Anaphylaxis After First Ingestion of Chapulines (Grasshopper) in Patients Allergic to House Dust Mite, Cockroach, and Crustaceans. Is Tropomyosin The Cause?
1University of California Irvine, Irvine, California, USA
2Indoor Biotechnologies, Inc., Charlottesville, Virgenia, USA
- *Corresponding Author:
- William N. Sokol
University of California Irvine, Irvine,
California, USA.
Tel: 9496511427
E-mail: wsokolallergy@aol.com
Received date: July 25, 2017; Accepted date: July 31, 2017; Published date: August 03, 2017
Citation: Sokol WN, Wünschmann S, Agah S (2017) Anaphylaxis After First Ingestion of Chapulines (Grasshopper) in Patients Allergic to House Dust Mite, Cockroach, and Crustaceans. Is Tropomyosin The Cause? Clin Immunol Infect Dis Vol. 1 No. 1:1.
Copyright: © 2017 Sokol WN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Two patients presented with a history of anaphylaxis (one with loss of consciousness, the other with laryngeal edema, urticaria, angioedema, and near syncope) immediately after eating chapuline from Oaxaca, Mexico.
Prick puncture testing to grasshopper antigen was 4+ in both patients and negative in five non-allergic controls. Both patients gave a prior history of urticaria/angioedema/laryngeal edema following ingestion of crustaceans. In vitro IgE specific antibodies to crustaceans, dust mites, and cockroach were positive in both patients. Total IgE was greater than 2000 IU/mL in one patient, and 92.6 IU/ mL in the other (nl<87 IU/mL). Tryptase levels in both patients were not elevated. Specific IgE inhibition studies reveal that grasshopper extract contains antigens capable of binding to patient's specific IgE to crustaceans, cockroach, and mites, indicating the presence of a cross reacting pan-allergen in grasshopper extract. Immunoblot analysis of the grasshopper extract revealed the presence of a 30 kD molecular weight protein in grasshopper and chapuline and a 38 kD molecular weight protein in shrimp, which bound patient-specific IgE antibody. Western Blot analysis of the extract probed with anti-tropomyosin antibody revealed those antigens to be tropomyosin.
Although previous reports in the literature of allergic rhinoconjunctivitis, contact urticaria, and asthma after inhalation of grasshopper are well known, this is the first well-documented report of anaphylaxis following ingestion of grasshoppers. Ingestion of insects is very popular in Asia, the Middle East, South and Central America, and particularly in Mexico and in southern California. The purpose of this report is to alert the medical community and the public to the fact that there is an increased risk of allergic reactions to the ingestion of grasshoppers in patients with a prior history of crustacean, house dust mite, and/or cockroach allergy.
Keywords
Grasshoppers freeze dried-Locusta migratoria; Cricket freeze dried- Acheta domesticus; Chapulines-Sphenarium mexicanum; sIgE-ImmunoCAP specific antibody; ImmunoCAP specific IgE; Fluorescent enzyme immunoassay
Abbreviation
GHFD: Grasshoppers Freeze Dried; CFD: Cricket Freeze Dried; FEIA: Fluorescent Enzyme Immunoassay; IgE: Immunoglobulin E; SDS-PAGE: Sodium Dodecyl Sulfate-Polyacrylamine Gel Electrophoresis; HDM: House Dust Mite; OAS: Oral Allergy Syndrome; w/v: Weight per Volume
Introduction
Grasshoppers are highly allergenic insects, which have been associated with allergic rhino-conjunctivitis, contact urticaria, and bronchial asthma after inhalation, contact, or high-level exposure in laboratory workers, hobbyists, from natural exposure, or from ingestion [1]. Although allergy from inhalation of grasshoppers is limited, the ingestion of grasshoppers is extremely common. Eighty percent of the countries in the world ingest over 1700 different insects, of which the most common are grasshoppers and cricket [2]. In Mexico, grasshoppers called chapulines from Oaxaca have been eaten since pre-Columbian times and today are served in restaurants and open markets throughout the country. Chapulines, plural for Chapuline, are grasshoppers of the genus Sphenarium mexicanum. The term is specific to Mexico and derives from the Nahuatl word 'chapulin'3. Southern California is known as the chapuline capital of North America [3] with numerous restaurants serving the insects in various forms. Despite this combination of high allergenicity and widespread ingestion of grasshopper, a search of the literature revealed no previous well-documented reports of anaphylaxis caused by the ingestion of grasshopper.
Within the last year, two patients presented to our southern California allergy clinic with a recent history of anaphylactic reactions after the consumption of chapulines, despite no previous occupational or natural exposure to chapulines and no history of having ever eaten chapulines before. This report consists of our findings on these two patients and the nature of these allergen(s) involved.
Materials and Methods
Preparation of extract
Freeze-dried commercial grasshoppers (Locusta migratoria) and freeze-dried commercial cricket (Acheta domesticus) were purchased from Fluker's Lab; Port Allen, Louisiana. Freshly prepared Chapulines (Sphenarium mexicanum) were purchased from the restaurant in which Patient #2 ate. All insects were ground with mortar and pestle to a fine consistency. Then 1:10 w/v saline for in vitro testing and 1:10 w/v 50% glycerinated saline solutions for skin testing were prepared. Solutions were stored immediately at 4°C and periodically shaken over 24 hours. Solutions were centrifuged at 3400 rpm for 40 min. The supernatants were removed and immediately passed through sterile 0.22 microns syringe filters (SimSIL, Inc., Irvine, California), and the filtered supernatant injected into sterile 10 mL vials and stored at 4°C.
Skin testing
Skin testing was carried out after obtaining informed consent from both patients and five nonallergic controls. Antigens of grasshopper, chapulines, and cricket in 50% glycerine were prepared as previously described. Other allergens were purchased from Hollister-Stier Laboratories (Spokane, Washington). Histamine dihydrochloride 10 mg/mL Hollister-Stier Laboratories (Spokane, Washington), glycerinated saline 50% w/v, sterile saline was purchased from Stallergenes (Lenoir, North Carolina), ALK-Abello were purchased. The prick puncture technique consisted of placing droplets of antigens on the skin and pricking through the drops with a stainless-steel lancet (Hollister-Stier Laboratories, Spokane, WA). The results were interpreted at 20 min and at 24 hours. The wheal and flare reactions, if present, were outlined with a pen, and paper tape was placed over the reaction site. The paper tape was then transferred to a white sheet of paper for measurement. The reactions were measured in length and width and the average taken as the result.
ImmunoCAP specific IgE (sIge) inhibition by grasshopper extract
2.5 mL of serum from both patients was incubated for 24 hours at 4°C with A.) No additives. B.) 200 μL of buffered saline. C.) 200 μL of grasshopper freeze-dried 1:10 w/v in buffered saline. The sera were then analyzed for sIgE to cockroach, crab, shrimp, lobster, clam, D. pteronyssinus, D. farinae, cat, dog, corn, and avocado.
Immunoblotting technique
The detailed method for immunoblotting has been described earlier [4] Briefly, the various extracts prepared (chapuline, grasshopper, cockroach, and cricket), along with purified natural shrimp tropomyosin (Indoor Biotechnologies, Inc.) were run on 4-20% pre-cast sodium dodecyl sulfate-polyacrylamide (SDSPAGE) gels (Mini-ProteanR TGXTM and pre-cast gels, Bio-Rad) in reducing conditions. Then the separated extract polypeptides were transferred to a PVDF membrane (Bio-Rad). The Western Breeze Chromogenic Western Blot Immunization Detection Kit (Invitrogen/Life Technologies) was used for blocking, antibody dilution and detection steps. Patient's serum was diluted 1:2 in the antibody dilution solution provided by the kit (Hammarsten casein solution in buffered saline containing detergent). Monoclonal anti-human IgE alkaline phosphatase produced in mouse (Sigma-Aldrich) was used as a secondary antibody with a 1:1000 dilution. The blots were developed using a ready-to-use BCIP/NBT substrate solution for alkaline phosphatase (Invitrogen/ Life Technologies) at room temperature for 20-30 min.
Western blotting technique
For the Western blot, several dilutions of the prepared grasshopper extract (3-30 μg) and the purified shrimp tropomyosin (200 Ng-200 μg) (Indoor Biotechnologies, Inc.) were run on a 4-20% pre-cast gel as described above. The blot was exposed to 1:1000 dilution of the anti-tropomyosin mouse monoclonal antibody (Indoor Biotechnologies, Inc.) for 1 hour at room temperature. A ready-to-use solution (10 mL) of alkaline phosphatase conjugated affinity purified anti-mouse antibody developed in goat (Invitrogen/Life Technologies) was used as a secondary antibody. A ready-to-use BCIP/NBP substrate solution for alkaline phosphatase (Invitrogen/Life Technologies) was used for developing the blot as described above.
Case Reports
Patient #1 - S.V. is a 43-year-old male with active allergic rhinoconjunctivitis and moderately severe persistent bronchial asthma. On two occasions, in 2006 and 2011, S.V. experienced urticaria and angioedema of the lips and tongue after eating soft shell crab. In 2013, he ate roasted cricket and experienced urticaria, angioedema, and laryngeal edema. In December 2014 in Mexico City, he ate two servings of Chapulines served over a corn tortilla with guacamole and took a picture of the meal. Within 10 min, he experienced itching and swelling of the lips and the tongue, itchy skin followed by hives, chest tightness, and light headedness. Over the next 15 min after leaving the restaurant, according to his 10-year-old son who was with him, the patient lost consciousness three separate times, about 1 minute each time. The patient has no recall of these events. After the third episode, he stood up, developed abdominal pain, had a sudden urge to defecate, and had watery diarrhea. Following this, the patient was taken to a facility where he was treated by a physician with Benadryl (possibly cortisol) and fluids and recovered with disappearance of the urticaria and angioedema over the next 24-36 hours. There were no delayed reactions.
Patient #2 - G.S.L. is a 50-year-old female with active allergic rhino-conjunctivitis, bronchial asthma, intermittent urticaria, and moderately severe atopic dermatitis. The patient had experienced three separate episodes of urticaria, angioedema, and laryngeal edema after eating various crustaceans. In May 2016 at a restaurant in Orange County, California, she ate a serving of approximately 140 Chapulines cooked on a comal with lime, salt, and garlic. The patient had the immediate onset of itching of the mouth and throat, swelling of the face, lips and periocular tissue, generalized itching, throat swelling, difficulty swallowing, difficult speaking, light headedness, and near syncope. She was taken to Urgent Care and was treated with fluids, Benadryl, and cortisol and improved over the next 3-4 hours and had no subsequent delayed reaction.
Results
These severe anaphylactic reactions after ingestion of chapulines in patients who had never eaten or even had been previously exposed to grasshopper but who had a history of anaphylactic reactions to crustaceans raised several questions.
• Were chapulines the cause of the reaction?
• Did the patients have IgE antibody against grasshoppers and chapulines?
• If so, how did the patients become sensitized without prior exposure?
• Was a cross reacting antigen or pan-allergen present in grasshoppers, chapulines, HDM, and crustaceans?
• If so, what was the pan-allergen?
Skin testing
Extracts of grasshopper (Locusta migratoria) 1:10 w/v, and chapuline (Sphenarium mexicanum) 1:10 w/v in 50% glycerinated saline were prepared (see Methods section) and skin tested. Skin testing was then carried out after informed written consent. Both patients and five control nonallergic patients were skin tested by the prick puncture method (see Methods section). All patients and controls had positive histamine and negative saline and glycerine controls (Table 1). Both patients had 4+ reactions to grasshopper FD and chapuline extract, and lesser but still positive reactions to cricket. All five control patients showed no reaction to grasshopper, chapuline, cricket, or clams. Additional skin testing of both patients showed 4+ reactions to cockroach, Dermatophagoides pteronyssinus, Dermatophagoides farinae, crab, shrimp, lobster, cat, and dog. There were negative skin test reactions to avocado and corn.
| Substances | Strength | Case #1 SV w/f* |
Case #1 GSL w/f* |
|---|---|---|---|
| Histamine | 10 mg/mL | 15/35 | 10/28 |
| Saline | -- | 0/0 | 0/0 |
| Glyserine | 50% Glycerin in saline | 0/0 | 0/0 |
| Grasshopper(fD) | 1:10 w/v | 18/36 | 16/32 |
| Chapulines | 1:10 w/v | 16/32 | 23/38 |
| Cricket(FD) | 1:10 w/v | 12/38 | 6/20 |
| Clam | 1:10 w/v | 0/0 | 0/0 |
| Cockroach mix | 1:10 w/v | 12/26 | 10/20 |
| D. pteronyssinus | 10000 au/mL** | 10/25 | 20/43 |
| D. farina | 10000 au/mL | 3/12 | 22/26 |
| Crab | 1:10 w/v | 10/24 | 9/20 |
| Shrimp | 1:10 w/v | 4/28 | 10/15 |
| Lobster | 1:10 w/v | 3/28 | 8/11 |
| Cat | 10000 bau/mL*** | 22/38 | 18/24 |
| Dog | 1:100 w/v | 9/15 | 8/13 |
| Avocado | 1:10 w/v | 0/0 | 0/0 |
| Corn | 1:10 w/v | 0/0 | 0/0 |
*Wheel/ Flare in millimeter
**Allergy units/milliliter
***Bioequivalent allergy units/Milliliter
Table 1: Allergy skin testing.
ImmunoCAP sIgE inhibition
sIgE and total IgE were measured in both patients (Table 2). Patient S.V. had a total IgE of 96.2 IU/mL, and patient G.S.L. had greater than 2000 IU/mL (normal 0.0-87 IU/mL). Both patients demonstrated sIgE to cockroach, crab, and shrimp (Table 2 Column A). Patient S.V. had low level specific IgE to D. pteronyssinus (0.46 kU/liter), but not to D. farinae (<0.35 kU/L). Patient G.S.L. had >100 kU/L for both mites. Patient S.V. was negative to lobster (<0.35 kU/L) whereas patient G.S.L. was positive (2.69 kU/L). Both patients were positive to cat and dog but negative to avocado and corn (Column A, Table 2). To answer the question as to whether grasshopper contains a cross-reacting antigen that has homologues in crustacean, HDM, and cockroach, inhibition assay was carried out (Table 2, Columns B and C for both patients).
| Substance | Range Units | SV A No add |
SV B 200 µL* Saline |
SV C 20 mgm GH/ 200 µL Saline** |
GSL A No add |
GSL B* 200 µL Saline |
GSL C 20 mgm GH/ 200 µL Saline** |
|---|---|---|---|---|---|---|---|
| IgE | 0.0-87.0 IU/ml | 96.2 | -- | -- | >2000 | -- | -- |
| Cockroach | <0.35 KU/L | 3.64 | 3.64 | <0.35 | 3.2 | 3.32 | 0.65 |
| Crab | <0.35 KU/L | 0.96 | 0.96 | <0.35 | 1.91 | 2.04 | 1.03 |
| Shrimp | <0.35 KU/L | 0.46 | 0.46 | <0.35 | 11.3 | 10.6 | 2.94 |
| Lobster | <0.35 KU/L | <0.35 | <0.35 | <0.35 | 3.87 | 3.76 | 0.98 |
| Clam | <0.35 KU/L | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 | <0.35 |
| D. pteronyssinus | <0.35 KU/L | 0.46 | 0.47 | <0.35 | >100 | >100 | >100 |
| D. farina | <0.35 KU/L | <0.35 | <0.35 | <0.35 | >100 | >100 | >100 |
| Cat | <0.35 KU/L | 22.9 | 22.9 | 23.9 | 34.9 | 34.1 | 35.2 |
| Dog | <0.35 KU/L | 2.52 | 2.52 | 2.07 | 2.85 | 2.7 | 2.99 |
*200 Microliter saline
**20 mgm Grasshopper/200 microliter
***kilo units/liter
Table 2: ImmunoCAP specific IgE and inhibition by grasshopper (FD).
ImmunoCAP specific inhibition by grasshopper FD
2.5 mL of serum from patients SV and GSL was incubated for 24 hours at 4°C with A) Without additives B) 200 μL buffered saline or C) 20 mg of grasshopper FD in 200 μL of buffered saline. The results show that the 200 μL of saline produced no inhibition of sIgE binding to any of the antigens tested in either patient. However, grasshopper (Table 2, Column C) bound to and completely eliminated sIgE, binding to cockroach, crab, shrimp, and D. pteronyssinus in patient SV In patient GSL, grasshopper markedly reduced binding to cockroach, crab, shrimp, and lobster. In patient SV, grasshopper completely eliminated binding to D. pteronyssinus, whereas in patient GSL, who had a sIgE to D. pteronyssinus and D. farinae of >100 KU/mL, no inhibition by grasshopper occurred. There was not non-specific inhibition of cat or dog specific IgE binding, showing that the effect of the grasshopper inhibition was specific. Therefore there is a cross-reacting antigen in grasshopper which binds to sIgE in the patient’s serum specific to mites, cockroach, and crustaceans, but not to cat and dog.
To identify the cross-reacting antigen, we carried out SDSPAGE followed by immunoblotting of grasshopper, chapuline, cockroach, HDM, and cricket to identify and separate proteins in the extracts and to identify sIgE antibodies in the patient's serum binding to those proteins.
SDS PAGE-immunoblotting technique
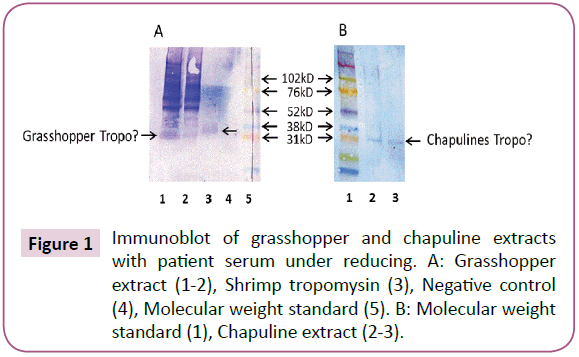
SDS PAGE immunoblotting showed that patients had specific IgE to a 30 kD protein in grasshopper extract (Figure 1, Experiment A, Lane 1 and 2 arrows). There was also IgE binding to purified shrimp tropomyosin in Lane 3 at 38 kD (molecular weight of shrimp tropomyosin). There was no binding to negative control protein (Lane 4). In Figure 1, Experiment B, IgE reactivity was seen to a 30 kD molecular weight protein in chapuline extract, which was similar to the IgE reactive 30 kD protein identified in grasshopper FD. Other higher molecular weight proteins that bound specifically to IgE were seen in grasshopper at 60 and 90 kD. chapuline extract, (Lane 2), showed faint IgE binding at these higher molecular weight levels, but the majority of the IgE binding was to the 30 kD protein.
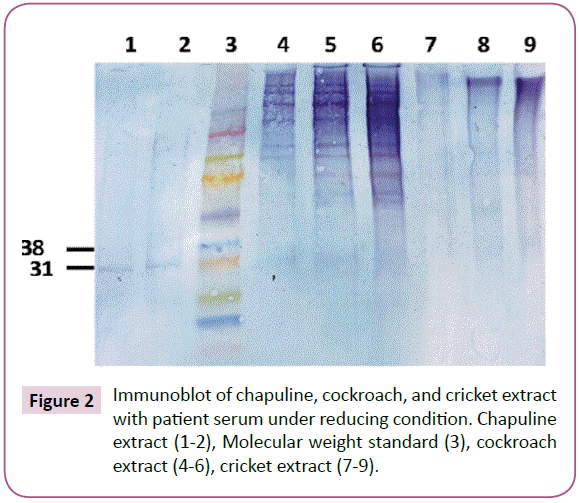
Immunoblotting of chapuline, cockroach, and cricket extracts was done (Figure 2). The results of this experiment showed a very clear reaction in Lanes 1 and 2 to chapuline extract. In Column 4, 5, and 6 are shown cockroach extract, which shows a faint band at 30 kD but a very similar high molecular weight binding pattern similar to that seen in grasshopper freeze dried extract (this may be a dimer of the 30 kD protein). There was no specific binding to cricket extract. Since we have found the protein in shrimp, grasshopper FD, chapuline, and cockroach to which the patients IgE bound, we did a Western Blot to see if that antigen was tropomyosin.
Western blot test
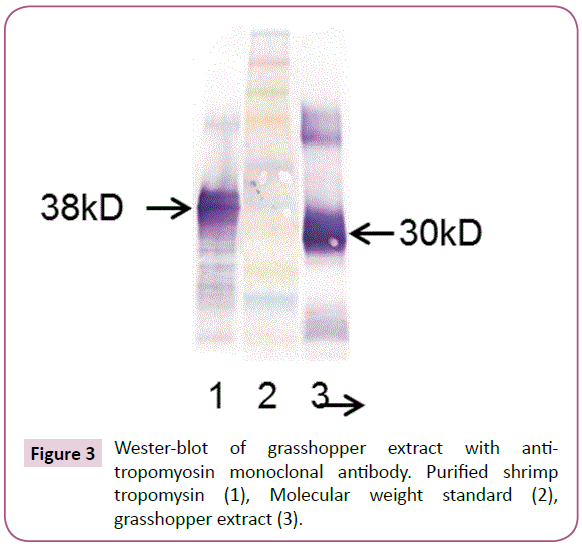
To see if the 30 kD IgE reactive protein in grasshopper and chapuline extract was tropomyosin, the Western Blot was done using a monoclonal antibody specific for tropomyosin. The antitropomyosin antibody bound to the same 30 kD protein in the grasshopper extract that the patient sera reacted to. This confirms that the 30kD protein that the patients IgE bound to in chapuline and grasshopper is tropomyosin (Figure 3).
Other high molecular weight IgE protein binding to IgE in grasshopper FD and cockroach consisted of a 60 kD protein, which may be a dimer of the 30 kD tropomyosin.
Discussion
Numerous reports of grasshopper causing allergic reactions are to be found in the medical literature 2 in patients in occupational and natural settings. Exposure and sensitization by inhalation of grasshopper (Locusta migratoria) in research laboratories has resulted in allergic rhinoconjunctivitis, bronchial asthma, and contact urticaria in 26-47% of exposed workers [5-8]. Allergy to grasshoppers in patients who fed grasshoppers to reptile pets or used grasshoppers as fishing bait have been reported [9] and massive exposure to grasshoppers in the field have been reported to cause allergic reactions in epidemic proportions [10]. Bhattacharya reported 26 asthma deaths and more than 1600 hospitalizations after swarms of locusts caused an allergic and asthma epidemic in the Sudan in 2003 [11].
Although occupational or natural sensitization to the inhalation of grasshopper is rare, ingestion of grasshopper is common. Eating of insects (entomophagy), the majority of which are grasshoppers is practiced in 80% of the countries in the world by approximately 2 billion people [12,13]. Entomophagy is widespread in Asia, the Middle East, Australia, southern and central Mexico, and southern California where numerous Mexican restaurants sell the insects imported from Oaxaca and prepared in various fashions as meals and snacks. Chapulines flour, as well as grasshopper and cricket protein bars and shakes, are increasingly sold on the internet as ecologically friendly, high-protein food options. In addition, the World Health Organization has recommended increased insect consumption as a solution to world hunger in developing countries due to its small ecological footprint, high protein content, and relatively low cost [14].
In Asia, anaphylaxis to ingested grasshopper has been reported. Piromrat et al. [15], in a retrospective review of records reported seven cases of anaphylaxis caused by fried grasshoppers and crickets in a two-year review of case records from an emergency room in Thailand. Ji et al. [16] in a retrospective review of hospital records, reported 27 cases of "anaphylactic shock" caused by consumption of grasshoppers in China between 1980 and 2007. No species identification or investigation of the nature of the antigen was carried out, nor whether the insects were grasshoppers, crickets, or katydids. Although not stated, given the widespread practice of entomophagy in Asia, it is assumed, although it cannot be confirmed, that these patients were sensitized by previous ingestion of insects.
Since our patients had no prior exposure to grasshopper through occupation, history, exposure to swarms, or by ingestion, their method of sensitization is distinct from these previously reported cases. Our patients did have a prior history of crustacean anaphylaxis and allergic sensitization to HDM and cockroach. We have shown in this report that the common IgE reactive antigen between the crustaceans, HDM, cockroach, grasshopper, and chapulines is tropomyosin and perhaps other higher molecular weight proteins. Tropomyosin found in cockroach [17] or in HDM has a 75-80% identity with the tropomyosin in shrimp [18]. Given the high degree of amino acid identity in the tropomyosins found in shrimp, HDM, and cockroach, it would be predicted that patients who have first been sensitized to tropomyosin in shrimp, HDM, or cockroach would, upon ingesting a food containing tropomyosin, experience an allergic reaction. Indeed, this has been reported in HDM allergic patients who developed an oral allergy syndrome (OAS) after the first -time ingestion of shrimp. In this study 78% of the HDM sensitized-shrimp reactive patients had specific IgE against tropomyosin (37-39 kD) [19]. Rame [20] found a high frequency of allergy to snails in patients who have never eaten snails but who had a high level of HDM allergy. Vuitton [21] reported several cases of systemic allergic reactions to the ingestion of snails which were eaten for the first time by patients who had HDM allergy. Fernandes [22] observed a cross reaction between HDM and crustaceans in orthodox Jews who observed strict kosher dietary rules prohibiting consumption of shellfish but who showed sensitization to shrimp due to a cross reacting tropomyosin allergen in HDM.
Cross-reactivity between different allergens occurs because of shared similar IgE epitopes. The structure and sequences of Tropomyosin are highly conserved, which explains the frequent cross-reactivity among distantly related allergen sources. [23] The results presented in this study suggest cross-reactivity between different invertebrate species which would explain reactivity to grasshoppers and chapulines in the absence of sensitization. It also suggests important clinical implications.
Conclusion
Our findings suggest that patients who have HDM, crustacean, and cockroach allergy are at risk for anaphylactic reactions from ingesting chapulines or perhaps even grasshopper products found in protein shakes, protein bars, or other sources of grasshopper protein due to the presence of a tropomyosin specific IgE antibody which cross-reacts with tropomyosin present in crustaceans, HDM, and cockroach.
We do not know the significance of these findings. Is a prior history of allergy to crustaceans or mites or cockroach alone or in combination enough to produce a reaction? Our patients both showed similar skin tests and sIgE levels to crustaceans and cockroach, but Patient #2 had much higher sIgE to HDM than did Patient #1. However, both patients had life-threatening anaphylaxis after eating chapulines. Therefore, the specific insect may not be as important as the total amount of sIgE to tropomyosin present.
Are these just two isolated patients who presented to our practice, or is this part of a larger phenomenon? The invertebrate pan-allergen tropomyosin is a potent, frequent cause of allergic reactions. When one considers that over 2 billion people eat insects, this phenomenon may not be rare.
References
- Javier C (2013) Los angeles is the chapuline capital of the US. Los Angeles Times, USA.
- Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitro-celluose sheets: Procedure and some applications. Proc Natl Acad Sci 76: 4350-4353.
- Pener MP (2014) Allergy to locusts and acridid grasshoppers: A Review. J Orthoptera Res 23: 59-67.
- Cohen JH, Sanchez NDM, Montiel-Ishimo F (2009) Chapulines and food choices in rural Oaxaca. Gastronimca 90: 61-65.
- Burge PS, Edge G (1980) Occupational asthma and rhinitis in locust workers. Clin Allergy 10: 346-347.
- Lopata L, Fenemore B, Jeebhay MF, Gade G, Potter PC (2005) Occupational allergy in laboratory workers caused by the African migratory grasshopper, Locusta migratoria. Allergy 60: 200-205.
- Soparkar GR, Patel PC, Cockcroft DW (1993) Inhalant atopic sensitivity to grasshoppers in research laboratories. J Allergy Clin Immunol 92: 61-65.
- Tee RD, Gordon DJ, Newman-Taylor AJ (1985) Allergy to locusts (Schistocerca gregaria and Locusta migratoria). J Allergy Clin Immunol 75: 122.
- Jensen-Jarolim E, Pali-Schoell I, Jensen SA (2015) Caution: Reptile pets shuttle grasshopper allergy and asthma into homes. World Allergy Organ J 8: 24.
- BBC News (2003) Locusts cause Sudan asthma deaths: Locust outbreak threatens Africa.
- Bhattacharya S (2003) Plague of locusts causes mass allergy attack.
- Hill JG, Goddard J (2012) Medical and veterinary importance of grasshoppers, katydids, and crickets. J Miss Acad Sci 57: 172-177.
- Gahukar RT (2011) Entomophagy and human food security. Int J Trop Insect Sci 31: 129-144.
- FAO (2014) Insects to feed the world. Wageningen UR, Netherlands.
- Piromrat K, Chinratanapisit S, Trathong S (2008) Anaphylaxis in an emergency department: A two-year study in a tertiary-care hospital. Asian Pac J Allergy Immunol 26: 121-128.
- Ji K, Chen J, Li M, Wang C (2009) Anaphylactic shock and lethal anaphylaxis caused by food consumption in china. Trends Food Sci Technol 20: 227-231.
- Santos ABR, Tobias KR, Fereriani VPL (1999) Identification of tropomyosin from Periplaneta americana as a major cockroach allergen. J Allergy Clin Immunol 103: 122.
- Leung PS, Chu KH, Chew WR, Ansari A, Bandea CA, et al. (1994) Cloning, expression, and primary structure of Metapenaeus ensis (tropomyosin), the major, heat-stable shrimp allergen. J Allergy Clin Immunol 94: 882-90.
- Gamez C, Zafra MP, Bouqete M, Sanz V, Mazzeo C, et al. (2014) New shrimp IgE-binding proteins involved in mite-seafood cross-reactivity. Mol Nutr Food Res 58: 1915-1925.
- Rame JM, Lavaud F, Staruska V (2002) Prevalence of the sensitization to snails and shrimps in patients allergic to house dust mite (hdm) - A prospective European multicenter study. J Allergy Clin Immunol 109: 654.
- Vuitton DA, Rance F, Paquin ML, Adessi B, Vigan M, et al. (1998) Cross-reactivity between terrestrial snails (helix species) and house dust mite (Dermatophagoides pteronyssinus) one in-vitro study. Allergy 53: 144-50.
- Fernandes J, Reshef A, Patton L, Patton L, Ayuso R, et al. (2003) Immunoglobulin E antibody reactivity to the major shrimp allergen, tropomyosin, in unexposed orthodox jews. Clin Exp Allergy 333: 956-961.
- Reese G, Ayuso R, Lehrer SB (1999) Tropomyosin: An invertebrate pan-allergen. Int Arch Allergy Immunol 119: 247-258.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences