Detailed Musculoskeletal Study of a Fetus with Trisomy-18 (Edwards Syndrome) with Langer’s Axillary Arch, and Comparison with Other Cases of Human Congenital Malformations
Malak A. Alghamdi1,2*, Rui Diogo1, Raque1 Izquierdo3, Francisco F. Pastor4, Feliz De La Paz4 and Janine M. Ziermann1*
1 Department of Anatomy, Howard University College of Medicine, Washington, DC, USA
2 Department of Basic Medical Sciences, King Saud bin Abdulaziz University for Health Sciences College of Medicine, Jeddah, KSA
3 Pediatrics Service, Rio Hortega University Hospital, Valladolid, Spain
4 Department of Anatomy, Valladolid University College of Medicine, Valladolid, Spain
- *Corresponding Author:
- Janine M. Ziermann
Department of Anatomy, Howard University
College of Medicine, Washington, DC, USA.
Tel: 2026608447
E-mail: jziermann@yahoo.de
Malak A. Alghamdi
Department of Basic Medical Sciences
King Saud bin Abdulaziz University for Health
Sciences College of Medicine, Jeddah, KSA.
E-mail: malak.alghamdi@bison.howard.edu
Received date: January 25, 2018; Accepted date: February 07, 2018; Published date: February 14, 2018
Citation: Alghamdi MA, Diogo R, Izquierdo R, Pastor FF, Paz FDL, et al. (2018) Detailed Musculoskeletal Study of a Fetus with Trisomy-18 (Edwards Syndrome) with Langer’s Axillary Arch, and Comparison with Other Cases of Human Congenital Malformations. J Anat Sci Res. Vol.1 No.1:1
Abstract
Background: Trisomy-18 is the second most prevalent autosomal aneuploidy after trisomy-21. Trisomy-18 individuals present with major multisystem alternations including craniofacial and musculoskeletal anomalies. The study of trisomic cases provides us with magnified clues to the complex history of individual muscles during development and also allows us to record the development of variable human phenotypes more accurately.
Methods and Findings: In this study, we describe in detail the musculoskeletal system of a premature female with trisomy-18 and compare it with previous studies of trisomies-13, -18 and -21 as well as karyotypically normal individuals. This study is a part of a long-term project aimed at contributing to the renaissance of comparative anatomy in general and comparative myology in particular and to a better understanding of both “normal” and abnormal development and its links to evolution, and birth defects. Five head features suggested developmental delay, supporting the idea that delayed development of the skeletal muscular system may be a crucial diagnostic feature for all human aneuploidy syndromes. More anomalies were found in the muscles of the upper and lower limbs than in the muscles of the head. These included the fusion of some muscles and the presence of supernumerary muscles such as the so-called "Langer’s axillary arch". In general, there is uniformity between the anomalies observed in the dissected fetus and in human fetuses with other trisomies. This supports Alberch’s ill-named “logic of monsters” theory because one finds the same malformations occurring in different syndromes, and moreover often mirroring phenotypes often seen in the normal configuration of other organisms. Furthermore, there are other clear cases of patterns found in human congenital malformations.
Conclusions: As noted above, the upper limbs often have more muscular defects than the lower limbs, and many of human congenital malformations in human trisomies are related with developmental delay. The study of trisomy thus provides us with magnified clues to understand the evolution, development, and pathology of human anatomical structures, and to discuss broader subjects with major implications for both evolutionary and developmental biology and for medicine.
Keywords:
Trisomy-18; Musculoskeletal; Anomalies; Variations; Birth defects; Comparative anatomy
Introduction
Trisomy-18 (Edwards syndrome) is the second most prevalent autosomal aneuploidy after trisomy-21 (Down syndrome) in liveborn infants [1]. Several studies estimated that the incidence of trisomy-18 is between 1:3600 and 1:8500 of live births [2-4]. The median survival of those born alive is 3 days with a survival rate of less than 50% after one week and less than 10% after one year of life [4]. Trisomy-18 results from disjunction of the maternal chromosomes during second meiosis leading to an extra copy of chromosome 18, which can produce different phenotypic outcomes throughout the body [5,6]. The first reports of musculoskeletal dissections of humans with trisomy-18 were published in 1960 by Edwards et al. and Smith et al. [7,8] and were followed by numerous clinical and autopsy studies that described a characteristic constellation of anomalies associated with the presence of an extra chromosome 18 [5,9-11]. Typical trisomy-18 cases have major multisystem alternations including craniofacial and musculoskeletal anomalies such as a prominent occiput, low-set ears, short palpebral fissure, downward sloping fissures, narrow oral opening, narrow palatal arch, narrow bifrontal diameter, clenched fist with overlapping fingers, hypoplastic skeletal muscles, underdeveloped thumbs, small fingernails, short sternum, club feet, and rocker-bottom feet [6,9]. Additionally, major heart malformations and profound intellectual disability in the surviving older children were reported [2].
Only a few, relatively detailed musculoskeletal descriptions of trisomic human individuals have been published so far [5,9,10,13- 16]. Therefore, information on the anatomy of these individuals is still scarce and there is a lack of detailed comparisons of anomalies between individuals with different trisomies as well as with other syndromes and with other species. Such comparisons between congenital malformations from humans with a wide range of syndromes as well as with non-human taxa, mainly primates and other mammals, provide us with the possibility to shed light not only on both normal and abnormal developmental mechanisms and disturbances, but also on the evolution of our own species [5,17-19]. This is the basis of a new field called Evolutionary-Developmental-Pathology (Evo-Devo-Path), which links development, comparative anatomy, human evolution, morphological variations and defects, and medicine [15-20]. Evo- Devo-Path research includes a combination of experimental/ developmental studies of non-human model organisms and primate/human evolution, genetics and anatomy, mostly concerning hard-tissues (e.g., skeleton and teeth). Moreover, it pays a special attention to data obtained from chordate comparative, developmental, and evolutionary anatomy, and from the direct study of normal/abnormal human development (using, e.g., cadaveric collections of hospitals, museums, and other institutions), with a major focus on soft tissues such as muscles.
Within the context of Evo-Devo-Path, in this study, we provide detailed descriptions of the musculoskeletal system of a trisomy-18 fetus, and compare them with other trisomic cases reported by us and by others as well as with karyotypically normal human individuals. This study is also part of a long-term project aimed at contributing to the renaissance of comparative anatomy in general and comparative myology in particular [5,16,21,22]. We hope that it will contribute to a better understanding of both “normal” and abnormal development, evolution, and birth defects. In particular, the study of trisomic embryos, fetuses, and neonates provides us with magnified clues to the complex history of individual muscles during development and also allows us to record the development of variable human phenotypes more accurately.
Material and Methods
A female fetus was delivered prematurely (33 weeks and 6 days) on December 17th, 2015, at Hospital Universitario Río Hortega, Valladolid, Spain. An urgent cesarean section was performed due to decreased fetal movement in the previous 24 hours. The birth weight was 1,200 g, crown-heel length 38 cm, and head circumference 27 cm. The following characteristics were reported directly after birth: pale skin, hypotonic muscles, small low-set ears, short palpebral fissure, underdeveloped thumbs, small toenails, and rocker-bottom feet. The mother had normal, controlled pregnancy, and her age at the time of delivery was 27 years. The fetus had serious medical conditions and she was diagnosed as a low birth weight premature fetus with trisomy-18. The parents were informed about the medical condition of the fetus, and a decision was made to euthanize the fetus on December 18th, 2015. The fetus was then donated to the Valladolid University, and legal licenses were obtained by one of us (FFP) for research on the fetus. The deceased fetus was stored in the freezer and dissected in FPP’s lab at Valladolid University, College of Medicine, Spain. Micro- and macro-dissections were done and photos were taken for documentation using a Canon EOS 70D camera with Canon EFS 18-200 mm lens. The fetus was compared with the literature and our own work [5,16]. CT scans were performed for the analysis of skeletal structures.
Results
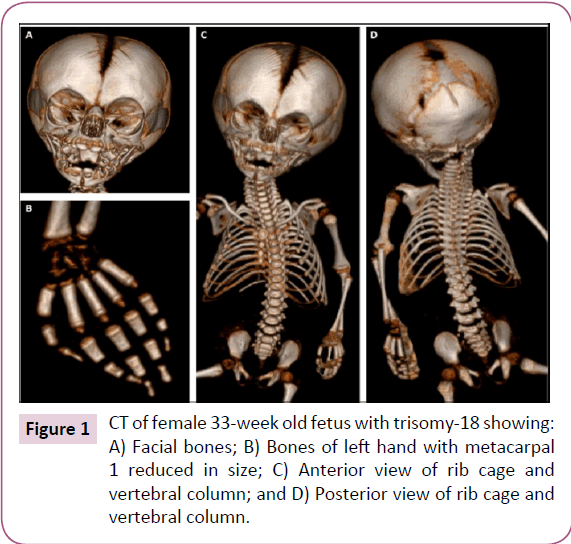
Here, we describe various musculoskeletal structures based on our dissections and based on CT scans for the analysis of skeletal structures. These scans (Figure 1) show all calcified bones, and were thus crucial to the preparation of the subsequent dissection of all muscular and skeletal structures. Except for the reduced size of the parietal bone and of the first metacarpal on both hands, the skeletal system appeared to be normal (Figure 1). The following textual anatomical descriptions of the muscles will focus on the observed anomalies/variations, and will be brief as they are complemented with the data provided in (Table 1) and in the supplementary tables (S Tables 1-3), which include information about the attachments and anatomy of each muscle. It should be noted that all nerves associated with the muscles described below were as described for the normal human fetal, neonate, and adult phenotype [18].
Face and neck
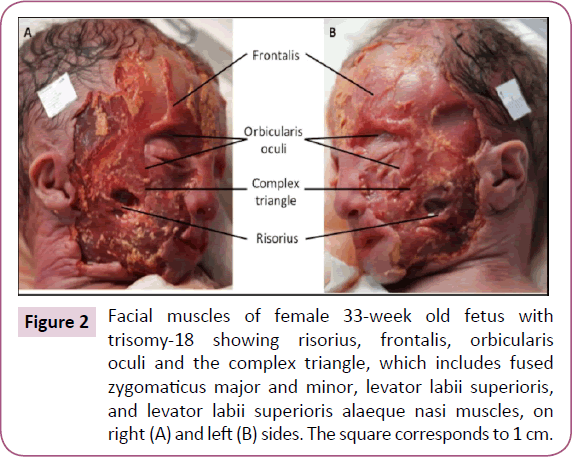
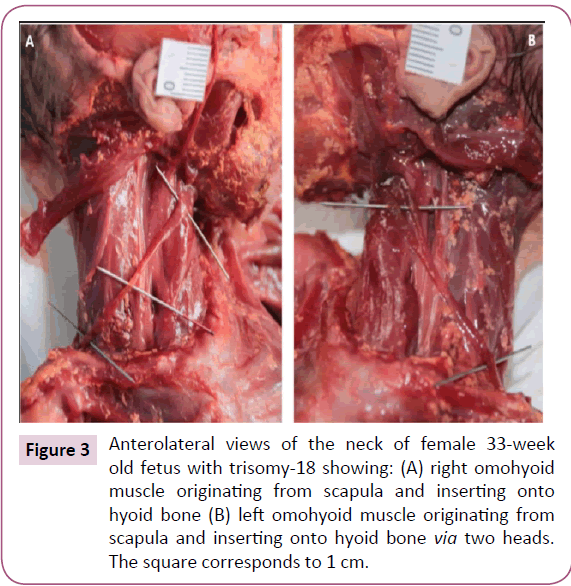
The two bellies of the frontalis were very thin and fused in the midline. The zygomaticus major and minor, levator labii superioris, levator labii superioris alaeque nasi, and levator anguli oris were fused on both sides forming one muscle sheet extending from the zygomatic bone and the inferior border of the orbicularis oculi to the upper lip (Figure 2). We designated this muscle sheet as the "complex triangle" (Figure 2). The left omohyoid had an extra head that originated anterior to its intermediate tendon and that runs lateral to the normal head to attach onto the greater horn of the hyoid bone (Figure 3). The stylohyoid was absent bilaterally. The depressor labii inferioris and mentalis muscles seemed to be fused on both sides.
Figure 2: Facial muscles of female 33-week old fetus with trisomy-18 showing risorius, frontalis, orbicularis oculi and the complex triangle, which includes fused zygomaticus major and minor, levator labii superioris, and levator labii superioris alaeque nasi muscles, on right (A) and left (B) sides. The square corresponds to 1 cm.
Figure 3: Anterolateral views of the neck of female 33-week old fetus with trisomy-18 showing: (A) right omohyoid muscle originating from scapula and inserting onto hyoid bone (B) left omohyoid muscle originating from scapula and inserting onto hyoid bone via two heads. The square corresponds to 1 cm.
Chest, back and upper limbs
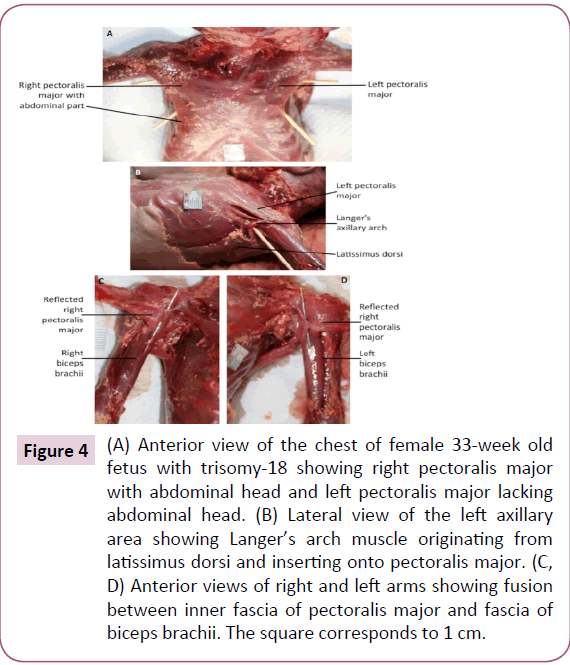
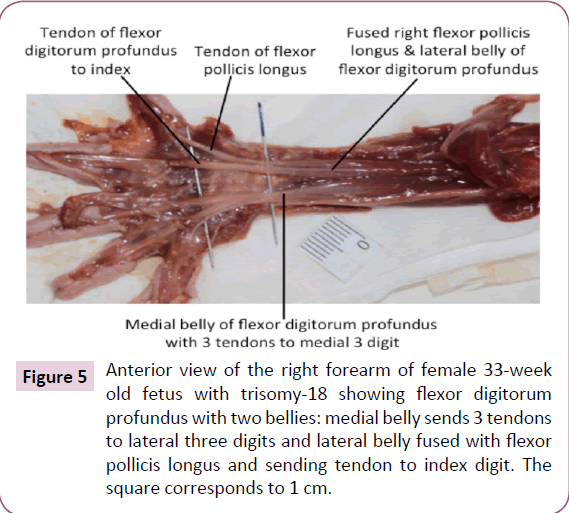
The infraspinatus and teres minor muscle were completely fused bilaterally. On the left side only, a Langer's axillary arch was observed, which is a muscle band extending from the lateral side of the latissimus dorsi to the inferior side of the pectoralis major near the insertion of the latter muscle onto the humerus (Figure 4b). The abdominal portion of pectoralis major was missing on the left side (Figure 4a). The deltoid muscle was fused with the pectoralis major on both sides, forming the deltopectoral complex. In addition, the fascia of the inner side of the pectoralis major seemed to be fused with the fascia of the short head of the biceps brachii (Figure 4c and 4d). The insertion of the right pectoralis minor extended inferiorly to the fascia of the coracobrachialis. The proximal end of the coracobrachialis was completely fused with the proximal end of the short head of the biceps brachii. The flexors of the forearm seemed to be fused proximally, near their attachment at the medial epicondyle of the humerus. The palmaris longus was absent on the right side. The proximal ends of the flexor carpi radialis and flexor digitorum superficialis seemed to be fused on both sides. The fourth tendon of the flexor digitorum superficialis was absent bilaterally. The four tendons of the flexor digitorum profundus split more proximally than normally on the left side. On the right side, the muscle had two bellies fused proximally, the lateral belly had one tendon to the index finger and the medial belly had three tendons to the medial three digits. Moreover, the lateral belly was fused proximally to the flexor pollicis longus (Figure 5). The brachioradialis was very thin on both sides. The extensor carpi radialis longus and brevis were fused proximally, bilaterally. The extensor pollicis brevis was fused proximally with the abductor pollicis longus on both sides. The four lumbricals were underdeveloped, in both hands.
Figure 4: (A) Anterior view of the chest of female 33-week old fetus with trisomy-18 showing right pectoralis major with abdominal head and left pectoralis major lacking abdominal head. (B) Lateral view of the left axillary area showing Langer’s arch muscle originating from latissimus dorsi and inserting onto pectoralis major. (C, D) Anterior views of right and left arms showing fusion between inner fascia of pectoralis major and fascia of biceps brachii. The square corresponds to 1 cm.
Figure 5: Anterior view of the right forearm of female 33-week old fetus with trisomy-18 showing flexor digitorum profundus with two bellies: medial belly sends 3 tendons to lateral three digits and lateral belly fused with flexor pollicis longus and sending tendon to index digit. The square corresponds to 1 cm.
Lower limbs
The gluteus medius seemed fused with the tensor fasciae latae bilaterally. The superior gemellus, obturator internus, and inferior gemellus appeared to be fused laterally. The extensor digitorum longus and fibularis tertius as well as the fibularis longus and brevis were fused proximally on both sides. The absence of the forth tendon of the flexor digitorum brevis to the little toe was observed bilaterally. The distal end of the abductor hallucis was fused with the medial head of the flexor hallucis brevis on both sides. A supernumerary muscle located lateral to the right extensor digitorum brevis, originated from the cuboid bone and sent a tendon to the dorsum of the little toe on the right side. The extensor hallucis brevis fused proximally with the extensor digitorum brevis bilaterally.
Discussion
Some features commonly present in trisomy-18 individuals such as the small low-set ears, short palpebral fissure, underdeveloped thumbs, small toenails, and rocker-bottom feet [6-11] were also observed in the dissected fetus. However, the trisomy-18 fetus described here seemed to have fewer muscle anomalies and skeletal deformities than other fetuses previously described [5,7,9,10,14]. The most common and distinctive abnormalities seen in the axial skeleton of trisomy-18 fetuses are the notching in the basilar part of the occipital bone and the reduced size or agenesis of the nasal bones. Partial clefting, dislocation and agenesis of vertebral bodies were observed in the axial skeleton of other trisomy-18 fetuses [23]. Abnormalities of the upper and lower extremities are not uncommon features of trisomy-18 fetuses, such as aplasia and hypoplasia of the radius and thumb that were found in 5-10% of trisomy-18 infants [24]. Aplasia of the radius, which is unique to trisomy-18 among human aneuploidy, is usually associated with the absence or hypoplasia of the first metacarpal and thumb, and is thought to be secondary to vascular disruption of the radial artery [24]. In the dissected fetus, hypoplasia of the first metacarpal was the only major observed anomaly in the skeletal system on both sides, without association with radius aplasia (Figure 1). The phenotype of trisomy-18 individuals may vary from typical trisomy-18 phenotype to anatomically normal adult. Approximately, 2% of trisomy-18 cases have partial trisomy due to balanced translocation or inversion inherited form one parent; mosaic trisomy-18 results in a highly variable presentation [6].
Although trisomies 18, 13, and 21 are associated with the existence of three different chromosomes, the reviewed data showed repeated patterns of muscle defects that occur in some individuals in each of the three conditions, which supports Alberch’s [25] ill-named “logic of monsters” (LoMo). According to this theory, phenotypic birth defects caused by completely different genetic syndromes would tend to be similar to each other and also to both natural variations and the “normal” phenotypes present in other taxa. This similarity between anomalies, variations, and the normal phenotype in different taxa would be associated with the regulation of a conserved developmental program (e.g., a set of genetic and/or epigenetic interactions) such that the structure of these internal interactions constrains the realm of the possible morphospace [25]. In principle, such a program can break down in the evolution of some clades, but within most clades this would lead to a very early death of the embryos [5,15,19,25].
Recent Evolutionary-Developmental-Pathology (Evo-Devo-Path) studies and comparisons [15,20] support the idea that internal constraints play a central role in not only normal, but also abnormal, human development, and thus Alberch’s LoMo [5,15,20]. For example, concerning the anomalies found in the present study, Smith et al. [5] described the absence of the stylohyoid muscle in 25% of trisomy-13 individuals and 41.2% of trisomy-18 individuals; variation of the omohyoid in 29.1% of trisomy-13 individuals, and 42.3% of trisomy-18 individuals; variation of the pectoralis major in 21% of trisomy-13 individuals, and 27% of trisomy- 18 individuals; fusion of the deltopectoral complex in 81% of trisomy-18 individuals; and variation of the pectoralis minor in 38.5% of trisomy-18 individuals. They also reported absence of the palmaris longus in 83% of trisomy-13 individuals, 88.5% of trisomy-18 individuals and 85.7% of trisomy-21 individuals; variation of the flexor pollicis longus in 8% of trisomy-13 individuals, 50% of trisomy-18 individuals and 14.3% of trisomy-21 individuals; variation of extensor carpi radialis longus and brevis in 12.5% of trisomy-13 individuals, and 38.5% of trisomy-18 individuals; variation of the flexor digitorum profundus in 21% of trisomy-13 individuals and 23% of trisomy-18 individuals; variation of the lumbricals in 21% of trisomy-13 individuals, 35% of trisomy-18 individuals and 14.3% of trisomy-21 individuals; and absence of the 4th tendon of flexor digitorum brevis to 5th digit in 25% of trisomy-13 individuals, 41% of trisomy-18 individuals and 40% of trisomy-21 individuals. In addition, some of the observed variations were reported in karyotypically normal individuals [26,27] such as the absence of the palmaris longus, which is a polymorphic feature within modern humans [13,15,28,29]. Other commonly observed malformations in trisomy-18 individuals were not observed in the trisomy-18 fetus examined by us, including the presence of the rhomboideus occipitalis in 30.8%, platysma cervicale in 76.5%, mentohyoideus in 23.5%, pectoralis minimus in 34.6%, tensor semivaginae scapulohumeralis (manubrium to humeral head/scapula) in 38.5%, and flexor carpi ulnaris accessorius (originating from middle of ulna and inserting with digiti minimi onto 5th digit) in 24.6% of trisomy-18 individuals [5]. The fact that many features found in some individuals are a) absent in other individuals with the same trisomy and/or b) present in individuals with other trisomies (as predicted by LoMo) makes it very difficult to establish a defined phenotype characteristic for each human trisomy.
It is often suggested in the literature about birth defects that the lower limbs have less defects than the upper limbs, but it is not clear if that suggestion reflects the reality of abnormal development, or instead a bias as most studies tend to focus more on the upper limbs. The compilation of anatomical network studies including data obtained exclusively in studies that described defects in both the upper limbs and lower limbs of human individuals with limb birth defects (thus avoiding such biases) suggest that the upper limbs tend effectively to have more gross anatomical defects than the lower limbs. Namely, within 316 defects compiled in studies including the head, upper and lower limbs, the proportion of upper limb defects (158, or 50%) was substantially higher than that of lower limb defects (64, or 20% ) and of head defects (94, or 30% ) [5,15]. Moreover, Wood [30,32] also reported that muscle variations are much more frequent in the upper than in the lower limbs of the normal human population (292 vs. 119 cases in his sample, i.e., 71% vs. 29%) [30-32]. In another study, a sample of 234 cases of muscle defects compiled from studies including both upper and lower limbs of humans with severe congenital malformations, the proportion was respectively 165 (34, 2, 22, 42, 37, and 28 for pectoral, dorsal and ventral arm, dorsal and ventral forearm, and hand, respectively) vs. 69 (4, 5, 2, 26, 12, and 20 for pelvic, dorsal and ventral thigh, dorsal and ventral leg, and foot muscles, respectively), so exactly also 71% vs. 29% [5,15]. Similar results were observed in the dissected fetus: a total of 13 anomalies/ variations were observed in the right upper limb, 11 in the left upper limb, 7 in the right lower limb, 6 in the left lower limb, and 5 in the head and neck, including fusion between muscles, absence of muscles/tendons, supernumerary muscles, hypotonic muscles, and variations in origin or insertion (Table 1). These data, including the information reported in the present study, thus allow us to start defining a pattern within human musculoskeletal malformations, which is crucial for further pathological and developmental studies and useful for surgical procedures.
| Absent | Stylohyoid | Bilaterally |
| Palmaris longus | Right | |
| Fourth tendon of flexor digitorum superficialis to 5th digit | Bilaterally | |
| Fourth tendon of flexor digitorum brevis to the little toe | Bilaterally | |
| Extra | Head of omohyoid | Left |
| Langer's axillary arch | Left | |
| Abdominal portion of pectoralis major | Right | |
| Supernumerary muscle lateral to extensor digitorum brevis | Right | |
| Fused | Frontalis | Bilaterally |
| Zygomaticus major and minor, levator labii superioris, levator labii superioris alaeque nasi, and levator anguli oris | Bilaterally | |
| Depressor labii inferioris and mentalis | Bilaterally | |
| Infraspinatus and teres minor | Bilaterally | |
| Fascia of inner side of pectoralis major and fascia of short head of biceps brachii | Bilaterally | |
| Proximal ends of forearm flexors | Bilaterally | |
| Proximal ends of flexor carpi radialis and flexor digitorum superficialis | Bilaterally | |
| Lateral belly of flexor digitorum profundus and flexor pollicis longus | Bilaterally | |
| Proximal ends of extensor carpi radialis longus and brevis | Bilaterally | |
| Proximal ends of extensor pollicis brevis and abductor pollicis longus | Bilaterally | |
| Gluteus medius and tensor fasciae latae | Bilaterally | |
| Lateral ends of superior gemellus, obturator internus , and inferior gemellus | Bilaterally | |
| Proximal 2/3 of fibularis longus and brevis | Bilaterally | |
| Distal end of abductor hallucis and medial head of flexor hallucis brevis | Bilaterally | |
| Muscle bellies of extensor hallucis brevis and extensor digitorum brevis | Bilaterally | |
| Variant | Insertion of pectoralis minor extends inferiorly to fascia of coracobrachialis | Right |
| Tendons of flexor digitorum profundus split more proximal than normal | Left | |
| Flexor digitorum profundus had two bellies fused proximally: lateral belly had one tendon to index finger and medial belly had three tendons to medial three digits | Right | |
| Thin brachioradialis | Bilaterally | |
| Underdeveloped hand lumbricals | Bilaterally |
Table 1: Muscle anomalies (absent, extra, fused) and variations observed in the trisomy-18 fetus
Accordingly, within these limb birth defects there was also a consistent pattern concerning muscle-skeleton attachments. Most of the defects found in the upper (65% ) and particularly lower (84% ) limbs are seen in the autopods and zeugopods, which are mainly evolutionary innovations of tetrapods; moreover, these more recent distal limb elements are positionally specified by members of the Hox gene family (i.e., Hox 9 to 13) that are seemingly unique to vertebrates [15]. This makes sense in an internalist view of evolution because these phylogenetically more recent innovations are the last limb regions to form in development and, therefore, more prone to developmental changes, variations, and defects. It also makes sense in a more externalist view of evolution, as the more distal limb regions are more prone to evolutionary adaptive changes due to their close contact with preys/substrate/objects. This link between the order in which morphological structures appear in evolution (phylogeny) and development (ontogeny), interactions with the environment (ecomorphology), and thus evolvability (adaptation) also supports the LoMo in the sense that it predicts a parallel between the normal and variant phenotypes of normal populations and defects in abnormal individuals [5,15]. Another significant point supporting the LoMo is that, within the total 1,540 human muscle defects that have been compiled, 1,044 (68% ) are found on the left (522) and right (522) sides of a same individual, while only 496 (32% ) are found on a single side [5,15].
The most interesting muscle structure observed in the described trisomy-18 fetus was the Langer’s axillary arch, which was not reported previously in trisomy-18 cases. Langer's axillary arch was observed on the left side only, and extended from the lateral side of the latissimus dorsi to the inferior side of the pectoralis major. According to some authors, this muscle structure was described first in 1783 by Bugnone as a muscular slip originating from the latissimus dorsi and inserting onto the humerus together with the pectoralis major [33,34]. Other authors reported that the first description was given by Alexander Ramsay in 1795 as being oblong and stretching from the pectoralis major to the latissimus dorsi and teres major [26,27,33,35-38]. In 1846 Langer noted its existence, and this muscle structure became of greater interest after that study [34,35]. Later, Testut (1884) observed what he referred to as the axillary arch of Langer [37]. More recently, Langer’s arch has also been described by other authors [23,33-42].
Langer’s arch was described as a rare muscular variation in the axillary region [37,38,40,42,43]. However, Daniels and Rovere [40] described it as the most common muscular variation in the axilla. A frequency of 7% of this variant muscular structure was mentioned in many anatomical texts. Nevertheless, some authors argue that its frequency varies between 0.25% and 37.5% [33,35-39,41,42]. According to Besana-Ciani and Greenall [36], the appearance of Langer’s arch is usually bilateral, but can occur unilaterally and is more common among Chinese than Caucasians and among females than males. However, in the fetus examined in the current study and in many cases within the reviewed literature [34,35,37-43] there is in fact a unilateral appearance of the muscle.
The origin, insertion, course, tissue composition, and dimensions of Langer's arch are variable. It commonly arises from the latissimus dorsi muscle, either directly as seen in our case or indirectly with an intermediate tendon, or less commonly from the serratus anterior muscle. Langer's arch most often inserts as a single muscular band onto the pectoralis major and less often onto the pectoralis minor, coracobrachialis, short head of the biceps brachii, teres major, coracoid process, axillary fascia, or the fascia of the arm [33,35,41,42]. In some rare cases it also extends between the coracoid process and the long head of triceps brachii [42]. Jelev et al. [33] summarized the three main characteristics of a typical Langer's arch in their literature review: (1) it has a constant origin from the latissimus dorsi muscle; (2) it inserts into structures around the anterosuperior part of the humerus; and (3) it crosses the axillar neurovascular bundle from dorsomedial to ventrolateral [33,34]. The Langer's arch encountered in the present study thus correspond to what seems to be its typical form.
Various nerves in the axillary region have been reported to innervate Langer's arch. The lateral pectoral nerve, the medial pectoral nerve, the intercostobrachial nerve, and the thoracodorsal nerve were reported to supply this structure in different studies [33,40-42]. Some authors postulated that innervation could vary depending on the site of insertion [35]. In our study the nerve supply of Langer's arch could not be determined.
The evolutionary and developmental derivation of Langer’s arch remains unknown, but the most reliable theory supports its origin from the “panniculus carnosus”, which is an embryologic remnant of a more extensive sheet of skin-associated musculature present in most mammals and primates, lying at the junction between the superficial fascia and the subcutaneous fat [17,36,38,40,41]. Some authors have however suggested an evolutionary and embryological origin from the pectoral muscle mass because they argue that the innervation of Langer's arch is frequently by the medial pectoral nerve [40,42]. Again other authors suggested that Langer's arch is a variation of the latissimus dorsi, due to its topological position [35].
The fusion of the zygomaticus major and minor and levator labii superioris was reported in 2 cases of trisomy-18 by Aziz [44,45]. The seemingly fusion of the head muscles (two heads of frontalis; zygomaticus major and minor, levator labii superioris, levator labii superioris alaeque nasi, and levator anguli oris; and depressor labii inferioris and mentalis) might be due to developmental delay [5,21,44], as all these facial muscles are 2nd branchial arch muscles that are developed from the same primordium. That is, they normally become differentiated in earlier developmental stages (in the embryo) [5,21,46], but a delay in development could have kept them undifferentiated until the later developmental stage of the fetus. The failure of deltoid and pectoralis major to separate from each other was reported only in trisomy-18 individuals, and is strongly suggested to be also due to delayed development [14,44,45], because the deltoid, teres minor, supraspinatus and infraspinatus arise from a common mass that is continuous with the pectoral mass [47].
The absence of the palmaris longus, the forth tendon of flexor digitorum superficialis, and the forth tendon of flexor digitorum brevis, which are common variations in karyotypically individuals and are more common features in trisomic fetuses, can be explained by the observation of Ramirez-Castro and Bersu [13]. According to these authors, it was observed that the primordia of such muscles tend to be small or difficult to distinguish as they develop from their respective anlagen, which is usually given as the reason for their frequent absence. Because of a probably decreased mitotic rate in human trisomic embryos, as evidenced from in vitro studies, the primordia of these muscles would be even smaller. This, in turn, could increase the frequency of their absence [13]. In addition, the missing 4th tendons of the flexor digitorum superficialis and flexor digitorum brevis in the hands and feet of the dissected fetus and in other reports of trisomic humans suggest developmental integration between digital flexors in the upper and lower limbs, lending support to the theory that hands and feet share similar developmental mechanisms [5], likely due to derived integration that occurred within the transitions from fins to limbs [48-51].
In summary, the present study of a trisomy-18 fetus and the literature review, undertaken here to put it within a broader context, thus allow us to start defining some patterns within human congenital malformations, and particular within human trisomies. In particular, there is a general uniformity between the abnormal features observed in the dissected fetus and the previously described cases of humans with not only trisomy-18 but also with trisomies 13 and 21. This support Alberch’s LoMo theory, which stated that there is a logic or inherent “order” even in severe birth defects and alterations of developmental processes due to developmental constraints [5,15]. By compiling the data presented in recent studies with the results obtained from our previous works, we found that there seems to be a very thin line between normal variations (which broadly corresponds to normal developmental plasticity, or the “norm of reaction” of Evo-Devo) and malformations (which can be due to either genetic causes, environmental, and/or other causes leading to developmental defects). Moreover, apart from the fact that these abnormal features are also seen in other human syndromes as well as normal variants or the normal phenotype of other species as predicted by LoMo, there are other clear cases of patterns found in human congenital malformations. For instance, the upper limbs often have more muscular defects than the lower limbs, and many of human congenital malformations in human trisomies are related with developmental delay. Studies such as the one presented here are thus crucial to inform future developmental and pathological studies and thus to potentially lead to a more comprehensive view of the evolution, development and pathology of human anatomical structures.
Acknowledgments
Special thanks go to the family who donated the fetus for scientific investigation. Furthermore, we are grateful to Anatomy Department of the College of Medicine at the University of Valladolid, Spain, for facilitating the donation and the dissection of the fetus. We acknowledge Howard University College of Medicine for the start-up funds assigned to Rui Diogo, as well as the King Saud bin Abdulaziz University for Health Sciences and Saudi Arabian Cultural Mission for Malak A. Alghamdi's PhD grant.
Funding
There was no funding supporting this specific study. RD and MAA were supported by their respective home institutions as acknowledged.
References
- Hui L, Slonim DK, Wick HC (2012) Novel neurodevelopmental information revealed in amniotic fluid supernatant transcripts from fetuses with trisomies 18 and 21. Hum Genet 131: 1751-1759.
- Root S, Carey JC (1994) Survival in Trisomy 18. Am J Med Genet 49: 170-174.
- Embleton ND, Wyllie JP, Wright MJ, Burn J, Hunter S (1996) Natural history of trisomy 18. Arch Dis Child Fetal Neonatal Ed 75: 38–41.
- Rasmussen SA, Wong LY, Yang Q, May KM, Friedman JM (2003) Population based analyses of mortality in trisomy 13 and trisomy 18. Pediatrics 111: 777-84.
- Smith CM., Diogo R, Ziermann JM, Molnar J, Gondre-Lewis MC, et al. (2015) Muscular and skeletal anomalies in human trisomy in an Evo-Devo context: Description of a T18 cyclopic fetus and comparison between Edwards (T18), Patau (T13) and Down (T21) syndromes using 3-D imaging and anatomical illustrations. CRC Press.
- Roberts W, Zurada A, Gielecki J, Loukas M (2016) Anatomy of trisomy 18. Clin Anat 29: 628-632.
- Edwards JH, Harnden DG, Cameron AH, Mary Crosse V, Wolf OH (1960) A new trisomic syndrome. The Lancet 275: 787-90.
- Smith DW, Patau K, Therman E, Inhorn SL (1960) A new autosomal trisomy syndrome: Multiple congenital anomalies caused by an extra chromosome. J Pediatr 57: 338-345.
- Bersu ET, Ramirez-Castro JL (1977) Anatomical analysis of the developmental effects of aneuploidy in man- the 18-Trisomy syndrome: I. anomalies of the head and neck. Am J Med Genet Part A 1: 173-193.
- Aziz MA (1979) Muscular and other abnormalities in a case of Edwards’ syndrome (18-Trisomy). Teratology 20: 303-312.
- Cereda A, Carey JC (2012) The Trisomy 18 Syndrome. Orphanet J Rare Dis 7: 81.
- Matsuoka R, Misugi K, Goto A, Gilbert EF, Ando M (1983) Congenital heart anomalies in the trisomy 18 syndrome with reference to congenital polyvalvular disease. Am J Med Genet 14: 657-668.
- Ramirez-Castro JL, Bersu ET (1978) Anatomical analysis of the developmental effects of aneuploidy in Man- The 18-trisomy syndrome: II. Anomalies of the upper and lower limbs. Am J Med Genet Part A 2: 285-306.
- Dunlap SS, Aziz MA, Rosenbaum KN (1986) Comparative anatomical analysis of human trisomies 13, 18, and 21: I. The forelimb. Teratology 33: 159-186.
- Diogo R, Smith CM, Ziermann JM (2015) Evolutionary developmental pathology and anthropology: A new field linking development, comparative anatomy, human evolution, morphological variations and defects, and medicine. Dev Dyn 244: 1357-1374.
- Alghamdi MA, Ziermann JM, Gregg L, Diogo R (2017) A detailed musculoskeletal study of a fetus with anencephaly and spina bifida (craniorachischisis) and comparison with other cases of human congenital malformations. J Anat 230: 842-858.
- Diogo R, Wood B (2012) Comparative anatomy and phylogeny of primate muscles and human evolution. Oxford, UK, Taylor & Francis.
- Diogo R, Noden D, Smith CM, Molnar, J.A., Boughner, J, et al. (2016) Learning and understanding human anatomy and pathology: An evolutionary and developmental guide for medical students. Oxford, UK, Taylor & Francis.
- Diogo R. (2017) Evolution driven by organismal behavior: A unifying view of life, function, form, mismatches and trends. New York, US, Springer.
- Diogo R, Guinard G, Diaz R (2017) Dinosaurs, chameleons, humans and Evo-Devo path: Linking Étienne Geoffroy's teratology, Waddington's homeorhesis, Alberch's logic of 'monsters', and Goldschmidt hopeful 'monsters'. J Exp Zool B (Molecular Developmental Evolution) 328: 207-229.
- Diogo R, Abdala V (2010) Muscles of vertebrates: Comparative anatomy, evolution, homologies and development. Boca Raton: Enfield, N.H: CRC Press.
- Diogo R, Walsh S, Smith C, Ziermann JM, Abdala V (2015) Towards the resolution of a long-standing evolutionary question: Muscle identity and attachments are mainly related to topological position and not to primordium or homeotic identity of digits. J Anat 226: 523-529.
- Kjaer I, Keeling JW, Hansen BF (1996) Pattern of malformations in the axial skeleton in human trisomy 18 fetuses. Am J Med Genet 65: 332-336.
- Sepulveda W, Treadwell MC, Fisk NM. (1995) Prenatal detection of preaxial upper limb reduction in trisomy 18. Obstet Gynecol 85 (5): 847-850.
- Alberch P (1989) The logic of monsters: evidence for internal constraint in development and evolution. Geobios 22: 21-57.
- Bergman RA, Thompson SA, Afifi AK, Saadeh FA (1988) Compendium of human anatomic variation: Text, atlas, and world literature. Baltimore, Md.: Urban & Schwarzenberg.
- Tubbs RS, Shoja MM, Loukas M (2016) Bergman’s comprehensive encyclopedia of human anatomic variation. John Wiley & Sons.
- Colacino SC, Pettersen JC (1978) Analysis of the gross anatomical variations found in four cases of trisomy 13. Am J Med Genet 2: 31-50.
- Bersu ET, Opitz JM (1980) Anatomical analysis of the developmental effects of aneuploidy in man: the down syndrome. Am J Med Genet 5: 399-420.
- Wood J (1866) Variations in human myology observed during the winter session of 1866-67 at King’s college, London. Proc R Soc Lond 15: 518-46.
- Wood J. 1867. On human muscular variations and their relation to comparative anatomy. J Anat and Physiology 1: 44-59.
- Wood J (1867) Variations in human myology observed during the winter session of 1867-68 at King’s college, London. Proc R Soc Lond 16: 483-525.
- Jelev L, Georgiev GP, Surchev L (2007) Axillary arch in human: Common morphology and variety. definition of ‘clinical’ axillary arch and its classification. Ann Anat 189: 473-481.
- Hirtler L (2014) Langer’s axillary arch – Case presentation and literature overview. Austin J Anat. 1 (4): 1020.
- Miguel M, Llusá M, Ortiz JC, Porta N, Lorente M, et al. (2001) The axillopectoral muscle (of langer) report of three cases. SRA 23 (5): 341-343.
- Besana-Ciani I, Greenall MJ (2005) Langer’s axillary arch: Anatomy, embryological features and surgical implications. The Surgeon 3: 325-327.
- Loukas M, Noordeh N, Tubbs RS, Jordan R (2009) Variation of the axillary arch muscle with multiple insertions. Singapore Med J 50: 88-90.
- Sharma T, Singla RK, Agnihotri G, Gupta R (2009) Axillary arch muscle. Kathmandu Univ Med J (KUMJ) 7: 432-434.
- Serpell JW, Baum M (1991) Significance of ‘langer’s axillary arch’ in axillary dissection. Aust N Z J Surg 61: 310-312.
- Daniels IR, Della Rovere GQ (2000) The axillary arch of langer- The most common muscular variation in the axilla. Breast Cancer Res Treat 59: 77-80.
- Bonastre V, Rodríguez-Niedenführ M, Choi D, Sañudo JR (2002) Coexistence of a pectoralis quartus muscle and an unusual axillary arch: Case report and review. Clin Anat 15: 366-370.
- Turgut HB, Peker T, Gülekon N, Anil A, Karaköse M (2005) Axillopectoral muscle (langer’s muscle). Clin Anat 18: 220-223.
- Lama P, Potu BK, Bhat KMR (2010) Chondrohumeralis and axillary arch of langer: A rare combination of variant muscles with unique insertion. Rom J Morphol Embryol 51: 395-397.
- Aziz MA (1981) Muscular anomalies caused by delayed development in human aneuploidy. Clin Genet 19: 111-116.
- Aziz MA (1981) Possible ‘atavistic’ structures in human aneuploids. Am J Phys Anthropol 54: 347-353.
- Gasser RF (1967) The development of the facial muscles in man. American J Anat 120: 357-375.
- Keibel F, Mall FP (1910) Manual of human embryology. Vol. 1. J. B. Lippincott company.
- Diogo R, Johnston P, Molnar J, Esteve-Altava (2016) Characteristic tetrapod musculoskeletal limb phenotype emerged more than 400 MYA in basal lobe-finned fishes. Sci Rep 6:37592.
- Diogo R, Molnar J (2014) Comparative anatomy, evolution and homologies of the tetrapod hindlimb muscles, comparisons with forelimb muscles, and deconstruction of the forelimb-hindlimb serial homology hypothesis. Anat Rec 297: 1047-1075.
- Diogo R, Linde-Medina M, Abdala V, Ashley-Ross M (2013) New, puzzling insights from comparative myological studies on the old and unsolved forelimb/hindlimb enigma. Biol Rev 88: 196-214.
- Miyashita T, Diogo R (2016) Evolution of serial patterns in the vertebrate pharyngeal apparatus and paired appendages via assimilation of dissimilar units. Front Ecol Evol 4:71.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences