preparation of the I-2 master seed lot (MSL)
One out of two ampoules containing the master seeds virus was then removed from -70oC and thawed at room temperature. Sound 10 -day old - embryonated chicken eggs were used to prepare the master seed-lot (MSL) from which the working seed lot (WSL) was produced.
The embryonated chicken eggs were candled and cleaned with 70% alcohol. Holes were drilled on the eggs shell, and the allantoic cavities of 15 embryonated chicken eggs were aseptically inoculated with 0.2ml of undiluted liquid allantoic fluid of the master seeds.
Five embryonated chicken eggs were inoculated with 0.2ml of antibiotics solution and kept as a negative control group. The inoculation sites were then sealed with paraffin wax and the eggs incubated at 38oC for 120 hours. The eggs were candled daily and any one with dead embryo was discarded. The infected allantoic fluid was harvested after the incubation period and chilled for 2 hours at 4oC, then the allantoic fluid was collected, and stored in 7ml aliquots, stored at -20oC.
Preparation of the I-2 working seed lot (WSL)
Using aseptic technique one aliquot of I-2 ND master seed bank prepared previously was removed and thawed at room temperature .A 5 ml of the thawed I-2 master seed was diluted in 20 ml Normal Saline (NS) with antibiotic mixture. Seventy-10- day-old embryonated chicken eggs were inoculated with 0.2ml of diluted I-2 master seed into the allantoic fluid using aseptic technique. A15 embryonated chicken eggs inoculated with 0.2ml (NS) were kept as control group.
Inoculated eggs were incubated at 38oC for 120 hours, next day all eggs were candled for the evidence of non specific death. After 120 hours incubation, 400 ml of infected allantoic fluid was harvested, and immediately tested using the rapid haemagglutination test to determine the presence of the ND virus (Grimes, 2002). The harvested allantoic fluid centrifuged in a cold centrifuge at 10,000 RPM for 5 minutes, then the pooled allantoic fluid dispensed into aliquots of 1.5 ml under laminar flow system, the ampoules were lypholised, sealed and stored at
-20oC. The working seed bank was tested for bacterial, fungal, and mycoplasma contaminations (OIE Manual. 2012).
Characteristics of the I-2 working seed virus
Identity test... (HI) test
Reference serum known to contain antibodies to ND virus was used to confirm the presence of Newcastle disease virus in three ampoules of the working seed virus.
Briefly a 25µl of sterile Normal Saline (NS) was dispensed to all wells of a 96 microtiter plate, then 25 µl of the reconstituted lyophilized ND hyper immune serum were dispensed in to 1st column, and then two fold dilutions carried out across the plate. A 25 µl of the 4HA unit (HAU) of I-2 virus suspension was added to each well of the 96 wells plate, then incubated at 37oC for 30 minutes, the test contains +ve control serum, then 25µl of 1% chicken RBCs had been added and the plate incubated at room temperature RT for 30 minutes (OIE Manual, 2012).
Assessment of I-2 virulence by intracerebral pathogenicity index (ICPI)
Two ampoules of lyophilized I-2 working seed vaccine were reconstituted in 1 ml sterile NS with no antibiotics for each. Using aseptic technique the reconstituted vaccine was pooled and diluted 1/10.
A aseptically 50µl of the diluted vaccine was injected intracerebrally into each of ten chicks of one- day old- , hatched from healthy flock. Further 5 one-day old- chicks were inoculated with 50ul of the reconstituting diluents and observed as a control group. The birds were examined every 24 hours for 8 days , for each observation, the vaccine treated birds were scored 0 if they were normal, 1 if sick, and 2 if they died.
The final ICPI was calculated as mean score per bird per observation over the 8 days of observations.
Control of seed lots
The tests for absence of bacteria, fungi, and mycoplasma, sterility tests have been done for the master seed lot (MSl) and the working seed lot (WSl) , as well as tests for safety (OIE, 2012).
The MSL and WSL used in this vaccine has been thoroughly investigated for its sterility, safety, and efficacy issues according to OIE terrestrial manual (OIE, 2012).
Quality of embryonated chicken eggs
This vaccine was produced using chicken embryonated eggs derived from a healthy flock and vaccinated against the major poultry disease. Palya (1991)
Strains of vaccines production
For phase I clinical trial. The vaccines were prepared by using two strains of avirulent ND virus of I-2 strain (Australian strain), and La-Sota strain. The intracerebral pathogenicity index (ICPI) of the I-2 strain is 0.125.
Pre-clinical Stage
Allantoic fluids of I-2 and Lasota strains containing 109.1 EID50 /ml and 108.5 EID50 /ml respectively were used as stock virus for the vaccine formulation.
Inoculation of vaccine strains
A three vials of each lyophilized I-2 and LaSota strain were obtained from their Working seed lots(WSL) and diluted in sterile normal saline i.e. 0.2ml containing 103 EID50 was inoculated into the allantoic cavity of 10-day-old embryonated chicken eggs, and then incubated at 37oc for 120 hours. Dead embryos were discarded within 24 hours.
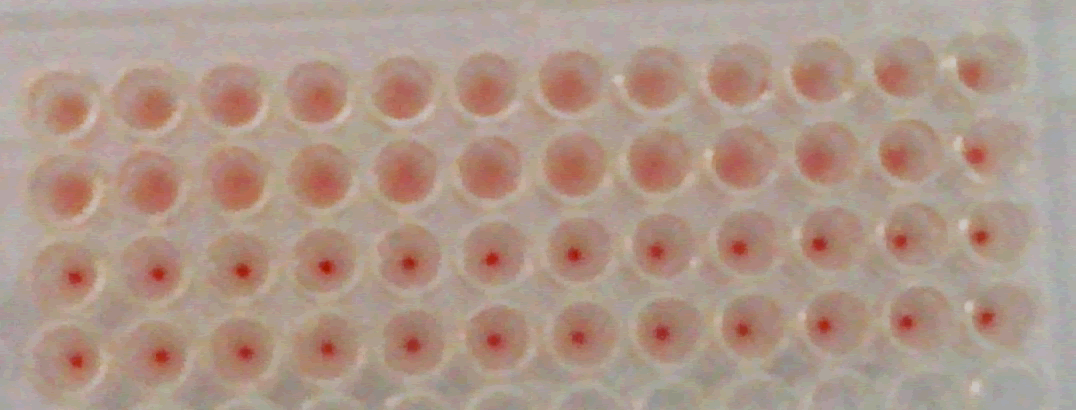
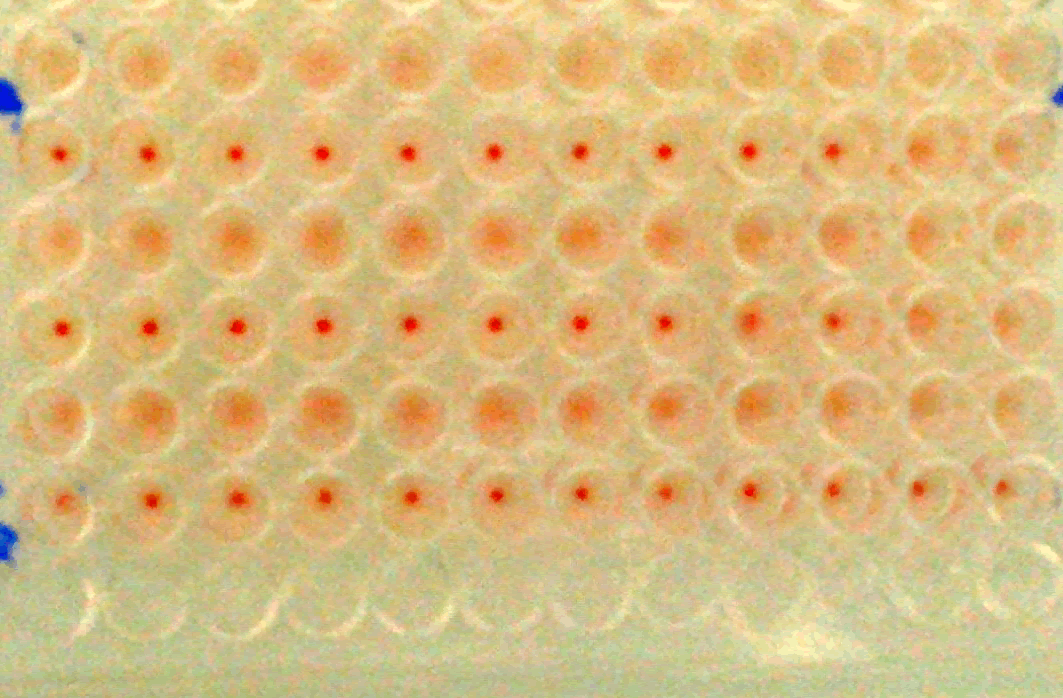
Figure 2 : (Identity test)the Haemagglutination Inhibition test (HI) carriedout for the working seed lots (WSL)using reference hyperimmune serum(diluted more than 1024 time) and 8 Haemagglutination unit (HAU) to test the identity of both I-2 and La-Sota srains. the top row shows the result of negative control serum while the bottom row shows the result of positive control serum, the rows in the middle show the result of the tested WSL of I-2 and Lasota strains respectively
Harvesting
At the end of the incubation period the infected eggs were chilled at 4oC over night before being harvested. The allantoic amniotic fluid (AAF) was aspirated using 10ml pipette .then the harvested AAF was centrifuged using cold centrifuge at 1000 RPM for 7 minutes, pooled and stored at 4oC.
Test of virus content
The HA test
This test was conducted according to OIE terrestrial manual (OIE, 2012).
Virus titration
The virus content of the WSl has been defined by making ten-fold dilution and inoculated in to 10-day-old embryonated chicken eggs. Titer estimated as embryo infectious dose of fifty (EID50)
Inactivation of the viruses
The two vaccines strains of I-2 and la-Sota were inactivated by treatment with 0.05% laboratory grade formaldehyde, this was according to Wisanu et al., (2009) and OIE, (2012). 75µl and 50µl of absolute laboratory grade formaldehyde were added to 150 ml, and 100ml AAF of I-2 and La-Sota strains respectively, and then the two bottles were shaken well, and incubated at 37oC for 16 hours. After incubation the bottles were stored at 4oC. Later on for the product escalation, a larger amount of 600 ml AAF of I-2 strain has been inactivated by adding 300ul of the concentrated formaldehyde using the same procedure.
Control tests on raw vaccines
Test for complete inactivation
The test has been performed on the formaldehyde treated allantoic fluid, immediately after the incubation for inactivation has been completed, The allantoic fluids of the two strains of I-2 and La-Sota were inoculated into 10-day old- embryonated chickens eggs and incubated at 37oC for 120 h. (Palya., 1991).
Sterility test for raw and final product
After verifying completion of the inactivation,the raw allantoic fluids and final product after emulsification were subjected to a simple bacteriological sterility test. 10 vials of thioglycolate broth media were each inoculated with 0.2ml inactivated allantoic fluid and oil the vaccines respectively and incubated aerobically at 37oC and at room temperature for 7 days.
Formulation of the water (W/O) in oil emulsion vaccines
phase I
The two formulations of the w/o vaccines were prepared according to Wisanu et al, (2009) the aqueous phase of the two emulsions consisted of the allantoic fluids according to the type of strain.
For each formulation the aqueous phase composes of 9.6 ml allantoic fluid, and 0.4ml tween 80 mixed in sterile plastic containers and stirred with magnetic stirrer, adjusted at low speed pace for 2 minutes.
The oil phases were purchased ready as incomplete Freund's adjuvant (sterile, oil-arlacel mixture) containing 9ml of paraffin oil plus 1ml manidmonoleate (span 80) as an oil phase emulsifier.
For each preparation the aqueous phase was added drop wise to the oil phase while the oil phase was constantly stirred at low speed in sterile container.
After the addition was completed, the mixture was emulsified by mixing with disposable 10ml syringe with 0.5mm needle. this process was repeated until all the aqueous phase has been incorporated into the oil phase.
On standing for a few moments no accumulation of the water phase has been seen at the bottom of the bottle.
phase II
For phase II clinical trials about 600 ml of W/O emulsion was prepared from a pharmaceutical grade white mineral oil ( paraffin oil) as follow: the aqueous phase was made up by adding 12 ml of tween 80 to the 288 ml allantoic fluid 1: 24 respectively ,then gently stirred for 30 minutes for proper mixing.
Equal volume of the oil phase was prepared by adding 30 ml manidmonoleate (span 80) to the 270 ml of the purified paraffin oil 1: 9 ratio respectively, then gently mixed for 45 minutes using
magnetic stirrer. Using the same procedure 300 ml aqueous phase was added drop wise to the 300 ml oil phase i.e. 1:1 ratio and thoroughly mixed by stirring for 20 minutes then using the rotary machine method , the product emulsified by using the machine Silverson emulsifier with head suitable for larger volumes, this process last for 20 minutes then a very homogenous product has been obtained. (Palya., 1991).
The physical characteristics of the emulsions
Testing the water in oil (w/o) emulsion
To determine the vaccine integrity, the finished w/o emulsion was tested by allowing a few drops to fall on the surface of tap water in Petri dish.
Stability test
The stability of this oily preparation was tested by incubating and observing the vaccines at 4oC for 6 month in tightly screwed tubes.
Viscosity Test
Relative viscosity was determined as a flow time at 24oc by discharging of 0.4 ml of the emulsion vaccine from a vertically mounted 1ml pipette, emulsion was drawn up to 1 ml mark. The time for discharge of the emulsion was measured in secounds
Clinical phase
Randomized-controlled trials for the two formulated inactivated Newcastle disease vaccines of I-2 and LaSota lentogenic strains in day-old-broiler chicks.
Study design
This phase I, and II double blinded randomized controlled trials which were conducted in 80 and 120 healthy day-old Commercial broiler chicks respectively at the veterinary research institute, department of viral vaccine production.
Experimental design
This study was parallel randomized controlled design.
Sample size
The sample size of this study was determined according to the method of manufacture, and the requirement for authorization stated by OIE terrestrial manual 2012. To prove that the ND formulated inactivated vaccines were safe and immunogenic, an over all sample size of 80, 120
chicks was generally considered appropriate for safety, and efficacy evaluation in phase I and phase II clinical trials for vaccines evaluation.
Chicks
Healthy day-old chicks were included regardless of their maternal antibodies level.
Chicks were excluded if they were layer breed or more than 48 hours age or derived from flocks that had any clinical signs attributed to ND or ND like diseases.
The vaccines
The formulated ND inactivated vaccines were prepared in this study from ND I-2 and LaSota lentogenic strains, and according to the manual of production of ND vaccine. (Palya., 1991).
Intervention
Tests for safety, immunogenicity, and efficacy were performed in host animal and done for both vaccine interventions used in this trials.
Each dose for immunogenicity and safety tests was 0.2ml or .4ml respectively.
Safety groups
Twenty and thirty Chicks in phase I and phase II trials respectively received the double recommended dose of 0.4ml using subcutaneous route (s/c).
Efficacy groups
Phase I
The chicks in group I, and group II, designated the efficacy groups, they received inactivated ND vaccines derived from I-2, and LaSota lentogenic strains.
Chicks in group I receive 0.2ml by s/c route at the nap of the neck. Chicks in group II received also 0.2ml via the same route of administration.
Control group of 10 chicks designed group V received no treatment, and remain as non-inoculated control.
Phase II
One hundred and twenty-day old- chicks obtained from the same source of the phase I chicks source were randomly assigned to four groups as follow , 30 chicks as a safety group, 40 chicks as efficacy group, 20 chicks as enhanced efficacy group ,and 30 chicks as non inoculated control group named group S, E1, E2, and C respectively.
Using the second preparation of I-2 inactivated vaccine the E1 group received 0.2ml subcutaneously and in the nap of chick,s neck (field dose), chicks in group E2 vaccinated simultaneously at day old with live I-2 vaccine via intraocular route and I-2 inactivated ND vaccines via the subcutaneous route, chicks in group C kept as unvaccinated control.
Out comes
Safety
Occurrence of local or systematic adverse events or tissue reaction were observed for 21 days post inoculation : Chicks inoculated with double recommended dose observed for any local or general adverse events, this test was done according to the OIE terrestrial manual (OIE, 2012).
Immunogenicity
Chicks were bled 3 times, on day-old to evaluate the maternal antibodies level, and after 21, and 45 days post vaccination in phase I trial , and at 18, 30 days post vaccination for phase II trial, to evaluate the vaccines derived antibodies. Accordingly the antibodies titers were measured on day, 1, 21, and 45 post vaccinations and on day 1, 18 and day 30 for phase I and phase II clinical trials respectively. Chicks wee inoculated with recommended dose of 0.2 ml then the seroconversion levels were evaluated using HI and ELISA tests (OIE, 2012).
Cross protection evidence
Virus antigens prepared from live La-Sota, and I-2 strains were used to test the Abs derived from I-2, and La-Sota inactivated vaccines respectively in both efficacy and safety groups.
Efficacy test (Challenge test)
Phase I
Three weeks post vaccination (21 days old) 5 chicks from group I and II (efficacy group), and 4 chicks from unvaccinated control group were selected randomly and challenged intraocularly with 106 EID50 of vv strain of (Shdi /12) according to the OIE terrestrial manual (OIE, 2012).
Morbidity and mortality rates were estimated after 10 days post challenge (Wisana et al., 2009).
Phase II
30 days post vaccination 15 chicks from group E1, and 8 chicks from group E2, and 12 chicks from group C were randomly selected and challenged intraocullarly with 107 ELD50 of vv (Shdi /12) strain (OIE,2012)
Randomisation
Sequence generation
Phase I: 80 one-day-old chicks were randomly allocated to 5 groups
Phase II: 120 one day old chicks were randomly assigned to 4 groups
Phase I Efficacy group
Group I inoculated with 0.2ml of I-2 inactivated vaccine (n=20)
Group II, inoculated with 0.2ml La-Sota inactivated vaccine( n=20)
Safety group
Group III 0.4ml I-2 inactivated vaccine (n=15) was used
Group (IV) 0.4ml La-Sota strain (n=5) was used
Control group
Group (V) unvaccinated chicks serving as control (n=5)
The sequence generation for the randomization was performed manually.
Phase II efficacy group
Group EI: 0.2ml of I-2 inactivated vaccine (n=40)
Group EII: 0.2ml of I-2 inactivated vaccine +40ul of live I-2 (n=20)
Group S: 0.4ml of I-2 inactivated vaccine (n=30)
Group C: neither vaccinated nor inoculated (n=30)
Allocation concealment and implementation
The intervention and control groups were assigned by research assistant who completely oblivious to the previous sequence generation to avoid the selection bias.
Blinding
This study is double blinded randomized control trial (RCT), the participant were unaware about either they receive the product or the placebo because of their animal nature.
Outcome evaluator and follow-up were masked about the group allocation and intervention.
Objectives
Phase I
This study designed to demonstrate the levels of safety and immunogenicity of the two prepared inactivated ND vaccines administered S/C to 1-day-old commercial broiler chicks at the nap of the neck as well as the survival rate after the challenge with vv ND strain.
Phase II
This trial conducted to confirm the result obtained from phase I clinical trial about safety and efficacy issues. moreover, in this study only I-2 inactivated vaccine which needed to be further investigated was produced in a larger scale for safety and efficacy concerns.
Statistical analysis
The data collected from various groups were compared by independent sample t.test .at 5% probability level.
Results
Pr-clinical testing
Control of seed lots
The master seed lot (MSl) and the working seed lot (WSl) demonstrated freedom from microbial contamination including bacterial, fungal, and mycoplasma contamination.
They were proved to be safe, and effective.
Control tests on vaccines
Sterility test for raw and final product
After 7 days observation there was no bacterial growth observed in any of the thyoglycolate broth vials inoculated with the inactivated allantoic fluid or the oil vaccines.
The physical characteristics of the emulsions
Stability and viscosity tests
The vaccine preparation were confirmed to be stable for 30 days at 37â° C and for 6 months at 4co The viscosity was 4ml/ 8 second.
Testing the water in oil (w/o) emulsion
the drops remained discrete on the surface of tape water without any dispersion (figure 6)
Figure 6: I-2 strains (ND) inactivated vaccines tested for complete water in oil emulsion, the vaccine drops remain discrete without any dispersion on the surface of tap water.
Immunogenicity Test
Phase I
The mean haemoagglutination inhibition ( HI) Abs titers for group EI after 18 and 30 days post vaccination were 35 HIU, and 28 HIU respectively, while the mean HI Abs titers for group EII after 18 and 30 days were 28 and 12 HIU respectively.The mean HI Abs titers for group C after 18 and 30 days were 29, and 6 HIU respectively.
Phase II
Elisa mean Abs levels after 18 days post vaccination were 5944, 5234, and 7139 for group EI, EII, and C respectively, while the maternal immunity level was 20400.
The mean Abs levels for ELISA after 30 days were 682, 329, and 695 for group EI, EII, and C respectively.
Efficacy test (challenge test)
Phase I
After 4 days post challenge 1 out of 5 chicks in group I (I-2 inactivated vaccine), and 2 chicks out of 5 in group II (La-Sota inactivated vaccine) developed ND clinical signs of depression, ruffled feather, prostration and sleepiness, but no chicks died.
In the control group 1 out 4 chicks was found dead.
On the seventh day post challenge, two out of the remaining 3 chicks of the control group were found dead with excessive salivation. In group I and group II of I-2, and La-Sota chicks demonstrate 80% protection for each group with 1 chick out of 5 died from each group after the first week of infection, later on the 10th day post challenge all control group of unvaccinated chicks died. At the end of observation period I-2 inactivated vaccine of group I demonstrate 80% protection.The group of inactivated La-Sota vaccine demonstrated only 40% protection after 10 days post challenge (Table 8).
High level of cross protection was observed. Abs derived from chicks immunized with I-2 inactivated vaccine neutralized La-Sota virus, and vice versa. Table 2
Phase II
Efficacy for group E1,E2, and C estimated after 21 post challenge, for group E1, within the first 3 days, 5 chicks out of 15 developed clinical signs, later on only one chicks died by the 7 th day post challenge while the remaining sick chicks recovered and withstand the test. For group E2 all chicks survived the challenge test only 2 chicks showed mild clinical signs and then completely recovered . For the non vaccinated control 9 chicks out of 12 died within 7 days post challenge. Table 5
Statistical analysis
Phase I
Part I
After 21days post vaccination Chicks vaccinated with 0.2ml of the I-2 inactivated vaccine (N=13), associated with Abs levels M=2217 (SD= (1657.9) , By Comparison, the non vaccinated control group (N=7) group C associated with numerically lower Abs levels, M=841.9 (SD= 1311).
Chicks vaccinated with inactivated La-Sota inactivated vaccine (N=15) associated with Abs levels M=2346.6 (SD=1931.5), By Comparison, the same non vaccinated control group (N=15) group C was also associated with numerically lower Abs levels M=841.9 (SD= 1311).
Part II
After 45 days post vaccination Chicks vaccinated with 0.2ml of the I-2 inactivated vaccine (N=6), associated with Abs levels M=3342 (SD= (2564.6) , By Comparison, the non vaccinated control group (N=3) group C associated with numerically lower Abs levels, M=0.67 (SD= 1.155).
Chicks vaccinated with inactivated La-Sota inactivated vaccine (N=8) associated with Abs levels M=4280.6 (SD=3273.3), By Comparison, the same non vaccinated control group (N=3) group C was also associated with numerically lower Abs levels M=.67 (SD= 1.155).
To test the hypothesis that the non- vaccinated, and vaccinated groups were associated with statistically significantly different means Abs levels, an independent samples t- test was performed. Additionally, the assumption of homogeneity of variances was tested, and satisfied via levene’s F-test.
In part I group I-2 against group C F(18) = 0.466, P =0.075, for group La-Sota against group C F(20) = 1.320, P=0.077
In part II group I-2 F (7) = 3.337, P=0.072, for group La-Sota F (9) =13.781, P=.056
In part I group I-2 and group La-Sota when compared together F (26) = 0.392, P =0.848.
In part II group I-2 and group La-Sota when compared together F (12) = 1.738, P =0.533.
Phase II
Part 1
After 18 days post vaccination Chicks vaccinated with recommended dose of the candidate vaccine (N=18) group EI, associated with Abs levels M=5943.5 (SD= (1835.4) , By Comparison, the non-vaccinated control group (N=15) group C was associated with numerically larger Abs levels, M=7139.4 (SD= 3134).
Chicks vaccinated with live vaccine alongside with recommended dose of the candidate vaccine (N=15) group EII, associated with Abs levels M=5233.5 (SD= 2057.9), By Comparison, the same non vaccinated control group (N=15) group C was also associated with numerically larger Abs
levels, M=7139.4 (SD= 3134)
To test the hypothesis that the non- vaccinated, and vaccinated groups were associated with statistically significantly different means Abs levels, an independent samples t- test was performed. Additionally, the assumption of homogeneity of variances was tested, and satisfied via levene’s F-test.
In part I group EI F (31) = 3.95, P =.056, for group E II F (28) = 2.5, P=1.25
In part II group EI F (24) = .124, P=.728, for group EII F (17) =4.9, P=.041
In part I Group EI and EII when compared together F (31) = .070, P =.794, in part II group EI and E II F (21) =2.39, P=.137
Part 2
After 30 days post vaccination immune responses of group EI (15) was associated with Abs levels M= 951.6 (SD= 681.8 , By Comparison, the non vaccinated control (N=11) group C was associated with numerically smaller Abs level M=641.9 (SD=695.2)
After 30 days post vaccination Chicks vaccinated concurrently with live vaccine alongside with recommended dose of the candidate vaccine (N=8) group EII, associated with Abs levels M=360.2 (SD= 328.6), By Comparison, the same previous non vaccinated control group (N=11) group C was also associated with numerically larger Abs levels, M=641.9 (SD=695.2)
After 18 days post vaccination immune responses of group EI (18) associated with Abs levels M=5943.5 (SD= (1835.4),By comparison group EII (N=15) was numerically associated with smaller Abs levels M=5233.5 (SD= 2057.9)
Twelve days latter group EI (15) associated with Abs level M=951.6 (SD=681.8), By comparison group EII (N=8) associated with numerically smaller Abs level M=360.2 (SD= 328.6)
To test the hypothesis that the non- vaccinated, and vaccinated groups were associated with statistically significantly different means Abs levels, an independent samples t- test was performed. Additionally, the assumption of homogeneity of variances was tested, and satisfied via levene’s F-test.
In part I group EI F (31) = 3.95, P =.056, for group E II F (28) = 2.5, P=1.25
In part II group EI F (24) = .124, P=.728, for group EII F (17) =4.9, P=.041
In part I Group EI and EII when compared together F (31) = .070, P =.794, in part II group EI and E II F (21) =2.39, P=.137
In part I group E I, and group C the independent sample t-test was associated statistically with insignificant effect, t (18) =1.89 P= .075, also in group EII, and C the independent sample t-test was associated statistically with insignificant effect (28) =1.969 P=.059
In part II, group EI and group C the independent sample t-test was associated statistically with insignificant effect t (24) = 1.135 P=.268, also in group EII, and C the independent sample t-test was associated statistically with insignificant effect t(17) =1.175P =0.258 equal variance not assumed with groupie
For part I Group EI and E II were not associated statistically with significant effect after 18 days post vaccination t (30) = 1.047, P =.303, but for part II, 30 days post vaccination t (21)= 2.297, P=.032 associated with statistically significant effect
Thus the group vaccinated with the inactivated vaccine only, and those vaccinated concurrently with live, and inactivated vaccines were associated with statistically insignificantly larger mean Abs level than non-vaccinated control group. (maternal immunity) except for group EI and EII in part II
A Graphical representation of the means and their coefficient variation were displayed in bar charts diagram
Discussion
Newcastle disease is most dangerous disease that continuously pose threat to chickens and poultry industry and thus due to it is high contagious and rapid spreading among chickens and other poultry species. (Rahman MM et al 2002)
To combat this deadly chickens disease an oil emulsion vaccines derived from thermostable ND strain and Lasota strain have been tried in this study.
For preparing a plausible oil emulsion, firstly , the syringe technique had been utilized, and for better performance stability and the duration of immunological activity of the prepared emulsion were greatly improved by breaking up the water phase into very small droplets i.e. less than 1µm in diameter. To avoid the unnecessary risks of using virulent ND strain these vaccines were prepared using lentogenic ND strains. The inactivated ND vaccine was developed according to the standard vaccine developmental procedure which mandate the vaccine clinical evaluation should undergo phases for safety and efficacy issues. In this study throughout phase I and phase II clinical trials the prepared vaccines were safe and immunogenic, the efficacy of candidate vaccines derived from La-Sota and I-2 strains was consistent when measured by HI, ELISA, and efficacy test, but I-2 derived vaccine gives better immune response which demonstrated by higher Abs levels and better protection. While I-2 strain showed longer lasting immune response the vaccine derived from La-sota gave slightly better homogenous immune response.
The results obtained from these trials confirm what the Italian and foreign workers have already found, i.e. that inactivated vaccine in oil emullion evokes very high antibody levels and durable resistance to massive challenge (Clara. 1965,) ( Zanella and Gervasi 1966) (Al Zubeedy 2009)
High level of cross protection was observed between the two vaccine strains which indicated vaccination with either strain could be protective against the other. This finding alludes to the fact that ND vaccine derived from any strain can produce consistently protective immune response against any other ND strain.
In phase II clinical trials there was a dramatic decrease in the maternal Abs of the control groups in the first 18 days, but still higher than in it is counterpart -the vaccinated group- within the same period of time. Over time more reduction of the Abs level was occurred in the unvaccinated control groups which might be due to the high metabolic rates of the broiler chicks but chicks still have protective levels ,while active and rising immune response were noticed in the vaccinated group according to the serological results obtained.
Although the GMTs derived from the La-Sota vaccine was arithmetically higher than that derived from the ND I-2 vaccine the difference remains statistically insignificant p>.05. Nevertheless, the I-2 vaccine performed better in the challenge test 100% protection, with higher survival rate; this might be due to higher virus yield of I-2 strain than that of La-Sota strain and better celluar immune response might be developed in case of I-2 rather than of La-Sota strain. This result inconsistent with Ezeifeka et al (2008) who tested many adjuvant with ND vaccines with 100% protective HI titer among 15 weeks of their study.
For all groups, the ELISA C.Vs were increased unproportionally against the level of immune response, with obvious decreasing among the vaccinated group which indicate the effect of the candidate vaccine upon producing more consolidated and homogeneous immune response. Generally the Abs driving immunity among vaccinated groups increased over time while the passive immunity of the non-vaccinated controls decreases over time.
After 45 days post vaccination,only the chicks received the candidate vaccine remain with protective Abs level while the maternal Abs of the non-vaccinated group have already vanished.
In phase II clinical trial only I-2 derived vaccine was further investigated.
The ELISA results were consistent with HI results; chicks vaccinated only with the candidate vaccine evoke Abs immune response higher than the chicks vaccinated with candidate and live vaccine, but achieved better protection than chicks vaccinated only with candidate vaccine. The decrease in the Abs levels might be due to the neutralizing effect of the live vaccine, but at the same time it might provoke better cellular and mucusal immunity which enhances the effect of the produced humoral immunity. Although, there is a decrease in the Abs level for the combination of live and in activated vaccines, the chicks perform better with higher weight gain and no deaths. This better immune response might be due to the live vaccine effect which replicates quickly in the mucosal membrane of the conjunctiva (harderian gland) and nostrils and stimulate cellular and humeral immune responses.
The function of adjuvant was elaborated more by Al-Zubeedy., (2006) ,Chedid et al., (1980) and Chansiripornchai and Sasipreeyajan (2006) who described the effects of the adjuvant is to stimulate the macrophages, which increases the antigen presentation capacity. In addition, another possible reason is that, unadjuvanted vaccines easy exposed to mop-up by neutralizing antibodies, whereas the oil adjuvant protected the unreleased antigens from the effect of antibodies (Roy et al., 1999).
Although the maternal Abs of the non-vaccinated control group keep vanishing over time, it remains higher than the group vaccinated with both live and inactivated vaccines at day 18 and day 30.However, only 33.3% of the chickens of the control group withstood the challenge, compared to 100% (no deaths) in chickens received live and inactivated combined vaccines.this finding is inconsistent with Wisanu Wanasawaeng et al( 2009) and Folitse et al.(1998)who explained the reasons for why vaccination of an inactivated NDV vaccine (s/c route) combined with live NDV vaccine (intranasal route) provided the higher HI antibody titer and this was because NDV from live vaccine can replicate rapidly on mucous membrane of ocular and nasal organs of chickens who had higher HI Abs titer than using only live vaccine, this result also favoured Wisanu Wanasawaeng1 et al 2009 who described the higher HI titer when combining live and inactivated ND vaccines, our finding in this study are partially goes with this finding regarding the better protection when combined live and killed vaccine and partially inconsistence regarding the higher HI titers ,this might be due to the differences in chickens, age used, while they use chicken with more than 35days old we used only on day old chicks i.e we already including the effect of the maternal immunity while they not.compared a single live vaccine and combined live and inactivated vaccines, we found that chickens received a live and inactivated vaccine produced HI antibody titers higher than chickens received a singly live vaccine.This considerable decrease in maternal immunity of control group might be due to rapid metabolic rate of the broiler chicks. While in case of treatment group, active immunization of both arms of the immune response were stimulated. On the other hand chicks received only inactivated vaccine stimulated higher Abs immune response compared to control group along the 30 days of observations. This is due to the active effects of the candidate vaccine, and allude to fact that the candidate vaccine did not interfere with the passive immunity “work progressively while passive immunity waned” and stimulate active immunity to the levels that protect chickens from the challenge with vv NDV. This result is in favor of Al Zubeedy ( 2009), who considered the humoral immunity as a key component in the
protection against ND, but this study demonstrated further the goodness of the collaboration between humeral and cellular immunity when enhanced concurrently. This result is more consistent with (Marangons & Busani., 2006) who tested inactivated vaccine in the presence of passive
immunity and found that, ND killed vaccines were more capable of eliciting an immune response in the face of existing antibody in spite of generally slower onset of immunity.
The non antagonistic effect of inactivated vaccines over the maternal immunity was illustrated by the insignificant differences between the ELISA means titers for the active and passive immunity throughout 30 days.
This indicated that the passive immunity always waned over time while the active immunity increased. The fact that chicks with vaccination keeps developing protective immune response with increasing Abs levels despite the the presence of maternal Abs and along the broiler life span is now more than ovious (PË? .05). Moreover, the results of this study supported the concept that both humeral and cellular immunity are the key component in the protection against ND. Therefore, vaccination programs should be directed toward eliciting and stimulating the both type of the immune response antibody in the birds’ flocks.
From this study also we have strong evidence that, the locally prepared inactivated vaccine is safe either with the recommended or the double recommended dose, and stimulated the same level of humoral immune response and.thus, there is no need to over dose the chicks for better immune response.
The candidate vaccine complied with the main quality control tests of sterility, viscosity, stability, safety and efficacy and has the potential to replace the imported inactivate ND vaccines.
References
- Alexander J, Bell J G , Alders R G (2004) A technology review: Newcastle Disease â??with special emphasis on it's effects on village chickens ch 1-3.
- Alders R G, Spradbrow P B, Young M P (2005) Village chickens, poverty alleviation and the sustainable control of Newcastle disease. Proceedings of an international conference held in Dar es Salaam Tanzania 5â??7 .
- Al-Zubeedy (2009) Immune response in day old broiler chicks vaccinated against Newcastle disease virus Iraqi Journal of Veterinary Sciences 23 143-146.
- Proceedings of the 5th Scientific Conference, College of Veterinary Medicine, University of Mosul.
- Bensink Z
- Clara I (1965) La vaccinazione contro la pseudopeste aviare con particolare riferimento aH'impiego di.
- Spradbrow P
- Chansiripornchai N and Sasipreeyajan J (2006) Efficacy of live B1 or ulster 2C Newcastle disease vaccines simultarneously vaccinated with inactivated oil adjuvant vaccine for protection of Newcastle disease. virus in broiler chickens. Acta Veterinaria Scandinavica. 48(2):1-4.
- Eisa M (1979) The isolation and partial characterization of a Newcastle disease virus. Sudan J. Vet. Sci.Anim. Husb. 20(1) 1-10.
- Ezeifeka G O, Nzewi K P , Amadi E S (2008) Effect of Oil Adjuvanted Newcastle Disease Vaccine on Immune Response in Chickens ,Nigerian Journal of Microbiology, Vol. 22(1):1754 â?? 1758.
- Vet Microbiol.
- Chedid L, Meischer P A, Muller-Eberhard H J (1980) Immunostimulation. Springer Berlin Heidelberg, New York. Pp. 20-32.
- Khalafalla A I , M A Fadol, O A Hameid, Y A Hussein, Mahasin El Nur (1992) Pathogenic properties of Newcastle disease virus isolates in the Sudan. Acta Veterinaria Hungaria 40:4 329-333.
- Khalafalla A.I. , S. Awad, (2001) Proceedings of the 10th Conference of the Association of Institutions for Tropical Veterinary Medicine,Copenhagen, Denmark. Â
- Marangons, Busani L (2006) The use of vaccination in poultry production Rev. Sc. Tech. off. Int. Epiz. 26(1): 265-274.
- Nichole L , Hines, Cathyl, Miller (2012) Avian paramyxovirus serotype -1: A Review of Disease Distribution, clinical symptoms and laboratory Diagnostics. Veterinary Medicine International, 2012 17.
- Osman, H. (2006). Immunological studies on live Newcastle disease vaccines used in Sudan with Emphasis on quality control aspects. M.Sc. Thesis, Sudan Academy of Science. Khartoum, Sudan.
- Office Intenationa de Epizootic (2012). Chapter 2.3.14. Newcastle disease in: Manual of Diagnostic tests and vaccines for Terrestrial Animals.
- Palya (1991). Manual for the production of marek's disease, Gumboro disease and in activated Newcastle disease vaccines FAO animal production and health paper Rome, 1991 part B, preparation and control of inactivated Newcastle disease vaccine p. 29-43.
- Roy, P., Venugopalan, A.T and Koteeswaran, A (1999). Efficacy of live adjuvanted Mesogenic Newcastle Disease Vaccine in Chickens. Vaccine 17:2674-2676.
- Rahman MM, Bar ASM, Giasuddin M, Islam MR, Alam J, Sil GC, Rahman MM( 2002) Evaluation of maternal and humeral immunity against Newcastle disease virus in chicken. Interna J Poul Sci;1(5):161163
- Vincent turblin,( 2009) the benefit of Newcastle killed vaccines in broilers, Ceva Animal Health Asia Pacific, Issue No.24
- Wegdan, H., Khair, A.M., Bontsi, M., Celia, A. (2010). Newcastle disease outbreaks in the Sudan from 2003 to 2006 were caused by viruses of genotype 5d, virus gene, 40:106-110.
- Wisanu Wanasawaeng1 Achara Tawatsin Jiroj Sasipreeyajan Prachak Poomvises2 Niwat
- Chansiripornchai2 (2009): Development of Inactivated Newcastle Disease Vaccine using Palm Oil as an Adjuvant: Thai J. Vet. Med.,. 39(1): 9-16.
- Young, M. Alders, R., Grimes, S., Spradrow, P., Dias, P., dasilva, A. and Lobo, Q. (2002). Controlling Newcastle disease in village chickens: a laboratory manual ACIAR Mono graph No 87142pp.
- Zanella A. and Gervasi E. (1967). Ricerche sull'impiego dei vaccini inattivati in veicolo oleoso.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences