Antiparasitic Activity of Fagopyrum esculentum Moench on Meloidogyne incognita
1Laboratory of Functional Physiology and Valorization of Bio-Resssources, Higher Institute of Biotechnology of Beja, University of Jendouba,Avenue Habib Bourguiba, B.P, 382-9000, Beja, Tunisia
2Department of Life and Environmental Sciences, University of Cagliari, via Ospedale 72, 09124, Cagliari, Italy
3Laboratory of Plant Improvement and Valorization of Agro-resources (LAPVA), National School of Engineering Sfax, University of Sfax, Km 4,Sukra Road, 3038 Sfax, Tunisia
- *Corresponding Author:
- Nadhem Aissani
PhD in Toxicology
Department of Life and Environmental Sciences
University of Cagliari, via Ospedale 72
09124, Cagliari, Italy
Tel: +39 3801481013
E-mail: aissaninadhem@gmail.com
Received date: July 12, 2018; Accepted date: August 14, 2018; Published date: August 17, 2018
Citation: Aissani N, Balti R, Sebai H (2018) Antiparasitic Activity of Fagopyrum esculentum Moench on Meloidogyne incognita. J Toxicol Anal. Vol.1 No.2: 8.
Copyright: © 2018 Aissani N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The nematicidal activity of common buckwheat (Fagopyrum esculentum Moench) on the root knot nematode Meloidogyne incognita race 1 was evaluated using serial dilutions. The methanolic extract of the dried plant presented an anthelmintic activity with an EC50=62.6 ± 26 and 40.8 ± 26 µg/ml after 48 and 72 h of immersion respectively. However, the extract from fresh plant was less active with EC50=127.7 ± 67 and 98.3 ± 54 µg/ml after the same period of immersion. Nematicidal activity of the most abundant aldehyde (salicylaldehyde) and other aldehydes with chemical similarities were then assessed according to fosthiazate control. The most active aldehyde was salicylaldehyde (2-hydroxy benzaldehyde) followed by 3-hydroxy benzaldehyde, 4-hydroxy benzaldehyde, benzene acetaldehyde and benzaldehyde with EC50 of about 11 ± 1; 31 ± 22; 75 ± 23; 168 ± 52 and 300 ± 110 µg/ml after 1 day of immersion, respectively. As a complementary experiment, the synergistic activity was evaluated for salicylaldehyde and 3-hydroxybenzaldehyde, salicylaldehyde and 4-hydroxybenzaldehyde and for salicylaldehyde and benzenacetaldehyde. Synergistic activity was observed in the first 2 cases with EC50 after 24 h of immersion of 8 ± 2.5 and 6 ± 2.3 µg/ml, respectively. The addition of salicylaldehyde to benzenacetaldehyde enhances significantly the activity of this latter to become 23 ± 4 µg/ml after the same immersion period. The obtained results show that Fagopyrum esculentum could be considered as potent nematicidal plant to face root knot nematodes and integrated in the pest management system.
Keywords
Root knot nematodes; Aldehydes; Buckwheat
Introduction
Allelochemicals are compounds produced by plants and have a role in the growth, health, and behavior of other organisms [1]. Root-knot nematodes (Meloidogyne spp.) are among a vast array of pests continually attacking plants and causing roughly US$70 billion annually of crop losses in fruit and vegetable production [2]. Meloidogyne spp. being the most common and widespread group of Root Knot Nematodes in the world, increases the severity of soil borne diseases [2-6]. Many scientific studies have reported data on the biological activity of plant secondary metabolites on root-knot nematodes. Aldehydes from Ailanthus altissima as (E,E)-2,4- Decadienal and (E)-2-Decenal and methyl isothiocyanate from caper were active on Meloidogyne javanica [6,7]
Buckwheat (Fagopyrum esculentum Moench) is a wellestablished special crop that has been grown on Eastern prairies for the last 40 years. Seeds of common buckwheat or sweet buckwheat are usually consumed in Asia, Europe, North America, South Africa, and Australia [8]. At the present time the only attributes used to evaluate buckwheat quality are color and flavor related to volatile aldehydes [9]. Buckwheat contains high levels of nutritionally beneficial components and can be processed into various functional foods. There have been a large variety of buckwheat-based food products available in the market such as buckwheat noodles, pasta, bread, tea, spirits, and vinegar [10].
Recent researchers have found that buckwheat seed extract has a strong anti-oxidant activity [11]. Besides, immunestimulant and anti-virulent activities of seeds were noted [12,13].
Except Sipes and Arakaki work dealing with the nematicidal activity of buckwheat against Meloidogyne javanica [14], there is no available report on the nematicidal activity of F.esculantum on Meloidogyne incognita, the most widespread Meloidogyne gender.
In the present investigation, we report (1) for the first time the antiparasitic activity of the methanolic extract of common buckwheat aerial part on Meloidogyne incognita with and without taking into account moisture (2), the nematicidal activity of the most abundant aldehyde in the extract (salicylaldehyde) and structurally related aldehydes and finally (3) their synergistic activity.
Materials and Methods
Chemicals
Aldehydes standards, fosthiazate of purity greater than 98%, Tween 20 and dimethyl sulfoxide were obtained from Sigma- Aldrich. Methanol and water were high performance liquid chromatography (HPLC)-grade.
Plant materials and extraction
Seeds of buckwheat were purchased from a local market in Cagliari-Italy in March 2016. Voucher specimens were deposited at the Laboratory of Functional physiology and valuation of bioresources, High Institut of Biotechnology of Beja-University of Jendouba-Tunisia, for species identification. Seeds were germinated in cotton for 2 week at a temperature of 24°C. Fresh aerial plant parts were collected and ground (100 g) and extracted with methanol (1:1 w/v) in a sonicator apparatus for 15 mints, filtered through a Whatman no. 40 filter (Thermo Fisher Scientific, Illkirch, France) and centrifuged for 15 mints at 28341 g. The extract was then used to assess the nematicidal activity. The same protocol was repeated after drying plant aerial parts at 105°C for 24 h and moisture determination.
Nematode population
A population of M. incognita originally reared from tomato roots (Solanum lycopersicum L.) cv. Belladonna, a cultivar that is very susceptible to root-knot nematodes, was collected from a greenhouse in Cagliari (Italy). All plants were maintained in a growth chamber at 25°C to 28°C, 60% relative humidity, and 16 h photoperiod, in plastic pots (18 cm diameter). Plants used for inoculations were 7-week-old, at the 5-leaf stage. After 40 days, the plants were uprooted, and the roots were washed free of soil and cut into 2 cm pieces. Eggs were extracted according to the sodium hypochlorite procedure, and secondstage juveniles (J2) were allowed to hatch in modified Baermann funnels at 28°C. All J2 hatching in the first 3 days were discarded, and thereafter J2 collected, after 24 h, were used in the experiments [15].
Nematicidal assay
The nematicidal activity of the extract and compounds, in terms of nematode juvenile infestive stage (J2) paralysis, was tested and the EC50 values were calculated. Stock solutions were prepared by dilution with dimethyl sulfoxide (DMSO), whereas working solutions were obtained by dilution with distilled water containing the polysorbate surfactant 20 (Tween-20). Final concentrations of dimethyl sulfoxide in each well never exceeded 2% v/v, because preliminary trials showed that paralysis of nematodes exposed at those concentration levels was similar to paralysis of nematodes maintained in distilled water [1]. Distilled water, as well as a mixture of water with dimethyl sulfoxide and Tween-20 at concentrations equivalent to those in the treatment wells, served as negative control while fosthiazate at the same concentrations was used as positive control. Twenty-five juveniles were used per treatment well in Cellstar 96-well plates (Greiner Bio-one, Milan, Italy). The plates were covered to prevent evaporation and were maintained in the dark at 28°C. Border wells containing plain water with nematodes were placed around the wells of each treatment to check the vapor drift among wells that could possibly interfere with the efficacy results. Juveniles were observed with the aid of an inverted microscope (Zeiss, Oberkochen, West Germany) at 40 x after 24 h and were ranked into 2 distinct categories: motile or paralyzed. After the assessment, the nematodes were transferred into plain water, after washing in tap water through a 20 μm pore screen to remove the excess of tested compounds, and they were assessed again after 24, 48 and 72 h for the re-obtainment of motility. Dead nematodes were characterized by the presence of internal vacuoles.
The same protocol with adequate concentrations was repeated for synergistic activity assessment.
Statistical analysis
Treatments of paralysis experiments were replicated 6 times, and each experiment was performed twice. The percentages of paralyzed J2 in the microwell assays were corrected by elimination of the natural death/paralysis in the water control according to the Schneider Orelli formula, corrected [16]:
%=[(paralysis % in treatment-paralysis % in control)/(100- paralysis % in control)] × 100
Then, they were analyzed (ANOVA) after being combined over time. Since ANOVA indicated no significant treatment by time interaction, means were averaged over experiments. Corrected percentages of paralyzed J2 treated with test compounds were subjected to nonlinear regression analysis using the log-logistic equation proposed by Seefeldt et al. [17]:
Y=C+(D-C)/{1+exp[b(log(x)-log(EC50))]}
where, C=the lower limit, D=the upper limit, b=the slope at the EC50, and EC50=the test compounds concentration required for 50% paralyzed nematodes after elimination of the control (natural death/paralysis). In the regression equation, the test compounds concentration (% w/v) was the independent variable (x) and the paralyzed J2 (percentage increase over water control) was the dependent variable (y). The mean value of the 6 replicates per compound concentration and immersion period was used to calculate the EC50 value.
Results
Moisture determination
The 2 week aged aerial part plants moisture was calculated at 60.5% dealing with the richness of this plant on water and allowing the assessment of the nematicidal activity of the extract before and after drying the plant.
J2 paralysis bioassays of methanolic extracts
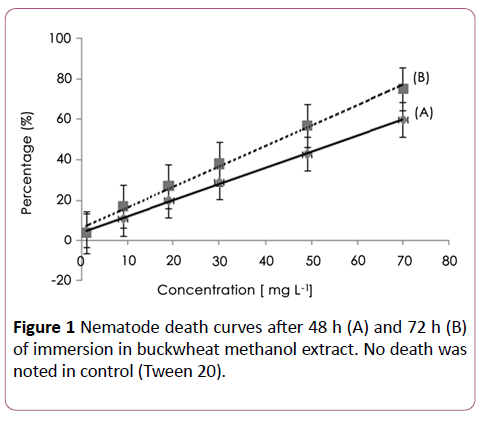
Without taking into account plant compound bioavailability or the synergetic effect, the extract was tested against M. incognita, a linear concentration-mortality relationship was established and significant paralysis/death of nematodes was evident after 48 and 72 h of exposure with an effective dose 50 (EC50) calculated value of about 62.6 ± 26 (R2=0.99) and 40.8 ± 26 μg/ml (R2=0.96), respectively (Figure 1). Nematicidal activity of the methanol extract without drying was EC50=127.7 ± 67 μg/ml and 98.3 ± 54 μg/ml after the same period of immersion respectively.
J2 paralysis of the tested compounds
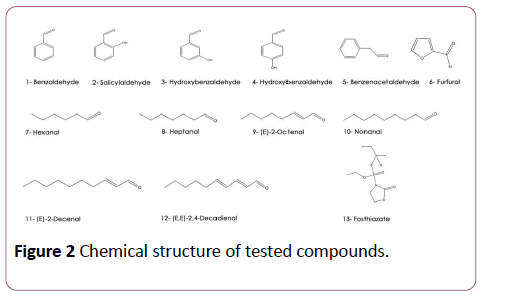
The analysis of the extract volatilome by headspace technique showed that this plant is rich in aldehydes and ketones according to a previous study [11]. Figure 2 and Table 1 present chemical structure and EC50 of the most important aldehydes found in this extract against M. incognita and M. javanica [5-7,18] as well as the control fosthiazate. Salicyl aldehyde (2-hydroxy benzaldehyde) was the most active aldehyde followed by 3-hydroxy benzaldehyde (3), 4-hydroxy benzaldehyde (4), benzeneacetaldehyde (5) and then benzaldehyde (1) with an EC50 after 24 h of immersion of about 11 ± 1; 31 ± 22; 75 ± 23; 168 ± 52 and 300 ± 110 μg/ml respectively (Table 1).
| S. No | Compound | Rt (min) | EC50/24 h (µg/ml) ± SD | |
|---|---|---|---|---|
| Meloidogyne incognita | Meloidogyne javanica | |||
| 1 | Hexanal | 26.7* | na | na‡ |
| 2 | Heptanal | 36* | na | na‡ |
| 3 | Furfural | 37.5* | 8 ± 1† | 21.79‡ |
| 4 | Benzaldehyde | 47.7* | 300 ± 110 | nt |
| 5 | (E)-2-octenal | 51.1* | 30 ± 17 | na‡ |
| 6 | Salicylaldehyde (2-hydroxy benzaldehyde) | 53.6* | 11 ± 1† | nt |
| 7 | Benzenacetaldehyde | 53.6* | 168 ± 52 | nt |
| 8 | Nonanal | 54.1* | na | na‡ |
| 9 | (E)-2-decenal | 67.7* | 22 ± 10 | 20.43‡ |
| 10 | (E,E)-2,4-decadienal | 72.9* | 12 ± 2 | 11.70‡ |
| 11 | 4-hydroxy benzaldehyde | 76.9* | 75 ± 23† | nt |
| 12 | Salicyladehyde+benzene acetaldehyde | - | 23 ± 4 | nt |
| 13 | Salicyladehyde+3-hydroxy benzaldehyde | - | 8 ± 2.5 | nt |
| 14 | Salicyladehyde+4-hydroxy benzaldehyde | - | 6 ± 2.3 | nt |
| 15 | fosthiazate | - | 0.4 ± 0.3† | 15.9‡ |
Table 1: Nematicidal activity of the tested compounds (n=4). Abbreviations: na: not active; nt: not tested, where *Resulst reported by Prosen et al. [11]. †Result reported by Caboni et al. [18]. ‡Result reported by Caboni et al. [5-7].
Synergistic activity assessment
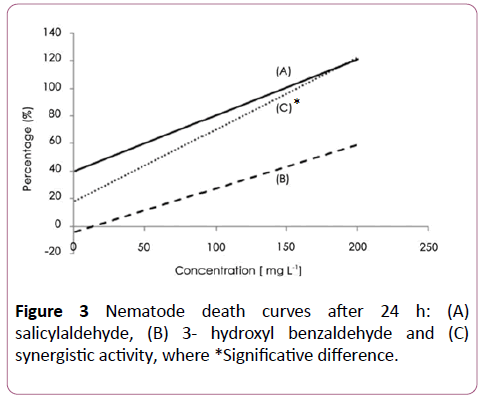
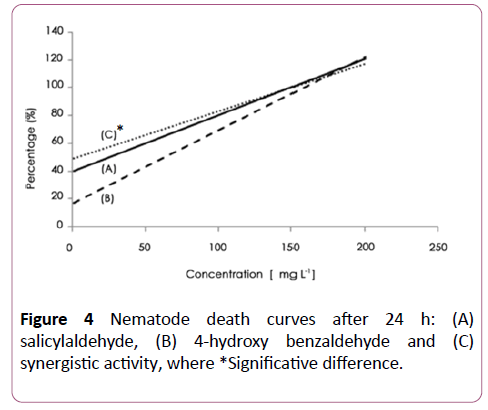
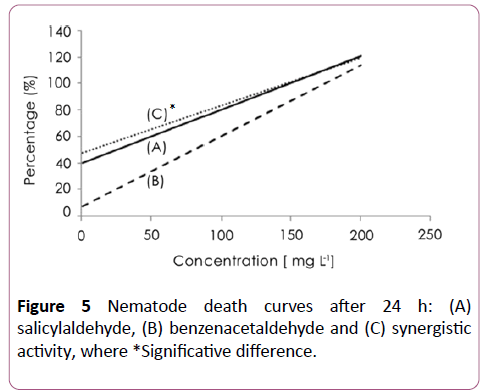
A synergistic activity of salicylaldehyde and 3-hydroxy benzaldehyde (Figure 3), with salicylaldehyde and 4- hydroxybenzaldehyde (Figure 4) was observed with EC50 of 8 ± 2.5 and 6 ± 2.3 μg/ml respectively. The addition of salicylaldehyde to benzenacetaldehyde enhances significantly the activity of this latter to become 23 ± 4 μg/ml after the same immersion period (Figure 5).
Discussion
In this study, aerial fresh and dried part of Common buckwheat (Fagopyrum esculentum) was extracted using methanol and the nematicidal activity of the extracts was assessed against juveniles of Meloidogyne incognita. Results showed that the extract was more active when the plant was dried with EC50=40.8 ± 26 μg/ml after 72 h. This nematicidal activity is high considering those of Capparis spinosa stems methanol extracts with an EC50 value of 215 ± 36 μg/ml [7].
The chemical analysis of buckwheat showed that this plant is rich in polyphenols such as rutin, quercitin and tannins [19-21] which can be responsible for this nematicidal activity. In a previous study, polyphenols from Melia azedarach presented a nematicidal activity against M. incognita and the most active compound was salicylic acid with EC50 of 379 ± 96 μg/ml after 24 h of exposure [5].
Different isomers of fagopyrin, a phototoxic agent which can act as photosensitizer upon excitation with visible light causing fagopyrism, were found in buckwheat [22]. Symptomatically, fagopyrism is manifested as a skin irritation that occurs with sunlight exposure following the ingestion of large quantities of this plant [22]. The phenomenon of skin irritation was observed when nematodes were treated with aldehydes such as phthalaldehyde and cinnamic aldehyde [18]. Aldehydes from Ailanthus altissima presented a strong nematicidal activity against root knot nematodes [6,7].
In the same fashion, the analysis of buckwheat volatilome by headspace technique showed that this plant is rich in aldehydes and ketones [11]. According to Janes and Kreft [23,24], salicylaldehyde is a characteristic aroma component of buckwheat and presents the highest concentration with 1.6 μg/ml. The structure-activity study showed that salicylaldehyde (2-hydroxy benzaldehyde) is 3 times more active than 3- hydroxyl benzaldehyde and 7 times more than 4- hydroxybenzaldehyde. Thus, the position 2 of the hydroxyl group in the benzene ring seems to be very important for the nematicidal power followed by the position 3 and 4. Using aldehydes with linear chains, our results clearly indicate that α,β,γ,δ- unsaturated C10 aldehydes are generally more nematicidal than their shortest C-chain counterparts versus M.incognita confirming those found by Caboni et al. [6,7] using M. javanica.
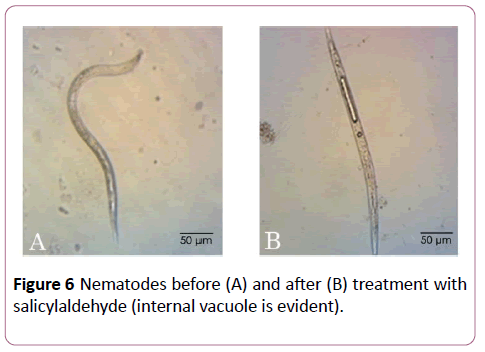
In a recent study, (E)-2-decenal degenerated the nematode pseudocoel cells and caused malformations of somatic muscles [25]. Caboni et al. [18] showed that nematodes treated with salicylaldehyde and aromatic aldehydes presented serious cuticle damages with marked macroscopic fractures and paralysis in a straight shape with evident internal vacuolization compared to the controls [18]. This observation was noted also after immersion of nematodes in buckwheat methanolic extract (Figure 6). Previous studies reported similar results when nematodes were treated with isothiocyanates and some vacuolar-type proton-translocating adenosine triphosphatase (V-ATPase) inhibitors such as pyocyanin [1,2].
Interestingly, salicylaldehyde produced an increased pH in lysosomal-like organelles on HeLa human cell line and this alteration was most likely related to a V-ATPase impairment [15].
This study deals for the first time with the nematicidal activity of common buckwheat methanol extract against M. incognita. This plant is rich in polyphenols, fagopyrin and aldehydes such as salicylaldehyde, which has nematicidal activity against this root-knot nematode. Results are promoters and allow us to use this plant for crop protection against pests.
Acknowledgment
Special thanks to Professor Pierluigi Caboni from the University of Cagliari-Italy for providing nematodes, chemical standards and his valuable technical assistance.
References
- Aissani N, Tedeschi P, Maietti A, Brandolini V, Garau VL, et al. (2013) Nematicidal activity of allylisothiocyanate from horseradish (Armoracia rusticana) roots against Meloidogyne incognita. J Agric Food Chem 61: 4723-4727.
- Aissani N, Urgeghe PP, Oplos C, Saba M, Tocco G, et al. (2015) Nematicidal activity of the volatilome of Eruca sativa on Meloidogyne incognita. J Agric Food Chem 63: 6120-6125.
- Hussain MA, Mukhtar T, Kayani MZ (2016) Reproduction of Meloidogyne incognita on resistant and susceptible okra cultivars. Pak J Agr Sci 53: 371-375.
- Kayani MZ, Mukhtar T (2018) Reproductivity of Meloidogyne incognita on fifteen cucumber cultivars. Pakistan Journal of Zoology 50: 1717-1722.
- Aoudia H, Ntalli NG, Aissani N, Yahiaoui-Zaidi R, Caboni P (2012) Nematotoxic phenolic compounds from Melia azedarach against Meloidogyne incognita. J Agric Food Chem 60: 11675-11680.
- Caboni P, Ntalli NG, Aissani N, Cavoski I, Angioni A (2012) Nematicidal Activity of (E,E)-2,4-Decadienal and (E)-2-Decenal from Ailanthus altissima against Meloidogyne javanica. J Agric Food Chem 60: 1146-1151.
- Caboni P, Sarais G, Aissani N, Tocco G, Sasanelli N, et al. (2012) Nematicidal activity of 2-thiophenecarboxaldehyde and methylisothiocyanate from caper (Capparis spinosa) against Meloidogyne incognita. J Agric Food Chem 60: 7345-7351.
- Li SQ, Zhang QH (2001) Advances in the development of functional food from buckwheat. Crit Rev Food Sci Nutr 41: 451-464.
- Przybylski R, Woodward L, Eskin NA, Malcolmson LJ and Mazza G (1995) Effect of Buckwheat Storage and Milling on Flavor Compounds. Current Advances in Buckwheat Research 2: 783-787.
- Ikeda K (2002) Buckwheat: composition, chemistry, and processing. Adv Food Nutr Res 44: 395-434.
- Prosen H, Kokalj M, Janeš D, Kreft S (2010) Comparison of isolation methods for the determination of buckwheat volatile compounds. Food Chem. 121: 298-306
- Bai CZ, Ji HJ, Feng ML, Hao XL, Zhong QM, et al. (2015) Stimulation of dendritic cell maturation and induction of apoptosis in lymphoma cells by a stable lectin from buckwheat seeds. Genet Mol Res 14: 2162-2175.
- Yuan S, Yan J, Ye X, Wu Z, Ng T (2015) Isolation of a ribonuclease with antiproliferative and HIV-1 reversetranscriptase inhibitory activities from Japanese large brown buckwheat seeds. Appl Biochem Biotechnol 175: 2456-2467.
- Sipes BS, Arakaki AS (1997) Root-knot nematode management in dryland taro with tropical cover crops. Supplement to the Journal of Nematology 29: 721-724.
- Caboni P, Tronci L, Liori B, Tocco G, Sasanelli N, et al. (2014) Tulipaline A: Structure-Activity aspects as a nematicide and V-ATPase inhibitor. Pest Biochem Physiol 112: 33-39.
- Puntener W (1981) Manual for Field Trials in Plant Protection (2nd ed.), Ciba Geigy, Basel, Switzerland, p(205).
- Seefeldt SS, Jensen JE, Fuerst EP (1995) Log-logistic analysis of herbicide rate response relationships. Weed Technology 9: 218-227.
- Caboni P, Aissani N, Cabras T, Falqui A, Marotta R, et al. (2013) Potent nematicidal activity of phthalaldehyde, salicylaldehyde, and cinnamic aldehyde against Meloidogyne incognita. J Agric Food Chem 61: 1794-1803.
- Verardo V, Arráez-Román D, Segura-Carretero A, Marconi E, Fernández-Gutiérrez A, et al. (2010) Identification of buckwheat phenolic compounds by reverse phase high performance liquid chromatography electrospray ionization-time of flight-mass spectrometry (RP-HPLCeESI-TOF-MS). J Cereal Sci 52: 170-176.
- Yao YP, Tian CR, Wei CA (2008) Anti-oxidative constituents of ethanol extract from buckwheat seeds by HPLC- Electrospray MS. Agric. Sci. China 7: 356-362.
- Kalinova J, Vrchotova N (2009) Level of catechin, myricetin, quercetin and isoquercitrin in buckwheat (Fagopyrum esculentum Moench), changes of their levels during vegetation and their effect on the growth of selected weeds. J Agric Food Chem 57: 2719-2725.
- Benkovic ET, Zigon D, Friedrich M, Plavec J, Kreft S (2014) Isolation, analysis and structures of phototoxic fagopyrins from Buckwheat. Food Chemistry 143: 432-439.
- Ladan MK, Straus J, Benkovic ET, Kreft S (2017) FT-IR-based method for rutin, quercetin and quercitrin quantification in different buckwheat (Fagopyrum) species. Sci Rep. 7: 7226-7234.
- Janes D, Kreft S (2008) Salicylaldehyde is a characteristic aroma component of buckwheat groats. Food Chem 109: 293-298.
- Ntalli N, Ratajczak M, Oplos C, Menkissoglu-Spiroudi U, Adamski Z (2016) Acetic Acid, 2-undecanone, and (E)-2-Decenal ultrastructural malformations on Meloidogyne incognita. Journal of Nematology 48: 248-260.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences