ISSN : 2348-9502
American Journal of Ethnomedicine
Anti-Oxidative and Anti-Metalotoxic Properties of Green Tea Catechin: A Preliminary Study
Department of Zoology, Radiation & Cancer Biology Laboratory, University of Rajasthan, Jaipur-302 004, India
- *Corresponding Author:
- Priyanka Sharma
Department of Zoology
Radiation & Cancer Biology Laboratory
University of Rajasthan
Jaipur-302 004, India
E-mail: priyanishtha.08279@gmail.com
Abstract
Aim of the study: Green tea catechin possesses antioxidant and free radical scavenging activity which stimulate detoxification system through selective induction or modification of phase I and phase II metabolic enzymes. This study was carried out to assess the anti- oxidative and anti- metalotoxic effect of green tea Catechin against cadmium chloride in mice.
Method: Adult Swiss albino mice were intoxicated with different doses of cadmium chloride in the presence (experimental) or absence (control) of green tea Catechin. Dose selection and DRF of Catechin were studied. The optimum dose of Catechin was determined by administering 1000, 1500, 2000, 5000, 7500μg/ k.g./ animal/ day of Catechin consecutively for 15 days, thence antioxidant enzymes (superoxide dismutase, catalase, & glutathione peroxidase), Phase I enzymes (Cytochrome P- 450 & Cytochrome b5), Phase II enzymes (Glutathione S- transferase & DT- diaphorase), antioxidant molecule (reduced glutathione) and lipid per- oxidation level were estimated in testes, liver and blood after 24 hrs, 7th day 16th and 31st day of Catechin administration.
Results: The dose of Catechin found to be most effective was 7500 μg, because this dose significantly increased the of Phase I & II enzymes, antioxidant parameters and decreased lipid per- oxidation. On the basis of survival data of mice DRF of Catechin against cadmium chloride was evaluated as 1.61. Conclusion: From the present results, it is evident that the green tea Catechin has the potential to reduce deleterious effects of cadmium chloride in mammals.
Keywords
Cadmiunm chloride, Catechin, Phase I & II enzymes, Antioxidant, Swiss albino mice.
INTRODUCTION
Heavy metals occur as natural constituents of the earth crust, and are persistent environmental contaminants since they cannot be degraded or destroyed. The diverse deleterious health effects upon exposure to toxic heavy metals in the environment are a matter of serious concern and challenge at the global level. The major hazardous metals of concern for India in terms of their environmental load and health effects are lead, mercury, chromium, cadmium, copper and aluminum. Their sources of such metals are mostly anthropogenic- industrial activity, vehicles, etc.
Cadmium is an extremely toxic metal commonly found in industrial work places. Cadmium occurs naturally in ores together with zinc, lead and copper [1]. Cadmium compounds are used as stabilizers in PVC products, color pigments, several alloys and, in re-chargeable nickel– cadmium batteries [2]. The detrimental effects of cadmium on physiological, biochemical and behavioral dysfunctions have already been documented in animals and humans. The higher levels affect the central and peripheral nervous systems, haematopoietic system, cardio-vascular system, kidneys, liver, and reproductive system [3,4].
Most common of those having potential implications in reproductive biology by interfering in the natural process of reproduction and fertilization include superoxide anion (O2-), hydrogen peroxide (H2O2), peroxyl (ROO-) radicals and the very reactive hydroxyl (OH-) radicals. Oxidative stress induced by Cd exposure affects many mammalian tissues including the testes, brain, liver and kidneys [5,6].
Cadmium has a toxic effect on many enzymes dependent on iron as a co-factor and one of these being Cytochrome P- 450 [7]. Testicular lesions from cadmium intoxication are primarily vascular, and the vascular damage determines the degree of lesion in the germ cells and induces Leydig’s cell tumors, tubular degeneration, atrophy, tissue necrosis and deficient androgen production [8,9].
To combat such deleterious effects of heavy metals, there is an urgent need to develop a protective formulation that is effective in multi directional manner to living cells. In spite of intense global research in the field of toxicology, no molecular or synthetic drug has been able to meet out the criteria of a clinically acceptable antidote because of accompanying toxic effects to one or more vital body systems at the effective concentration [10]. This has shifted the focus of researchers towards natural products and neutraceuticles for their prophylactic and therapeutic uses against heavy metal intoxication. A phyto- therapeutic approach to modern drug development can provide many invaluable drugs from traditional medicinal plants.
In Ayurvedic system of medicine, many potential drugs of plant origin have been studied to combat the toxic effects as they are rich of antioxidants and act as immune stimulants and capable to terminate free radical reactions that prevent our body from oxidative stress, conferring less side effects and compatible to body physiology [11]. Plants respond to heavy metal toxicity through immobilization, chelating and compartmentalization of the metal ions. A number of metal-binding ligands have now been recognized in plants.
Camellia sinensis, (family: Theaceae) is an evergreen heavily branched shrub or tree with dark green, hairy oblongovate leaves, which have been originally discovered and grown in Southeast Asia. Green tea is inexpensive non toxic and a popular beverage consumed world wide. Chemically green tea leaves contain polyphenolic compound, more commonly known as Catechin [12].
Mechanism of the action of Catechin includes antioxidant and free radical scavenging activity which stimulate detoxification system through selective induction or modification of Phase I and Phase II metabolic enzymes [13]. In addition, green tea may inhibit biochemical markers of tumors initiation and promotion including the rate of cell replication and thus inhibits the growth and development of neoplasm [14]. Another potential effect due to the antioxidant activities of green tea polyphenols such as Catechin, which binds with Cd ions to form an insoluble complex - ionic salt that is used to remove Cd from biological tissues [15].
Therefore, the aim of the present study was to throw a light on possible prophylactic and therapeutic potential of green tea extract against the toxic effects of cadmium chloride on the testes of mammals.
MATERIALS AND METHODS
Animal care and handling
Swiss albino male mice, 6- 8 weeks (Mus musculus) old & weighing 25 ± 2 gm, were selected from an inbreed colony. They were maintained under controlled conditions of temperature and light (light: dark, 10 hrs: 14hrs), and were fed with balanced diet in the form of pellets manufactured by Ashirwad industries, Chandigarh, and water was provided ad libitum. Tetracycline water was also given to them, once in a fortnight, as preventive measure against infections.
The following experiments were conducted:
Experiment-1: Dose selection of catechin
Catechin was administered by oral gavage to normal mice in different doses (1000, 1500, 2000, 5000, 7500μg/k.g./ animal/day ) till 15 days thence the changes in the body weight, food & water consumption, general behavior were observed daily till 30 days. For biochemical analysis, testes were surgically removed at each autopsy interval from the necropsied animals of each group and weighed. One part of it was fixed in Bouin’s fluid, and slides were prepared by routine procedure. The remaining part of testis was used for biochemical analysis to antioxidant enzymes viz. glutathione peroxidase [16], Phase I enzymes viz. cytochrome P-450 & cytochrome b5 [17], Phase II enzymes viz. glutathione- s- transferase [18] & DTdiaphorase [19], antioxidant molecule viz. reduced glutathione [20,21] and lipid peroxidation [22] in testes, liver and blood serum on 24 hrs, 7th day 16th and 31st day of Catechin administration. The dose of Catechin in which the highest level of Phase I & II enzymes, antioxidant parameters and the lowest level of lipid per- oxidation were measured and considered as optimum dose, and the further experiments were performed by using the same dose.
Experiment-2: Determination of LD 50/30 & dose reduction factor (DRF)
To ascertain the efficacy of Catechin, the animals were divided into two groups.
• Intoxicated Group (DDW + Cadmium Chloride).
Animals of this group were administered with DDW before intoxication of CdCl2 at different doses (2.5, 5.0, 7.5, 10, 20 mg/ Kg./ b.wt ).
• Experimental Group ( Catechin + Cadmium Chloride )
Animals of this group were administered with the optimum dose of Catechin (7500 μ gm/kg b.wt/day), once orally for 15 consecutive days and then on 15th day these were treated with CdCl2 (dose similar to Group 1) after 30 minutes of the Catechin administration.
The animals of both the groups were monitored daily for the weight, sickness, gait, morbidity, behavior, mortality and abnormality (if any) up to 30 days postintoxication. The percentage of mice surviving at each cadmium chloride dose till 30 days following such intoxication were used to construct survival dose response curves. Regression analysis was done to obtain LD 50/30 and the dose reduction factor (DRF) was calculated as following:

Statistical analysis
All the data generated were subjected to statistical analysis to determine the validity of the results. The values were expressed as mean ± standard error and compared in various groups. The values of various groups were compared by using Students’ “t” test.
RESULTS
Selection of the optimum dose of catechin
The optimum dose of Catechin was selected on the basis of the changes in the body weight, food & water consumption, general behavior and different antioxidative parameters, where the animals were treated with Catechin by oral gavage with different doses (1000, 1500, 2000, 5000, 7500μg/k.g./animal/day) till 15 days and then they were observed daily till 30 days of post- treatment.
Antioxidant enzymes ( glutathione peroxidase), Phase I enzymes (Cytochrome P-450 & Cytochrome b5) and Phase II enzymes (Glutathione S- transferase & DTdiaphorase) were measured in liver and testes both while antioxidant molecule (reduced glutathione) and lipid peroxidation levels were estimated in the testes, liver and blood at 24 hrs, 7th day, 16th and 31st day of Catechin administration.
Body weight
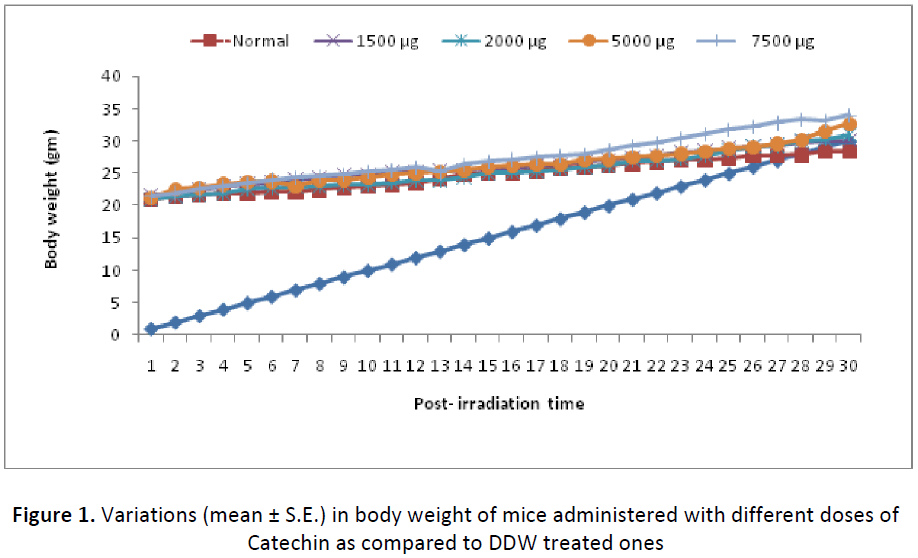
Administration of different dose of Catechin (1000, 1500, 2000, 5000, 7500μg/kg. b.wt/day for 15 consecutive days) to Swiss albino mice exhibited a regular weight gain till day 31st by reaching 139.66%, 142.41%, 147.61%, 154.24% and 158.13% higher than the initial body weight, respectively. No noticeable sign of sickness, mortality, morbidity and change in general behavioral were observed throughout the experiment. More over, these animals appeared quite healthy in all respect (Fig. 1).
Phase I enzymes
Cytochrome P- 450
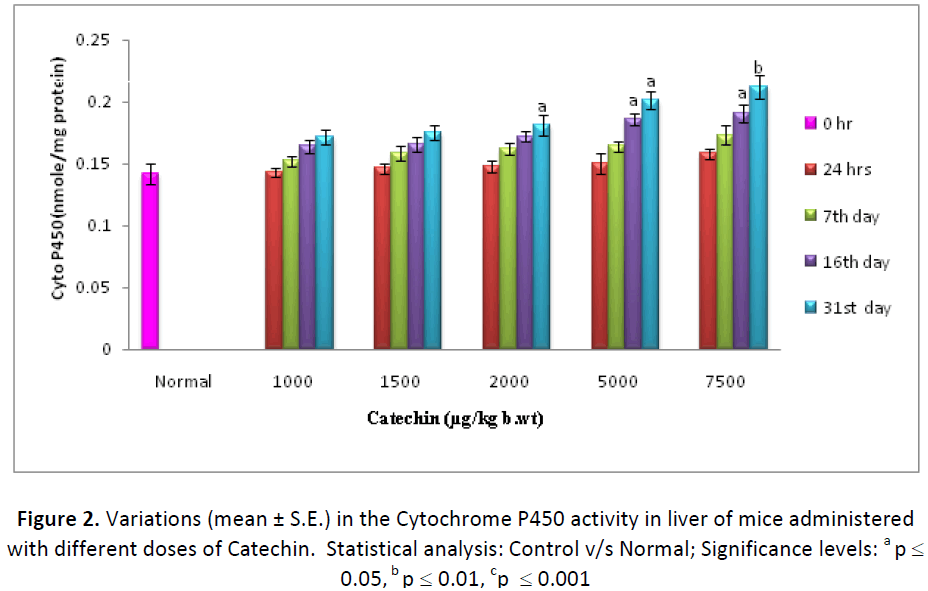
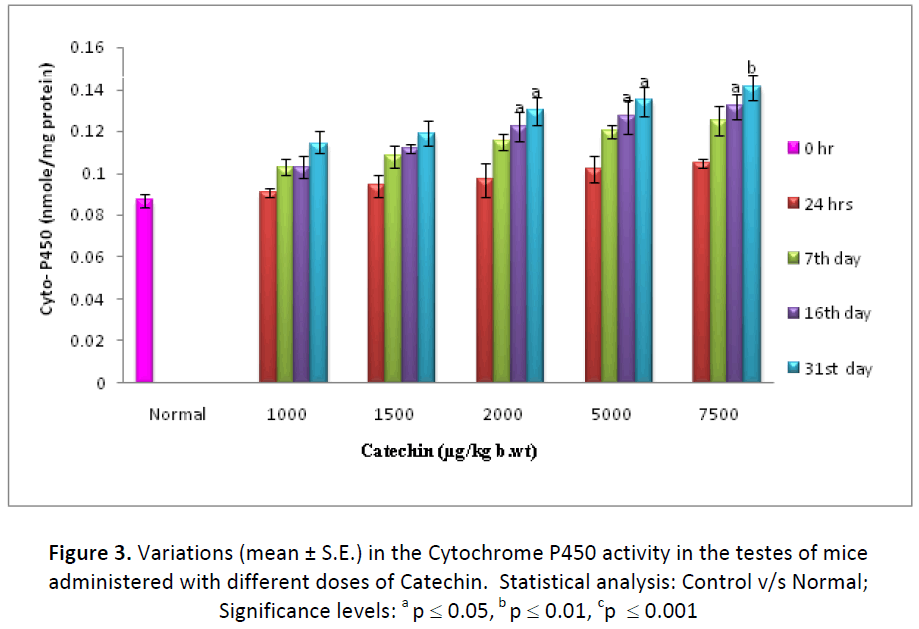
Cytochrome P 450 level was found to be increased progressively from 24 hrs to 31st day of experiment both in liver and in testes of animals treated with different doses (1000, 1500, 2000, 5000 & 7500 μg ) of Catechin as compared to normal (DDW treated). By the end of experiment, percent increase in Cytochrome P-450 was observed as 120.42, 123.23, 127.46, 141.54 and 149.29 in hepatic tissue of animals treated with 1000, 1500, 2000, 5000, 7500μg/k.g./ animal/day, respectively as compared to DDW treated ones (Fig. 2). Similarly, testicular tissue also showed a similar mode of elevation and the highest increase (162.06 %) was noted on 31st day with 7500 μg dose of Catechin as compared to normal (Fig. 3).
Cytochrome b5
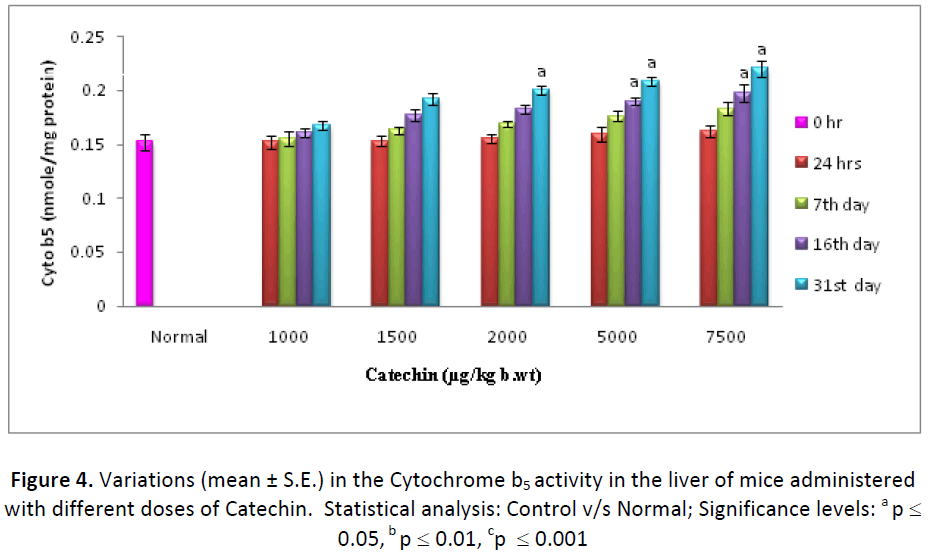
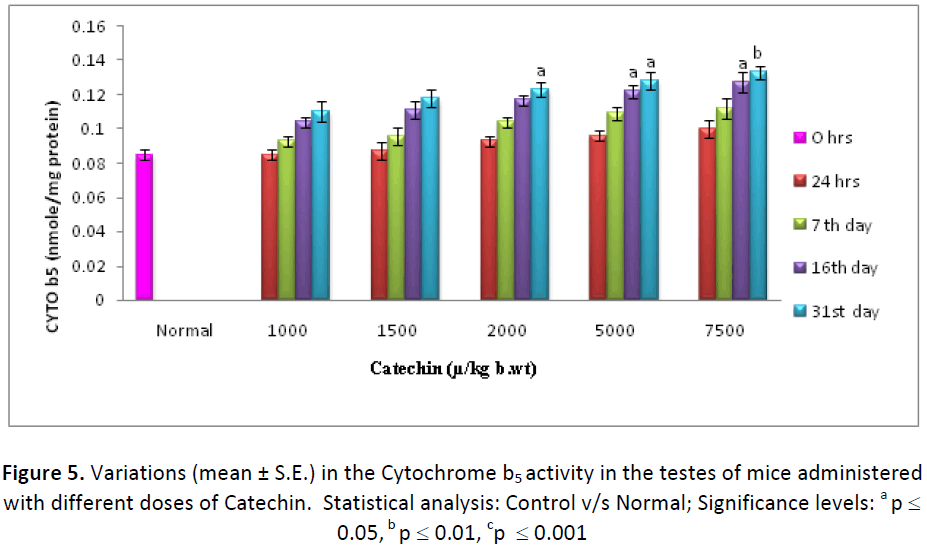
Cytochrome b5 levels in liver and testes were also found to be increased, gradually from the beginning of experimentation till the last autopsy interval, in the animals treated with different doses (1000, 1500, 2000, 5000 & 7500 μg) of Catechin as compared to normal. In the liver, the activity of Cytochrome- b5 was observed as 110.52%, 113.07%, 114.19%, 115.72% and 118.51% higher than DDW treated ones, with 1000, 1500, 2000, 5000 & 7500 μg of Catechin, respectively (Fig.4). Further, testes also exhibited a similar pattern of change, with the maximum increase (156.47 %) at 31st day post- treatment of Catechin (7500 μg) as compared to normal (Fig. 5).
Phase II enzymes
Glutathione -s -transferase (GST)
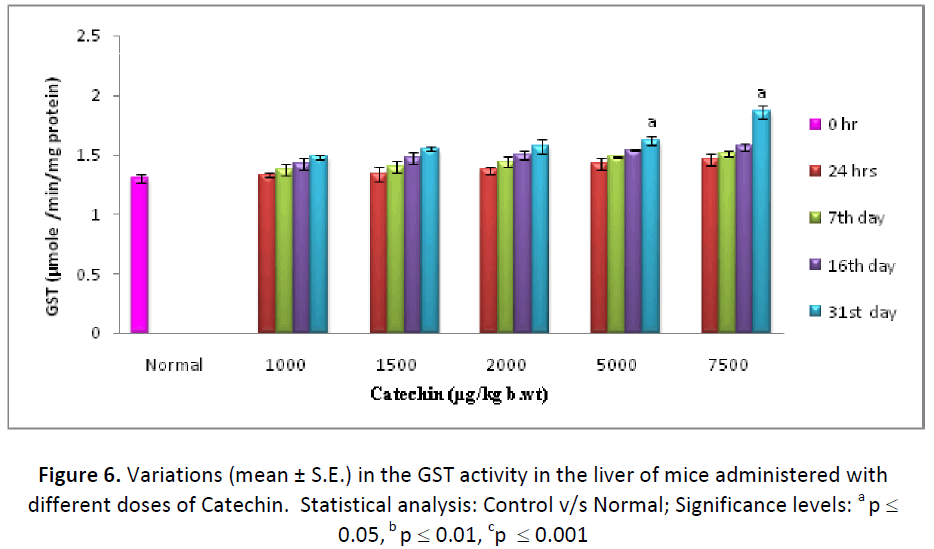
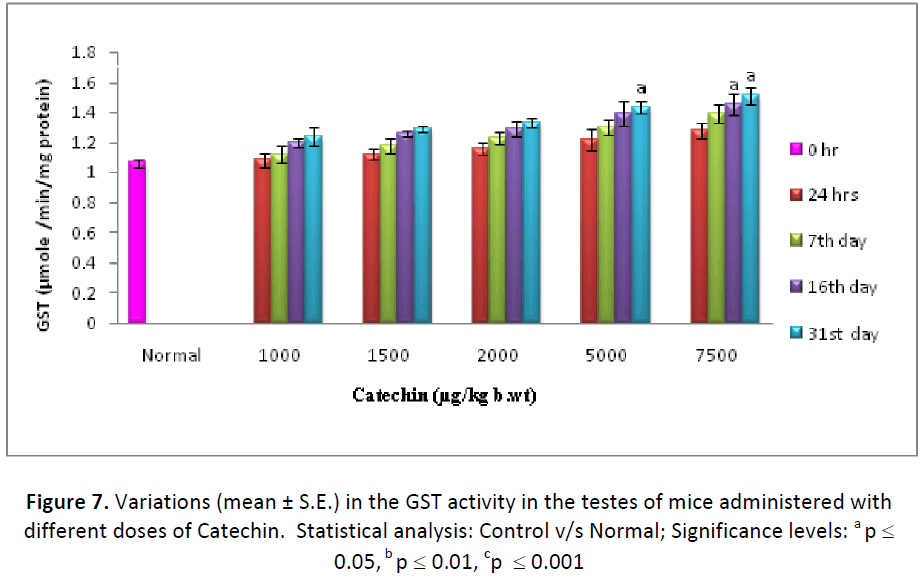
Similar to Phase I enzymes, GST activity (μ mole CDNB-GSH conjugate formed/min/mg protein) also showed a noticeable increase at all the dose levels of Catechin when compared to normal. It increased continuously from 24 hrs and maintained till the end of experimentation. However, the maximum elevation in GST concentration was observed with the dose of 7500 μgm Catechin. It was noted considerable higher in hepatic (146.07 %) as well as testicular (139.81 %) tissues in comparison to DDW treated animals (Figs. 6 & 7).
DT-diaphorase
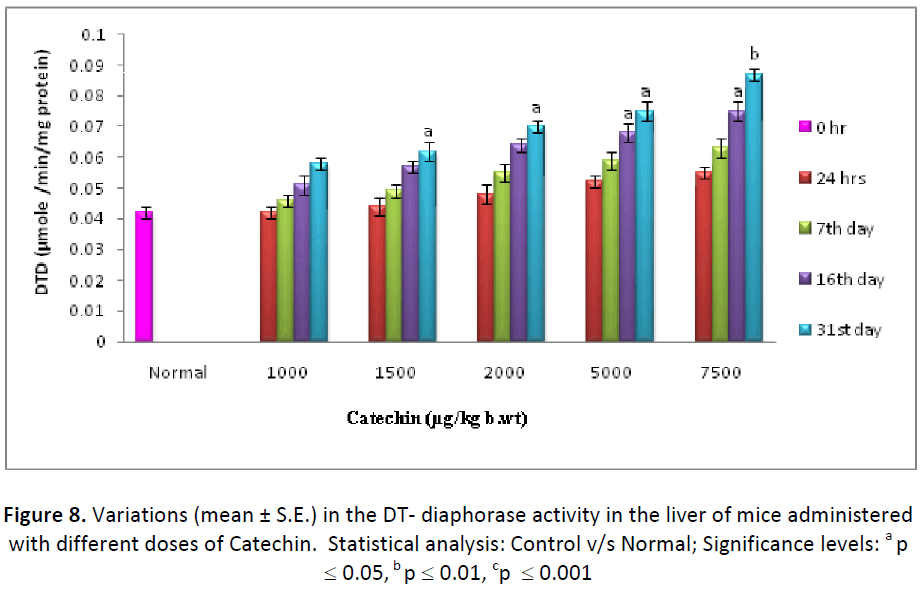
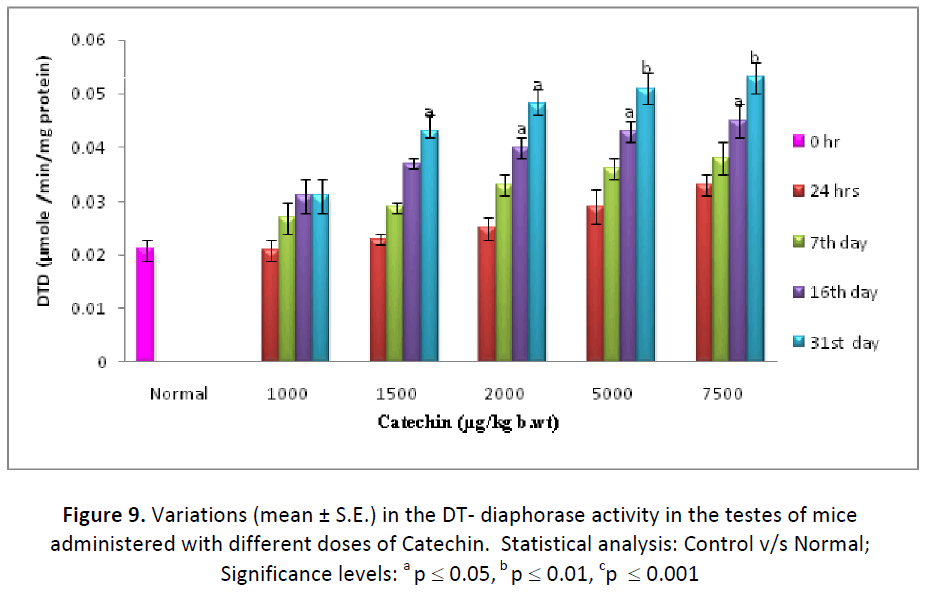
The activity of DT- diaphorase enzyme (μ mole of DCPIP reduced/min/mg protein) was found to be increased in liver as well as testes after administration of different dose (1000, 1500, 2000, 5000, 7500μg/kg. b. wt/day) of Catechin. Similar to other detoxification enzymes, DTdiaphorase also exhibited the maximum augment with the dose 7500 μg of Catechin in both the tissues as it elevated from 152.38 % (24 hrs) to 261.90 % (31st day) in liver and from 147.61% (24 hrs) to 252.38 % (31st day) in testes (Figs. 8 & 9).
Glutathione (GSH)
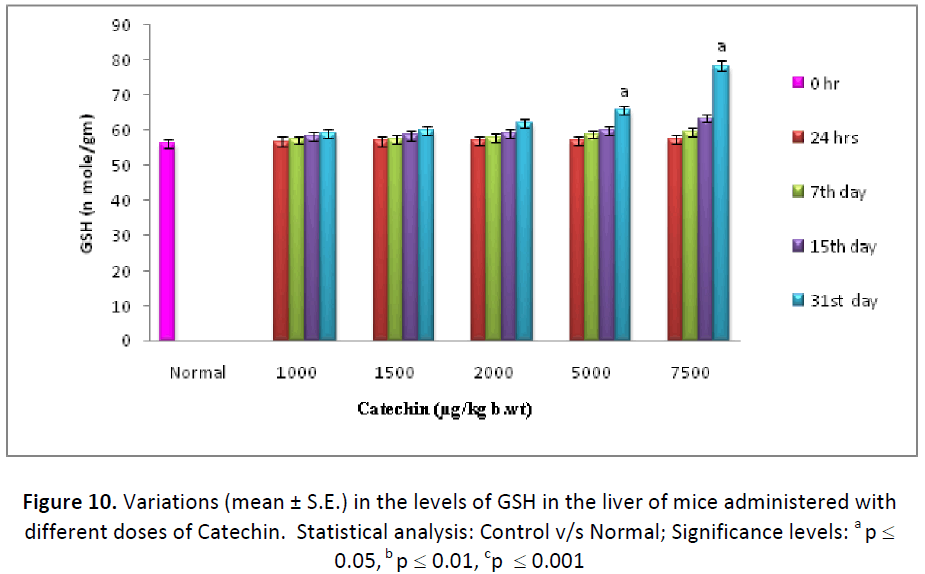
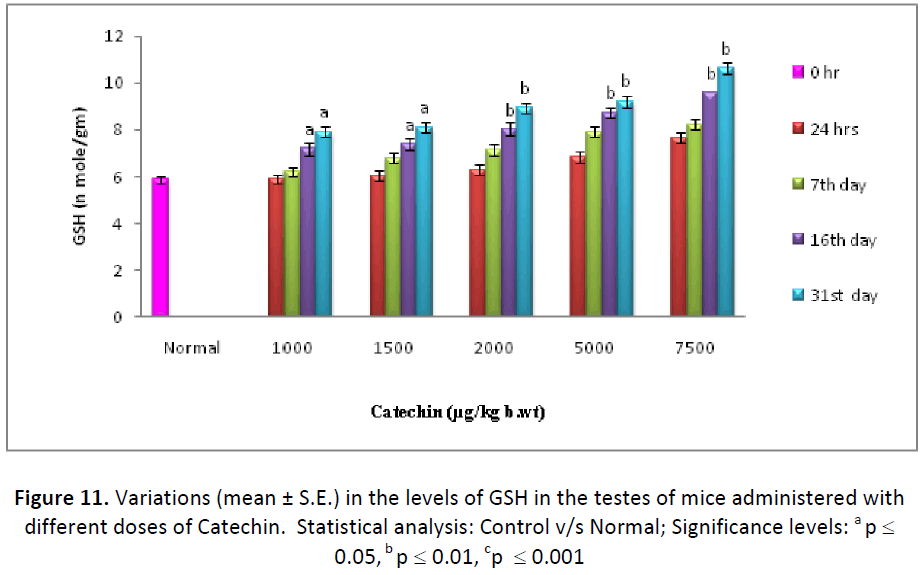
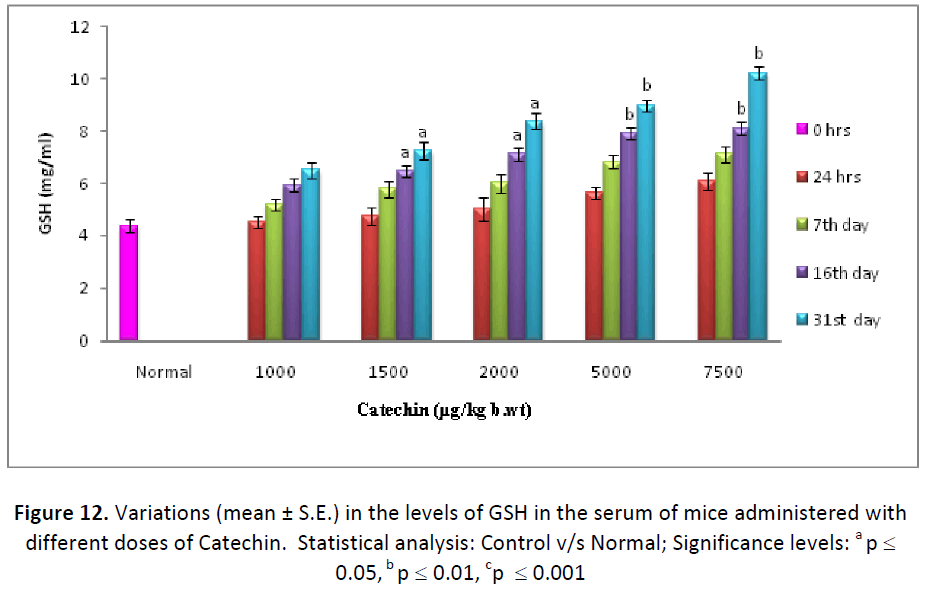
In the present experiment, glutathione levels exhibited a consistent increase in liver, testes and blood serum with all the doses of Catechin, from 24 hrs to last autopsy interval, as compared to their respective normal. By the end of experiment (31st day), the maximum increase was observed with the dose of 7500 μg Catechin, where it was estimated as 139.74%, 181.16% and 234.09% higher than the DDW treated animals in the liver, testes and blood, respectively (Figs. 10- 12).
Lipid peroxidation (LPO)
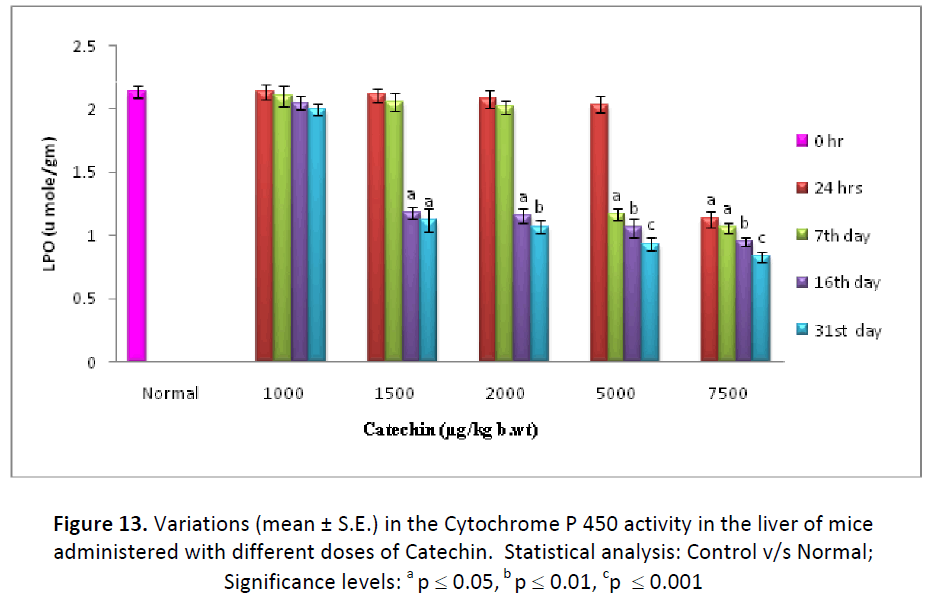
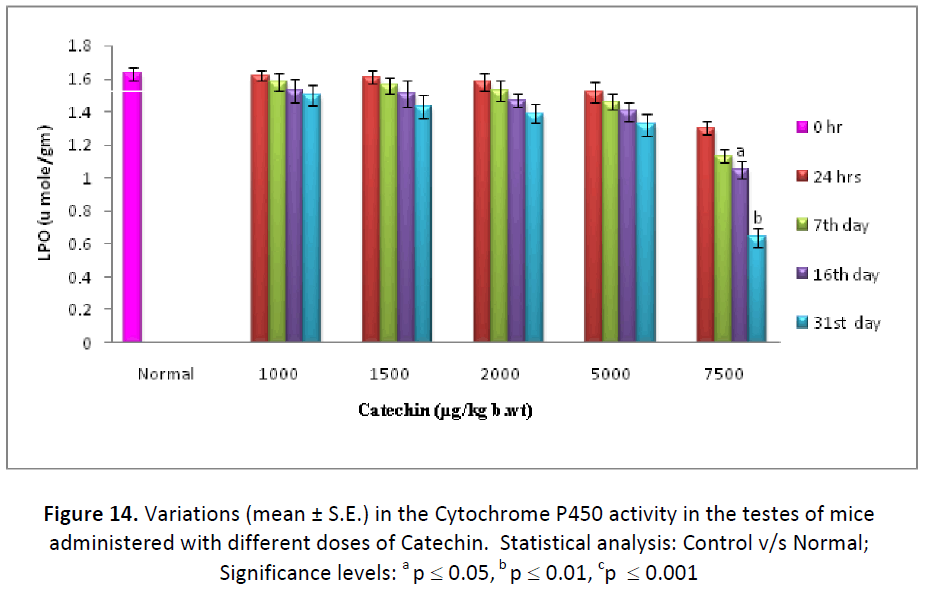
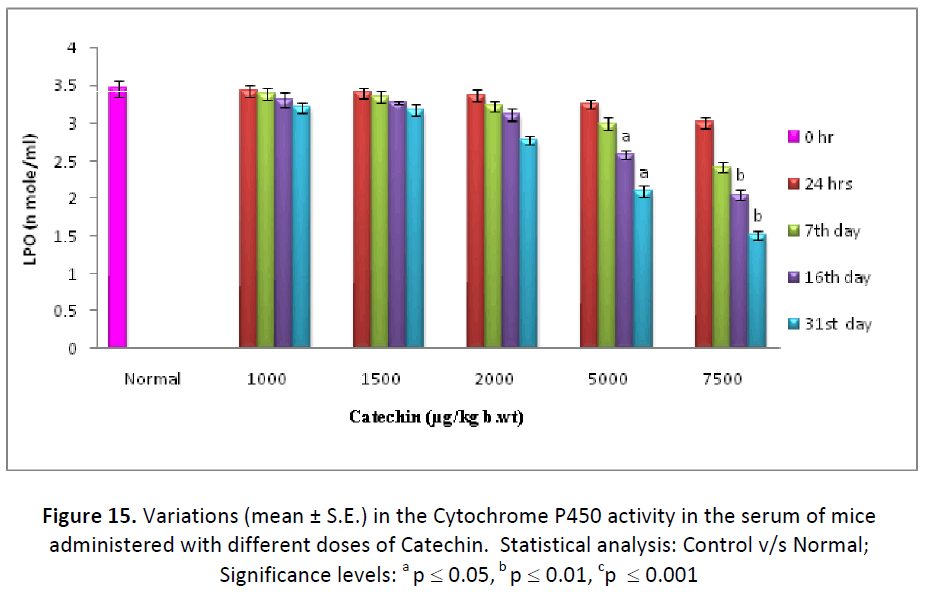
On contrary to GSH, hepatic, testicular and blood LPO levels decreased continuously from the beginning till the end of study after the different doses (1000, 1500, 2000, 5000, 7500 μg/kg. b. wt/day) of Catechin as compared to DDW administered ones. The concentration of LPO was measured in the form TBARS and it was found to be lowest after 31 days of Catechin treatment (7500 μg). Such levels were found to be declined from 52.80 % (24 hr) to 38.78 % (31st day) in liver, 92.02% (24 hr) to 76.07% (31st day) in testes and 95.94 % (24 hr) to 84.89 % (31st day) in blood. (Fig. 13- 15).
On the basis of the above results, dose of Catechin i.e. 7500 μg/kg b wt. /day were found as the optimum dose against Cd intoxication in Swiss albino mice. The highest activity of Phase I & Phase II enzymes as well as antioxidant parameters and the lowest level of lipid per-oxidation were measured at this particular dose, therefore, it was considered as positive optimum dose.
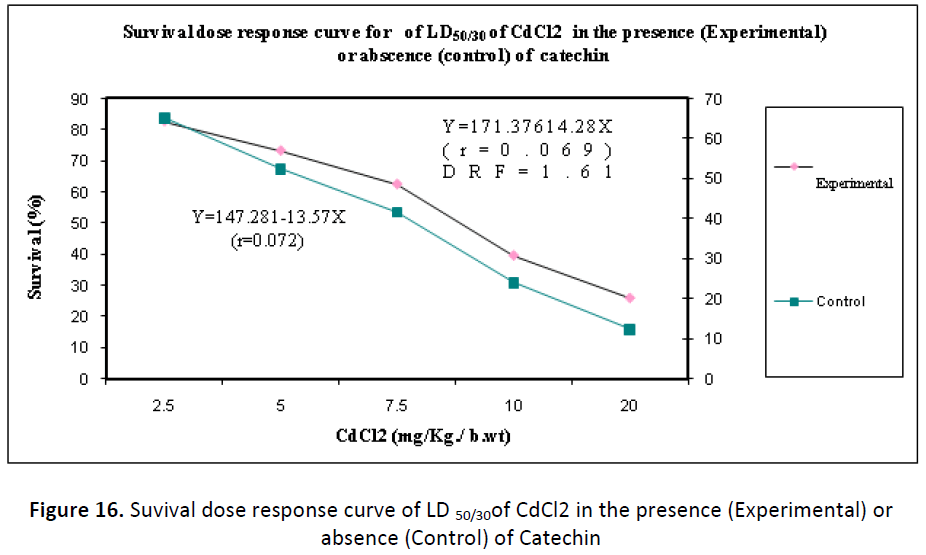
LD 50/30 & Dose reduction factor (DRF)
In order to establish the survival dose response of Swiss albino mice to cadmium chloride intoxication, in the presence or absence of Catechin, the following two groups of animals were used:
(i) Animals of one group (n= 10 for each dose) were administered with DDW before intoxication of CdCl2 with different dose (2.5, 5.0, 7.5, 10 or 20 mg/Kg./ b.wt) to serve as control. The animals (n= for each dose) of another group (experimental) were administered with optimum dose of Catechin (7500 μg/kg b.wt/day) once orally for 15 consecutive days and then on 15th day, they were intoxicated to CdCl2 (dose simililar to group I) after 30 minutes of the Catechin administration.
(ii) Animals of the other group, which were intoxicated to cadmium chloride showed 65.24, 52.40, 41.66, 24.00 & 12.30 percent survival up to 30 days with 2.5, 5.0, 7.5, 10 and 20 mg/Kg./b. wt of cadmium chloride respectively; while in Catechin pretreated animals, survival was noted to be significantly increased and found as 82.63, 73.20, 62.46, 39.62 and 26.00 %. When survival data were fitted on regression line equation, then LD50/30 values for CdCl2 treated animals (control) and for Catechin treated animals (experimental) were determined, and the calculation of dose reduction factor (DRF) was computed as 1.61 (Fig.16).
DISCUSSION
Various phytonutrients are known to be good antioxidants that can inhibit the propagation of free radical reactions and bring to an end the development of various degenerative diseases. Any natural compound with anti- oxidant properties may help in maintaining health when continuously taken as components of dietary foods, spices or drugs [23]. Now- a-days, tea is considered as a source of dietary constituents endowed with biological and pharmacological activities with potential benefits to human health. The present study thus investigates the induction of the activities of hepatic, testicular and hematopoietic detoxification enzymes system and antioxidant enzyme profiles in mice by Catechin extract against oxidative damage and variations in survival rate induced by cadmium chloride.
The results from the present study indicate that pre-treatment of green tea Catechin protects the mice from the deleterious effects of CdCl2. The metallotoxic effect of Catechin has been demonstrated by the increased body weight and survival rate. A significant protection was achieved when Catechin was given orally at the dose of 7500 μ gm/kg b. wt/ day for 15 consecutive days prior to cadmium intoxication. Its protective effect was demonstrated by determination of LD50/30. In the present study, a significant and dose dependent decrease in survival rate was evident in Cadmium-intoxicated control animals in 30 day survival assay, however, Catechin pre-treated intoxicated animals showed a significant and gradual recovery in survival rate within 30 days of intoxication. In toxicological studies, body, organ and relative organ weights are important criteria for evaluation of organ toxicity [24,25]. A dose dependent body weight gain in mice was recorded till the end of experiment at all the treated dose of Catechin in the present experiment. Such improvement in survival rate and body weight against CdCl2 intoxication may be due to antioxidant activities of green tea Catechin which binds with Cd ions to form an insoluble complex– ionic salt that was used to remove Cd and restrict the interaction of the metal ion with membrane lipids, thus avoiding oxidative damage to membrane lipids and proteins as also suggested by Paul, (2008) [15].
Protection provided by Phase I and Phase II enzymes against several metals may be the initiation of antioxidant enzymes which assist their degradation from the body [26]. The findings of the present study demonstrates that administration of the Catechin extract at all the dose levels (1000, 1500, 2000, 5000, 7500μg/k.g./animal/day) for 15 days have elevated the levels of hepatic as well as testicular Cytochrome b5, Cytochrome P450, glutathione-S- transferase and DT-diaphorase significantly in a dose and time dependent manner. Such effects elucidate that Catechin serves as bifunctional inducer as it provokes both Phase- I and Phase- II system enzymes. Cytochrome P450 is the major component of Cytochrome P450 system and its induction/inhibition is analogous to induction/ inhibition of Phase I system. Cytochrome b5, Cytochrome P450 reductase, and Cytochrome b5 reductase function in a synergistic manner, and allow the appropriate functioning of the Cytochrome P450 system.
The exact mechanism underlying the synchronized elevation of Phase I and Phase II enzyme system by the Catechin extract has not been entirely understood, it may be inferred that it may have acted as a “blocking agent” and increased the sequential reduction of xenobiotic substrates preparing it for Phase- II metabolism. Such variations in Phase I and Phase II enzyme systems by green tea extract are consistent with the results of Dasgupta et al, (2003) [27], who also recorded similar mode of variation by using Henna leaf (Lawsonia inermis) against cellular oxidative stress.
The cellular antioxidant status determines the susceptibility to oxidative damage primarily occurs through production of reactive oxygen species and is usually altered in response to oxidative stress. Lipid per oxidation in biological membranes causes impairment of membrane functioning decreased fluidity, inactivation of membrane- bound receptors and enzymes as well as increased non-specific permeability to ions [28]. Catechin is a polyphenolic antioxidative, therefore, it reduce the level of tissue MDA in the present study by inhibiting lipid peroxidation caused by free radicals. This observation is in conformation with Bursill et al, (2007) [29] and Rahman El- Shahat et al, (2009) [30].
Glutathione is a powerful intracellular antioxidant and its levels in the cells are dependent upon the rates of biosynthesis and utilization in oxidation and/or reduction reactions [31]. In the present study, increased level of GSH in the testes of mice that consumed the green tea Catechin supplement may be as a result of a cascade involving endogenous antioxidants which react differently according to their polarity and redox potential as also suggested by the others also [31,32]. Polyphenols have the potential to upregulate the expression of ß glutamylcysteine synthetase, the rate limiting enzyme in the biosynthesis of GSH and this may explain the increase of GSH level in the experimented animals [33]. These findings are in accordance with TÜrk et al, (2008) [34] and Khan & Ahmed, (2009) [35] who also reported an increase in the GSH level in the testes of rats treated with pomegranate juice and Digera muricata, respectively, which are rich in flavonoid and polyphenolic compounds.
The protective system includes chain-breaking antioxidants capable of reducing oxidant radical levels and blunting the propagation of free radical chain reactions [36]. Phytochemicals are known for inducing Phase II antioxidant enzymes and thereby increasing the synthesis of antioxidants and detoxification enzymes and major cellular antioxidants [37]. The exact intracellular mechanisms mediating the induction of antioxidant enzymes by tea flavonoids are not known. In the present study, anti- oxidative mechanisms of catechin can include: (i) suppressing ROS formation either by inhibition of enzymes or chelating trace elements involved in free radical production; (ii) scavenging reactive oxygen species; and (iii) up regulating and protecting antioxidant defenses and (iii) suppressed lipid peroxidation and /protected the cell membrane from oxidative damage. As also suggested by (Halliwell et al, 1999; Ola-Mudathir et al, 2008 & Suresh et al, 2010) [38-40].
The alterations in the levels of the enzymes in the present study may be due to the damage and dysfunction of the hepatic, hematopoietic and testicular tissues due to free radicals generated in biochemical reactions. Catechin showed protective effects on the above studied tissues, by up regulating the Phase I & II enzyme and GSH level along with the down regulating the level of LPO near to control level. Green tea has been found to aid in heavy metal detoxification by inhibiting its absorption and promoting excretion. The increase in the levels of biotransformation enzymes and antioxidant profiles by Catechin may be attributed to have biological significance in eliminating reactive free radicals that may affect the normal functioning of cells.
CONCLUSIONS
The findings of present investigation conclude that green tea Catechin treatment reduces cadmium- induced sickness, mortality and oxidative stress in mice by virtue of its antioxidant properties for improving the structural integrity of cell membranes and alleviating the biochemical perturbations.
Conflict of interests
The authors declare that there is no conflict of interest regarding the publication of this paper.
ACKNOWLEDGEMENTS
Authors are grateful to Indian Council of Medical Research (ICMR), New Delhi, India for financial assistance in the form of the research project (Ref No. 5/10/FR/5/2010/RHN) sanctioned to Prof. P.K. Goyal.
REFERENCES
- Razinger J, Dermastia M, Koce JD, Zrimce Oxidative stress in duckweed (Lemna minor L.) caused by short-term cadmium exposure. Environ Pollut. 2008; 153:687- 694.
- Waisberg M, Joseph P, Hale H, Beyersmann D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology. 2003; 192:95-117.
- Yu H N, Shen S R, Yin J J. Effects of metal ions, catechins, and their interactions on prostate cancer. Crit. Rev. Food Sci. Nutr. 2007; 47(8):711-9.
- Diana P H. Effect of green tea polyphenols on cadmium toxicity in Coenorhabaditis elegons. NCDR, Poster session, No. 2, 2008.
- Oteiza P, Adonaylo V N, Keen C L. Cadmium induced testes oxidative damage in rats can be influenced by dietary zinc intake. Toxicology. 1999; 137(1):13-22.
- R. C. Patra, Amiya K. Rautray, D. Swarup. Oxidative Stress in Lead and Cadmium Toxicity and Its Amelioration. Veterinary Medicine International; 2011, 9: doi:10. 4061/2011/457327.
- Maines MD. Characterization of hame oxygenase activity in Leydig and Sertoli cells of the rat testes: differential distribution of activity and response to cadmium. Biochemical Pharmacology. 1984; 33:1493- 14502.
- Lafuente A, Marquez N, Perez-Lorenzo M, Pazo D, Esquifino AI. Pubertal and postpubertal cadmium exposure differentially affects the hypothalamic- pituitary-testicular axis function in the rat. Food Chem Toxicol. 2000; 38:913–923.
- Siu ER, Mruk D.D., Porto CS, Cheng CY. Cadmium- induced Testicular Injury. Toxicol Appl Pharmacol. 2009; 238(3):240– 249.
- Yadav RK. Possible role of calcium antagonists in modifying brain lesions induced by heavy metal and ionizing radiation in mice. 2003, A Ph.D. thesis submitted to the University of Rajasthan, Jaipur, India.
- Hsu CY. Antioxidant activity of extract from Polygonum aviculare. L. Biol Res. 2006; 39:281-88.
- Steele V, Kelloff G, Balentine D, Boone C, Metha R, Bagheri D, Sigman C, Zhu S, Sharma S (2000): Comparative chemopreventive mechanisms of green tea, black tea and selected polyphenol extracts measured by in vitro bioassays. Carcinogenesis. 2000; 21:63-67.
- Kim J H, Kang B H, Jeong J M. Antioxidant antimutagenic and chemopreventive activities of a phyto- extract mixture derived from various vegetables, fruits and oriental herbs. Food Science and Biotechnology. 2003; 12:631-638.
- Jung Yd, Ellis Lm. Inhibition of tumour invasion and angiogenesis by epigallocatechin gallate (EGCG), a major component of green tea. Int. J Exp Pathol. 2001; 82:309-16.
- Paul, D. H. Effect of green tea polyphenols on cadmium toxicity in rats. Dominican Edu J. 2008; 6(2):1-10.
- Paglia DE, Valentine WM. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 70:158–169, 1967.
- Omura T, Sato R. The carbon monoxide binding pigment of liver. J Biol Chem. 1964; 239:2370–2378.
- Habig WH, Pabst MJ, Jokoby WB. Glutathione S-transferases – the first step in mercapturic and formation. J Biol Chem. 1974; 249:7130–713.
- Ernest L, Danielson L, Ljunggren M. DT- diaphorase- purification from the soluble fraction of rat liver cytoplasm. Biochem Biophys Acta. 1962; 58:171–188.
- Moron MA, Depierre, J.W.; Mannervick, B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochimica et Biophysica Acta. 1979, 582, 67-78.
- Beutler E, Duron O, Kelly BM. Improved method for the determination blood glutathione. J Lab Clin Med. 1963; 61:582- 888.
- Ohkhawa H, Ohishi N, Yogi K. Assay for lipid peroxidation in animal tissue by thiobarbituric acid reaction. Analytical Biochemistry. 1979; 95:351-358.
- Singh RP, Padmanathi B, Rao AR. Modulatory influence of Adhatoda vesica (Justicia adhatoda) leaf extract on the enzymes of xenobiotic metabolism antioxidant status and lipid peroxidation in mice. Mol Cell Biochem. 2000; 213, 99-109.
- Heikal TM, Ghanem HZ, Soliman MS. Protective effect of green tea extracts against dimethoate induced DNA damage and oxidant/antioxidant status in male rats. Biohealth Science Bulletin. 2011; 3(1):1– 11.
- Crissman JW, Goodman DG, Hildebrandt PK, Maronpot RR, Prater DA, Riley JH, et al. Best practice guideline. Toxicologic histopathology. 2004; 32:126–131.
- Miller EC. Some current perspectives on chemical carcinogenesis in humans and experimental animals. Cancer Res. 1998; 38:1479-96.
- Dasgupta T, Rao AR, Yadava PK. Modulatory effect of Henna leaf (Lawsonia inermis) on drug metabolising phase I and phase II enzymes, antioxidant enzymes, lipid peroxidation and chemically induced skin and forestomach papillomagenesis in mice. Molecular and Cellular Biochemistry. 2003; 245:11–22.
- Mayer C, Schmezer P, Freese R, Mutanen M, Hietanen E, Obe G, Base S, Bartsch H. Lipid peroxidation status, somatic mutations and micronuclei in peripheral lymphocytes: A case observation on a possible interrelationship. Cancer Lett. 2000; 152:169–173.
- Bursill CA, Abbey M, Roach PD: A green tea extract lowers plasma cholesterol by inhibiting cholesterol synthesis and upregulating the LDL receptor in the cholesterol-fed rabbit. Atherosclerosis. 2007; 193:86-93.
- Shahat AR, Gabr A, Meki AR, Mehana S. Altered Testicular Morphology and Oxidative Stress Induced by Cadmium in Experimental Rats and Protective Effect of Simultaneous Green Tea Extract. Int J Morphol. 2009; 27(3):757-764.
- Wild AC, Mulcahy RT. Regulation of gamma-glutamylcysteine synthetase subunit gene expression: insights into transcriptional control of antioxidant defenses. Free Radic Res. 2000; 32:281-301.
- Pietta, P G. Flavonoids as antioxidants. Journal of Natural Products. 2000; 63:1035- 1042.
- Moskaug JO, Carlsen H, Myhrstad MCW, Blomhoff R. Polyphenols and glutathione synthesis regulation. Am J Clin Nutr. 2005; 81:277-283.
- TÜrk G, Sönmez M, Aydin M, Yüce A, Gür S, Yüksel M. Effects of pomegranate juice consumption on sperm quality, spermatogenic cell density, antioxidant activity and testosterone level in male rats. Clin Nutr. 2008; 27:289-296.
- Khan MR, Ahmed D (2009). Protective effects of Digera muricata (L.) Mart. on testis against oxidative stress of carbon tetrachloride in rat. Food Chem Toxicol. 2009; 47:1393-1399.
- Heikal TM, Mossa AT, Rasoul MA, Marei GK. The ameliorating effects of green tea extract against cyromazine and chlorpyrifos induced liver toxicity in male rats. Asian Journal of Pharmaceutical and Clinical Research. 2013; 6 (1):48-55.
- Rebrin I, Zicker S, Wedekind KJ, Paetau- Robinson I, Packer L, Sohal RS. Effect of antioxidant- enriched diets on glutathione redox status in tissue homogenates and mitochondria of the senescence-accelerated mouse. Free Radical Bio Med. 2005; 39:549-557.
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 3rd ed. Oxford: Oxford University Press: 1999. pp. 1059- 1094.
- Ola-Mudathir KF, Suru SM, Fafunso MA, Obioha UE, Faremi TY. Protective roles of onion and garlic extracts on cadmium- induced changes in sperm characteristics and testicular oxidative damage in rats. Food Chem Toxicol. 2008; 46:3604-3611.
- Suresh S, Prithiviraj E, Prakash S: Effect of Mucuna pruriens on oxidative stress mediated damage in aged rat sperm. Int J Androl. 2010; 33:22-32.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences