Antimicrobial Activity of Lactic Acid Bacteria Isolated from 'Pupuru': An African Fermented Staple against Food Borne- Pathogens
Food and Industrial Microbiology Unit, Department of Microbiology, Obafemi Awolowo University, Ile-Ife, Nigeria
- *Corresponding Author:
- Adeyemo SM
Food and Industrial Microbiology Unit
Department of Microbiology, Obafemi Awolowo University
Ile-Ife, Nigeria
E-mail: adeyemostella@gmail.com
Received date: July 2, 2018; Accepted date: September 11, 2018; Published date: September 21, 2018
Citation: Adeyemo SM, Agun TF, Ogunlusi ED (2018) Antimicrobial Activity of Lactic Acid Bacteria Isolated from ‘Pupuru’: An African Fermented Staple against Food Borne- Pathogens. J Mol Biol Biotech Vol.3: No.1:5
Abstract
Lactic acid bacteria (LAB) have been found to produce antimicrobial agents that contribute to inhibition of pathogens and spoilage microorganism in food. This research aims at assessing and determining the antimicrobial substances produced by LAB isolated from ‘pupuru’ against food borne pathogens. Samples of ‘pupuru’ were collected from Ondo State, Nigeria and different species of LAB were isolated from them. LAB were identified using standard morphological and biochemical tests. They were tested against pathogenic organisms using the Agar well diffusion method. Antibacterial substances produced were also monitored for five days.
A total of 18 LAB were isolated and identified as L. casei, L. delbrueckii, L. brevis, L. plantarum, L.pentosus, L. fermentum and L. acidophilus. Production of antimicrobial substances; Lactic acid, Diacetyl and Hydrogen peroxide ranges from (0.010–0.0196 g/L). The highest yield was obtained from L. plantarum and L. acidophilus; the least being L. pentosus. Reduction in pH was observed in the fermenting cassava from 5.8–3.5 on day five.
LAB species from ‘pupuru’ had a considerable inhibitory effect on food borne pathogens Four species were tested for antagonistic activity and L. plantarum showed the highest zone of inhibition on Staphylococcus aureus (24 mm) and Pseudomonas aeruginosa (20 mm) respectively while the antimicrobial activity decreases with time. The antimicrobial activities of LAB were shown to significantly (p<0.05) compare with standard antibiotics used as control.
LAB demonstrated high antimicrobial properties against food borne pathogens. This potential can be employed as starters in food industry to inhibit food spoilage microbes and contaminants.
Keywords
Lactic acid bacteria; Antimicrobial properties; Pupuru; Food spoilage microorganisms; Fermented foods
Introduction
Antibiotic drug resistance in pathogenic microorganism has developed due to indiscriminate use of commonly prescribed antibiotics. This has forced scientists to carry out research for new antimicrobial component present in microorganisms isolated from fermented foods [1-3].
Food contamination, food poisoning and food borne- illnesses is a major public health concern in developing countries, hence the awareness about food safety and hygiene has increased over the years. The awareness about the roles being played by probiotic organisms’ especially lactic acid bacteria and the bioactive compounds they produce in fermented foods has also increased over the years.
Lactic acid bacteria (LAB) on the other hand display numerous antimicrobial activities in the fermented foods. This is mainly due to the production of bacteriocins, organic acids, ethanol, Hydrogen peroxide, Diacetyl and reuterin. Lactic acid bacteria play an important role in food industry by increasing nutritional values of food and food safety [4,5]. The antimicrobials produced by LAB have been used widely as bio-preservatives and shelf life extender and has found application in many industries and various commercial purposes.
Pupuru’ is a fermented cassava product that is consumed by at least 4-6 million people in Nigeria and more in some African countries [6]. Cassava is usually processed into various forms in order to increase the shelf life of the products, facilitate transportation and marketing, reduce cyanide content and improved palatability [7]. One of such processed form of cassava is ‘pupuru’.
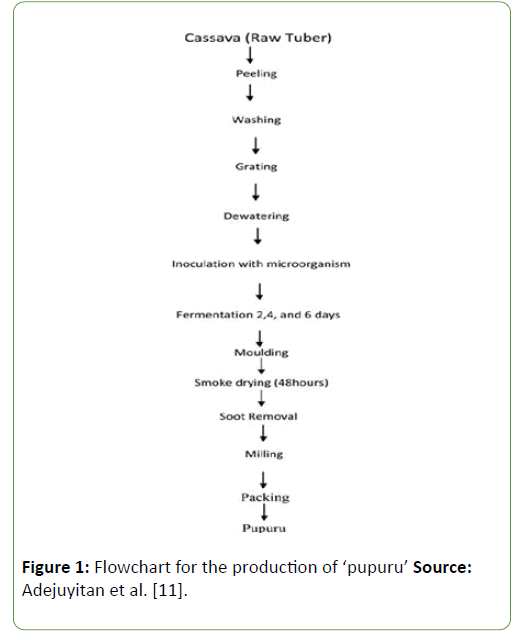
Locally, rural women process cassava into ‘pupuru’ by steeping peeled cassava tubers in stream water and fermenting it for 4-6 days. This method of processing does have some side effects such as fouling the water and also increasing the level of microbial contaminants in the fermented cassava product. Hence there is need to allow complete fermentation of the product by LAB [8]. ‘Pupuru’ can be classified as a functional food which contains LAB that has probiotic and antimicrobial properties. Evidence suggests that functional foods derived from the fermentation of such foods with probiotic strains could prevent infectious diseases in man [2,9-11]. The aim of this work therefore is to assess and determining the antimicrobial substances produced by LAB isolated from ‘pupuru’ against food borne pathogens. The flow chart representing the production of ‘pupuru’ is shown below:
Materials and Methods
Samples of ‘pupuru’ were collected in a sterile nylon from Ondo town in Ondo state, Nigeria. Thereafter the ‘pupuru’ were transported into the laboratory and therefore homogenized using a stomacher at room temperature. After homogenization, a tenfold serial dilution using maximum recovery diluent (MRD) was carried out.
Isolation of the LAB from the samples was done using De man Rogosa and Sharpe (MRS) Agar using Pour plate technique. Samples were incubated at 30oC for 48 hrs using an anaerobic jar [12]. Isolation process and growth of LAB was directly observed in different forms in terms of shape, size, opacity, elevation, surface, edge, consistency and the pure isolates were subjected to different biochemical tests.
Identification of lactic acid bacteria
.Lactic acid bacteria were characterized by Gram staining (positive) and catalase negative reaction using 3% hydrogen peroxide. The morphology and other biochemical tests for the Identification of Lactic acid Bacteria were also carried out. The organisms were identified using Bergey’s Manual of Determinative Bacteriology [13].
Determination of lactic acid produced by the isolates
Estimation of lactic acid was determined by titration of 25 ml of broth cultures of the test organism (24 h old) with 0.1N NaOH with three drops of phenolphthalein (1% w/v) as indicator. The NaOH solution was added slowly to the sample until a pink colour appeared. Each ml of 0.1 N NaOH is equivalent to 90.08 mg of lactic acid (Association of Official Analytical Chemist Methods, 2000). Lactic Acid was calculated using the formula below:
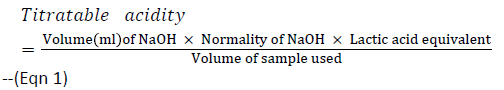
Normality of NaOH=0.1
Lactic acid equivalent = 90.08 mg
Volume of sample used = 25 mL
Determination of diacetyl produced by the lab isolates
The amount of diacetyl produced by the LAB isolates was estimated following the method of [14].
To 25 ml of 24 hour old MRS broth culture of the test isolates in conical flasks was added 7.5 ml hydroxyl amine (0.1M) solution which served as substrate for residual titration. The content of the flasks were then titrated with 0.1N HCl to a green-yellow end-point using bromophenol blue as indicator. Each 1.00 ml of 0.1N HCl is equivalent to 21.52 mg of diacetyl (AOAC Methods, 2000). Diacetyl was calculated using the formula below:
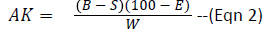
AK=Percentage of diacetyl
B=ml of 0.1N HCl consumed in titration of the sample
E=Equivalent factor of 1 mL of 0.1N HCl to diacetyl=21.52 mg
W=Volume of sample used
S=Volume of ml 0.1N HCl consumed in titration of 7.5 mL Hydroxyl amine.
Determination of hydrogen peroxide production by lab isolates
Twenty millilitres of 0.1 M H2SO4 was added to 25 mL of the 24 h old MRS broth cultures of the test isolates. Titration was carried out with 0.1N potassium permanganate. Each ml of 0.1M H2SO4 is equivalent to 1.70 mg of Hydrogen peroxide and decolourization of the sample was regarded as end point [14]. Hydrogen peroxide concentration was calculated using the formula below:
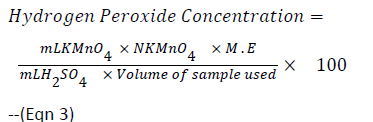
ml KMnO4=Volume of KMNO4 used
N KMNO4=Normality of KMNO4
ml H2SO4=Volume of H2SO4 added
M.E=Equivalence factor of H2O2=1.701 mg
Determination of pH
The pH of the fermenting cassava was measured at 24 hrs intervals using an electronic digital pH meter (HANNA INSTRUMENT 8021). Ten ml of the fermenting water was poured into a beaker and a pH meter was used to take the readings [14].
Standardization of test isolates
The LAB isolated were tested on standard culture of Gram positive and Gram negative bacteria obtained from National Center for Biotechnology Information (NCBI). S. aureus and P. aeruginosa from contaminated food sources were stored at the culture collection unit of Microbiology Department, Obafemi Awolowo University, Ile-Ife, Osun State. S. aureus and P. aeruginosa respectively were standardized to 0.5 McFarland standard at a wavelength of 600 nm and 3.0 × 105 cfu/ml using a colorimeter [15].
Antimicrobial assay
Antimicrobial assay was carried out using the Agar well diffusion method. Petri dishes were prepared with 20 ml of sterile Mueller Hinton Agar (MHA). The test culture (1.00 ml) was swabbed on the top of solidified media and allowed to dry for 10 mins. About 6mm diameter holes were made into the Agar gel using a cork borer, 1.00 ml of cell free supernatant which was concentrated using a rotary evaporator after centrifugation was introduced into the well. The plates were incubated for 24 h at 37oC under aerobic conditions. After incubation, confluent growth of bacteria was observed and inhibition of growth was measured in mm. A control experiment was set up using streptomycin at 25 mg/ml.
Results and Discussion
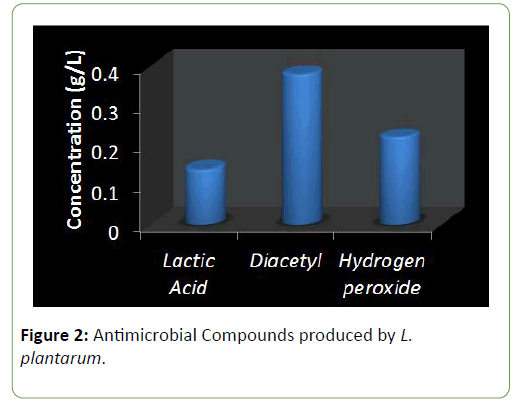
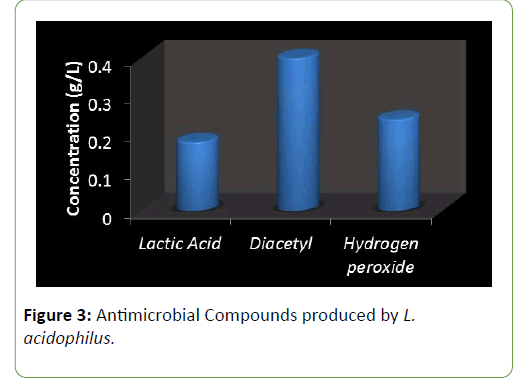
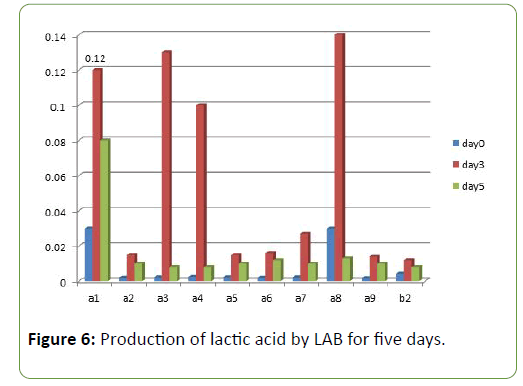
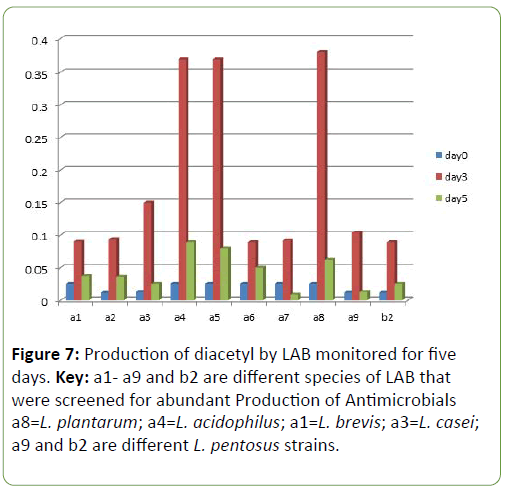
Results and Discussion There was a reduction in the pH of the fermenting cassava from 5.8 to 3.5 during fermentation of cassava for the production of ‘pupuru’ (Table 1). Production of antimicrobial agents such as Lactic acid, Diacetyl and Hydrogen peroxide ranges from (0.010–0.22 g/L). L. plantarum was observed to produce the highest antimicrobial compounds of lactic acid, diacetyl, and hydrogen peroxide (0.14, 0.38, 0.22 g/L) respectively (Figures 1 and 2), the least being L. pentosus (0.0019, 0.10, 0.129 g/L).
Figure 1: Flowchart for the production of ‘pupuru’ Source: Adejuyitan et al. [11].
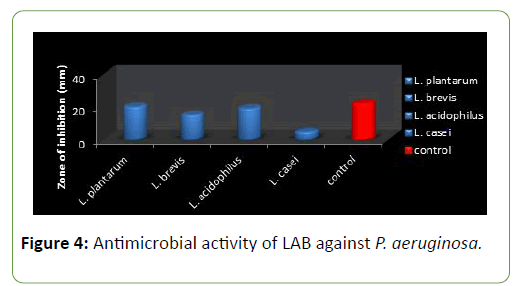
Four species of LAB were tested for antagonistic activity namely L.plantarum, L. acidophilus, L. brevis and L. casei, and and all showed zones of inhibition. For P. aeruginosa (20 mm, 15 mm, 19 mm and 4.5 mm respectively) (Figure 3) and S. aureus (22, 16 mm, 19 mm and 5 mm) (Figure 4) respectively. L. plantarum showed the highest zone of inhibition while the antimicrobial activity decreased with time. The antimicrobial activity of LAB competes favourably with standard antibiotics used as control. The production of lactic acid and Diacetyl by LAB was also monitored over a period of five days (Figures 4-7).
Discussion
‘Pupuru’ is a fermented cassava product that is consumed by some people in Nigeria and more in some African countries [6]. Cassava is usually processed into various forms in order to increase the shelf life of the products, reduce cyanide and antinutritional content and improve nutrient composition, flavour and palatability [7].
This research work revealed the antimicrobial compounds present in strains of LAB isolated from ‘Pupuru’ and its antimicrobial effect against some bacterial isolates from food sources. All the lactic acid bacteria isolated from ‘Pupuru’ had
The entire LAB isolated during this research produced lactic acid, diacetyl and hydrogen peroxide at varying degrees. This observation is in accordance with the report of Ogunbanwo et al. [17] who reported that LAB produced the antimicrobial bacteriocin against some pathogens. Their importance is associated mainly with their safe metabolic activity while growing in foods and utilizing available sugar for the production of organic acids and other metabolites. Their common occurrence in foods and feeds coupled with their long-lived use contributes to their natural acceptance as GRAS (Generally Recognized as Safe) for human consumption [3,9].
Only three out of the LAB were used because a form of screening was carried out using some technological properties such as production of metabolites by the selected LAB strains and production of antimicrobials against selected pathogens and enzyme production in abundance. The other three organisms did not produce these metabolites significantly when compared to the other three isolates that were chosen. These were used as the basis for selection and screening out the other three isolates.
The production of antimicrobial compounds by these LAB strains implies that they can serve as a source of novel preservative products in food industries. This is in agreement with an earlier report by Shao-Bin et al. [18] and Kaskokiene et al. [2]. Considering the increasing importance of LAB as antibiotics alternative, the knowledge of the antimicrobial activity of LAB species especially L. plantarum and L. acidophilus in particular is of high significance. The antimicrobial activity of L. plantarum and L. acidophilus shows that it can be used as a food preservative to reduce contaminants in food. There are three mechanisms that could explain the antimicrobial activity of LAB especially L. plantarum and L. acidophilus; the production of bacteriocins; the yield of organic acids and other inhibitory substances such as ethanol, carbon dioxide and hydrogen peroxide; and the competition for nutrients [3,19]. These cannot be overemphasized.
The LAB strains isolated were tested against food pathogens and the zones of inhibition were observed. L. plantarum showed the highest zones of inhibition on Gram positive and Gram negative bacteria from food sources this was followed by L. acidophilus. The report of these findings is similar to the work reported by Dal Bello et al. [20] and Rodríguez et al. [21] who reported that antimicrobial compounds such as phenyl-lactic acid and lactic acid were effective against many Gram-negative and Gram-positive pathogenic bacteria such as Escherichia coli, Proteus vulgaris, Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus subtilis, Enterococcus faecalis. The antagonistic activity of LAB metabolites against the spoilage bacteria also agrees with the findings of Vasiee et al. [22].
The inhibitory effect caused by L. plantarum and L. acidophilus can be considered that the LAB produced organic acids, especially lactic and acetic acids, exerting a strong inhibitory effect on Gram-negative and positive bacteria [3,23]. They stated further that the low pH observed during the fermentation of ‘pupuru’ may not be the sole reason for the observed inhibition effects. It could however be an important condition for the passage of organic acids through the membrane to the intracellular environment, where they will accumulate and exert inhibitory activity [24-26]. The lowering of the pH of fermented foods by LAB to below 4 through acid production inhibits the growth of pathogenic microorganisms which can cause food spoilage, food contamination and food poisoning. LAB because of their potential use as natural antimicrobial agents have been used to enhance the safety of food products. Most chemical preservatives used in processed foods have been found to contribute to health hazards among consumers when used in high doses [27]. Some preservatives have also been reported by Food and Drug Administration (FDA) to produce allergic reactions [27].
The increasing resistance of food spoilage microorganisms to current preservatives, the consumer’s high demand for safe, minimally processed foods and the hazards associated with the use of high doses of chemical preservatives has led to the need for finding safer alternatives in food preservation. The application of LAB with the simultaneous control of factors that affect microbial growth can help to minimize food spoilage. The selection and addition of novel isolates of LAB may be the key to reducing the use of chemical preservatives, enhancing/ improving nutrients and extending the shelf life of food products [3,10].
L. plantarum and L. acidophilus strains are effective against a variety of bacterial pathogens and some food –borne microorganisms; they can serve as alternative antimicrobial agents and food preservatives against the corresponding food borne pathogens. This potential can be harnessed by the food industries on a large scale as bio-preservatives instead of the chemical preservatives that we use which have some side effects.
Conclusion
The increase in food poisoning and contamination is a major challenge in developing countries. Food safety and hygiene can be enhanced by the development of new organic antimicrobial drugs from alternative and natural sources. Bioactive compounds from microorganisms especially LAB are a source of bio-preservative agents. Also, the increasing rate of antibiotic resistance and side effect of chemical and synthetic preservatives, calls for a need to redirect our minds to the use of probiotics as alternative remedy. Metabolites produced by LAB can be of probiotics and bio- preservative benefits.
The promising results of these study underline the important role that antimicrobial compounds obtained from lactic acid bacteria may play in food industry so as to improve food quality and safety; also it can serve as source of novel antimicrobial agents in food industries and natural bio-preservative compound.
References
- Mokoena MP, Mutanda T, Olaniran AO (2016) Perspectives on the probiotic potential of lactic acid bacteria from African traditional fermented foods and beverages. Food Nutr Res 60: 12-19.
- Kaskokiene V, Stankevicius M, Bimbiraite SK, Naujokaityte G, Serniene L, et al. (2017) Current state of purification, isolation and analysis of bacteriocins produced by lactic acid bacteria. Appl Microbiol Biotechnol 101: 1323-1335.
- Muruzović MŽ, Mladenović KG, Žugić-Petrović TD, Čomić LR (2018) Characterization of lactic acid bacteria isolated from traditionally made Serbian Cheese and evaluation of their antagonistic potential against Enterobacteriaceae. J Food Process Preserv 13: 57-61.
- Adeyemo SM, Onilude AA (2013) Enzymatic Reduction of Anti-nutritional factors in soybeans by Lactobacillus plantarum isolated from fermenting cereals. NIFOJ 31: 84-90.
- Adeyemo SM, Onilude AA (2014) Reduction of Oligosaccharide content of soybeans by the action of L. Plantarum isolated from fermented cereals. Afr J Biotechnol 13: 3790-3796.
- Odetokun SM, Aiyesanmi AF, Esuoso KO (2008) Enhancement of the nutritive value of pupuru, a fermented cassava product. La Rivista Italiana Delle Sostanze Grasse 75: 155-158.
- Oboh G, Akindahunsi AA, Oshodi (2002) Nutrient and anti-nutrient contents of Aspergillus niger fermented Cassava products (Flour and Gari). J Food Comp Anal 15: 617-622.
- Sawadogo-Lingani H, Lei V, Diawara B, Nielsen DS, Moller PL, et al. (2007) The biodiversity of predominant lactic acid bacteria in dolo and pito wort for production of sorghum beer. J Appl Microbiol 103: 765-777.
- Garneau S, Martin NI, Vederas JC (2002) Two-peptide bacteriocins produced by lactic acid bacteria. Biochimie 84: 577-592.
- Belal J.M, Zaiton H, Nazamid S (2013) Lactic Acid Bacteria in Bio-preservation and the Enhancement of the Functional Quality of Bread. Int J Food Microbiol 16: 77-113.
- Adejuyitan JA (2015) Influence of fermentation with Rhizopus species on the properties of selected cassava- based foods. Ph.D Thesis, Ladoke Akintola University of Technology, Ogbomoso, Nigeria.
- Cheesebrough M (2003) District Laboratory Practices in Tropical Countries. Cambridge University Press, Edinburgh, UK, pp. 382-407.
- Schillinger U, Lucke FK (1989) Antibacterial activity of Lactobacillus sake isolated from meat. Appl Environ Microbiol 55: 1901–1906.
- AOAC (2000) Official Methods of Analysis of the Association of Official Analytical Chemists, 25th edition AOAC, Washington DC.
- Andrews JM (2001) Determination of minimum inhibitory concentration. J Antimicrob Chemother 48: 5-16.
- Ogunbanwo ST, Sanni AI, Onilude AA (2003) Characterization of bacteriocin produced by Lactobacillus plantarum F1 and Lactobacillus brevis OG1. Afr J Biotechnol 2: 219-227.
- Ogunbanwo ST, Sanni AI, Onilude AA (2004) Effect of bacterocinogenic Lactobacillus spp on the shelf life of fufu, a traditional fermented cassava product. World J Microbiol Biotechnol 20: 57-63.
- Gu SB, Zhao LN, Wu Y, Li SC, Sun JR, et al. (2015) Potential probiotic attributes of a new strain of Bacillus coagulans CGMCC 9951 isolated from healthy piglet faeces. World J Microbiol Biotechnol 31: 851-863.
- Magnusson J, Ström K, Roos S, Sjögren J, Schnürer J (2003) Broad and complex antifungal activity among environmental isolates of lactic acid bacteria. FEMS Microbiol Lett 219: 129-135.
- Fooks LJ, Gibson GR (2002) Probiotics as modulators of the gut flora. Br J Nutr 88: 39–49.
- Rodríguez N, Salgado JM, Solana RR, Cortés S, Domínguez JM (2012) Biotechnological production of cell-free extract obtained by fermentation of cheese whey and phenylpyruvic acid using Lactobacillus plantarum and antimicrobial effect against pathogen bacteria. New Biotechnology 29: 59-65.
- Vasiee AR, Mortazavi A, Tabatabaei-yazdi F, Edalatian M (2018) Detection, identification and phylogenetic analysis of lactic acid bacteria isolated from Tarkhineh, Iranian fermented cereal product, by amplifying the 16s rRNA gene with universal primers and differentiation using rep-PCR. Int Food Res J 25: 423-432.
- Markas L, vuyst LD (2006) The in-vitro inhibition of Gram- negative pathogenic bacteria by bifidobacteria is caused by the production of organic acids. Int Dairy J 16: 1049- 1057.
- Fooks LJ, Gibson GR (2002) Probiotics as modulators of the gut flora. Br J Nutr 88: 39–49.
- Patel A, Shah N, Ambalam P, Prajapati JB, Holst O, et al. (2013) Antimicrobial profile of Lactic Acid Bacteria isolated from vegetables and indigenous fermented foods of India against clinical pathogens using micro dilution method. Biomed Environ Sci 26: 759-764.
- Puniya M, Sangu KPS, Bhardwaj A, Gupta D, Kumar S, et al. (2012) Probiotic and functional attributes of Lactobacillus spp. isolated from human faeces. Journal of Reseach in Anti microbials 1: 32-42.
- Badis A, Guetarni D, Boudjemaa BM, Henni DE, Tornadijo ME, et al. (2004) Identification of cultivable lactic acid bacteria isolated from Algerian raw goat’s milk and evaluation of their technological properties. Food Microbiol 21: 343-349.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences