AEURA, A Novel Homeopathic Agent, Shows High Level Protection Against Viral Infection and Stress Induced Neuronal Toxicity
Prentice H1*, Weiss A2, Marshall ML2 and Wu JY1*
1Department of Biomedical Sciences, Charles E Schmidt College of Medicine, Atlantic University, Florida, USA
2AEURA Trust, 2525 Arapahoe Ave E4-138, Boulder, Colorado 80302, USA
- *Corresponding Author:
- H. Prentice
Department of Biomedical Sciences
Charles E Schmidt College of Medicine
Florida Atlantic University, Boca Raton, Florida, USA
Tel: 5612970362
E-mail: hprentic@health.fau.edu
- Jang-Yen W
Department of Biomedical Sciences
Charles E Schmidt College of Medicine
Florida Atlantic University, Boca Raton, FL, USA.
Tel: 5612970167
E-mail: jwu@health.fau.edu
Received Date: February 19, 2017 Accepted Date: March 13, 2018 Published Date: March 15, 2018
Citation: Prentice H, Weiss A, Marshall ML, Wu J.-Y (2018) AEURA, A Novel Homeopathic Agent Shows High Level Protection Against Viral Infection and Stress Induced Neuronal Toxicity. J Biomed Sci Appl Vol.2 No.1:3
Abstract
In our analysis of the protective actions of the novel homeopathic agent AEURA we have 1) investigated the capacity of AEURA to show antiviral effects using virally infected PC12 cells (a neuronal model system) and we have 2) analyzed the effect of AEURA in preventing cell death caused by high extracellular glutamate or by hypoxia/re-oxygenation exposure. AEURA treatment was found to significantly decrease the cell death caused by HSV-1 infection. Similarly, when cells were infected with rhinovirus (the viral agent responsible for the common cold), treatment with AEURA significantly increased cell survival back to control uninfected levels. Under conditions that elicit cell death caused either by glutamate excitotoxicity or by hypoxia/ re-oxygenation it was found that pretreatment with AEURA elicited high level protection against both forms of cell stress and increased cell viability back to the levels of healthy control cells.
Keywords
AEURA; Rhinovirus; Glutamate excitotoxicity; Hypoxia
Introduction
Infection of mammalian cells with a variety of different classes of virus, including herpes simplex virus and rhinovirus, is known to elicit cell death and tissue toxicity. Such tissue damage may involve inflammatory processes involving cytokines or intracellular kinase signaling that can lead to cell death through induction of pro-apoptotic processes. In our recent studies novel homeopathic formula AEURA was found to protect against cell death caused by viral infection using infection with either a herpes simplex virus viral vector or with rhinovirus [1]. Furthermore AEURA was found to be highly neuroprotective and was capable of enhancing survival of the PC12 neuronal cell line in the face of toxicity caused by high levels of glutamate or by hypoxia /reoxygenation exposure [1,2].
Antiviral actions of aeura
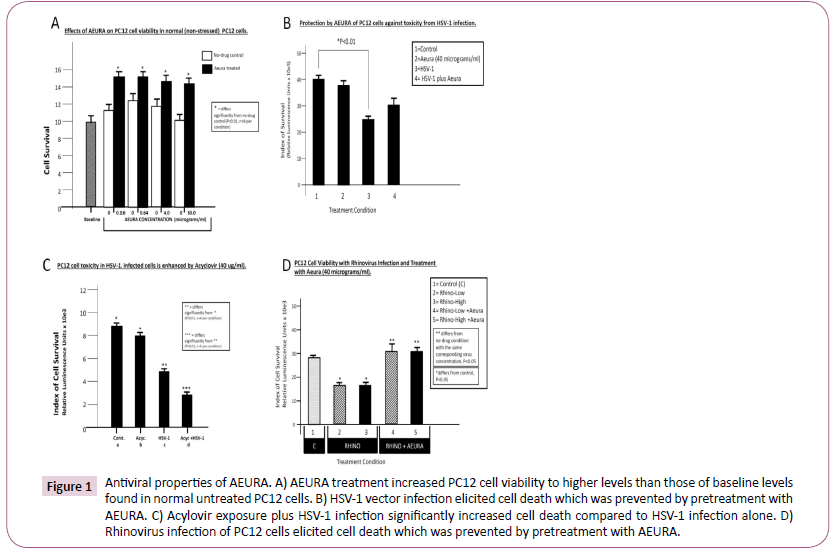
AEURA is a novel homeopathic agent that is often used by individuals infected with herpes simplex virus. We aimed to evaluate the effects of AEURA on the PC12 cell line which is a commonly used neuronal cell culture model derived from adrenal pheochromocytoma cells. To obtain an initial insight into the effect of AEURA on normal cells in an initial study we examined the baseline effect of AEURA on the health and survival of normal non-stressed PC12 cells at a range of concentrations from 0.16 microgram/ml to 30 microgram/ml (Figure 1A). Interestingly, at all doses analyzed, AEURA increased cell viability compared to untreated control cells. Many studies have demonstrated cellular toxicity resulting from viral infection with the resulting apoptosis as a key contributor to the overall cell death. For example, elevated pro-apoptotic processes in virally infected cells have been reported for such viruses as herpes simplex virus (HSV), adenovirus, adeno-associated virus, enterovirus and influenza virus [3-7]. In investigating the anti-viral effects of AEURA we pretreated PC12 cells with AEURA at 40 microgram/ ml concentration and then infected the cells with HSV-1 vector. PC12 cells were subsequently evaluated for % cell survival at 24 hours after viral infection. Our standard concentration of HSV-1 vector was found to cause approximately 50% cell death whereas pre-treatment with AEURA resulted in significantly improved PC12 cell survival compared to infected cells that were not exposed to AEURA (Figure 1B). Acyclovir is an antiviral agent that shows potent toxicity against virally infected cells. To confirm the effect of acyclovir in our experimental culture model and to compare the functional effects with those of AEURA we exposed PC12 cell cultures to acyclovir plus HSV-1 treatment or individually to acyclovir treatment alone or HSV-1 infection alone. Our data clearly indicated that acyclovir treatment plus HSV-1 infection resulted in greater cell death than infection with HSV-1 alone. These data when taken in combination with our findings on antiviral actions of AEURA (Figure 2) demonstrate that acyclovir is toxic to cells infected with HSV-1 but, by contrast, AEURA is protective to the HSV-1 infected cells and increases cell survival relative to HSV-1 infected PC12 cells that were not treated with AEURA (Figure 1B and 1C). To further elucidate the antiviral actions of AEURA we infected cultures of PC12 cells with rhinovirus, the viral agent responsible for the common cold, at low or high titers in the presence or absence of treatment with AEURA. While infection with rhinovirus caused 50% cell death in PC12 cells by comparison to uninfected cells, when PC12 cultures were pre-treated with AEURA and then infected with low or high titers of rhinovirus, the experiment demonstrated markedly enhanced levels of cell viability (indistinguishable from uninfected control levels) (Figure 1D).
Figure 1: Antiviral properties of AEURA. A) AEURA treatment increased PC12 cell viability to higher levels than those of baseline levels found in normal untreated PC12 cells. B) HSV-1 vector infection elicited cell death which was prevented by pretreatment with AEURA. C) Acylovir exposure plus HSV-1 infection significantly increased cell death compared to HSV-1 infection alone. D) Rhinovirus infection of PC12 cells elicited cell death which was prevented by pretreatment with AEURA.
Pharmacological approaches for eliciting protection against neuronal toxicity
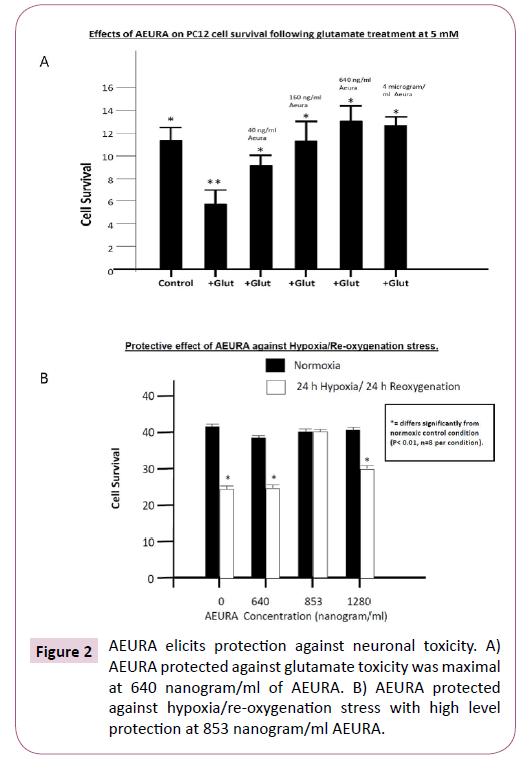
Several experimental neuroprotective agents are known to diminish the extent of neuronal death under conditions of pathological stress by preventing glutamate excitotoxicity or inhibiting mitochondrial free radical generation. Such neuroprotective agents from our own previous studies include DETC-MeSO, a disulfiram metabolite and glutamate receptor partial antagonist and taurine, an amino acid that prevents calcium overload and mitochondrial dysfunction [8,9]. Our experiments have also analyzed the protective actions of the homeopathic agent AEURA against cell stress using the PC 12 cells subjected to glutamate induced excitotoxicity or alternatively subjected to toxicity caused by hypoxia/re-oxygenation exposure. An extensively studied cause of toxicity for neuronal cell types is over-excitation from elevated extracellular glutamate. Exposure of neurons to high levels of extracellular glutamate is known to activate ionotropic glutamate receptors as well as metabotropic glutamate receptors. Glutamate receptor partial antagonists that elicit their effects via the N-methyl-D-aspartate (NMDA) receptor show very good potential for neuroprotection against glutamate toxicity and for preventing pro-death signaling resulting in apoptosis or the ER stress response. The drug memantine binds selectively to the NMDA receptor and, because it is a partial antagonist of the receptor memantine is capable of preventing the pathological effects of increased glutamate while maintaining the physiological effects of glutaminergic neurotransmission. In our recent studies on a different NMDA receptor partial antagonist, the drug DETC-MeSO (the active metabolite of disulfiram) we have demonstrated that pretreatment of mice with this drug will prevent the induction of seizures that were caused either by ethanol, by NMDA or by ammonium acetate [10]. The neuroprotective effect of DETC-MeSO was also seen in a rat stroke model (the middle cerebral artery occlusion model) as shown by a potent reduction in infarct size. The amino acid taurine (2-aminoethane sulfonate), a potent neuroprotective agent, is found at high levels in brain, skeletal muscle and cardiac muscle. Using PC12 cells in culture we have demonstrated that taurine is capable of protecting neuronal cells against damage from oxidative stress by inhibiting key endoplasmic reticulum (ER) stress components. To analyze the effects of taurine in protecting neuronal cells against glutamate induced toxicity we preincubated primary rat neuronal cultures with a range of concentrations of taurine (1 mM, 5 mM or 10 mM taurine) for 1 hour and then treated the neurons with 100 micromolar glutamate for 1 hour. Our findings indicated that taurine was highly protective against glutamate induced toxicity with significant levels of protection by taurine treatment at 1 mM and high level increases in cell viability to greater than 90% of control levels with 10 mM taurine treatment. Interestingly taurine decreased the ER stress that resulted from glutamate exposure by inhibiting the ATF6 and IRE1 pathways of the ER stress response. To establish whether AEURA was capable of preventing neuronal toxicity caused by excessive glutamate exposure we employed PC12 cell cultures that were pretreated with a range of concentrations of AEURA for 24 hours and then exposed cells to glutamate at 5 mM for an additional 4 hours. Under these experimental conditions AEURA showed strong protection at a range of concentrations from 4 ng/ml up to 4 micrograms/ml with highest cell survival (indistinguishable from survival of control cells without glutamate exposure) at 640 ng / ml AEURA (Figure 2A). Neuronal stress resulting from hypoxia/ re-oxygenation exposure involves prodeath signaling responses resulting from elevated mitochondrial reactive oxygen species release. Our previous analyses on protective interventions for combatting hypoxia/reoxygenation damage in neuronal cell lines have included assessment of protection by the NMDA receptor partial antagonist DETC-MeSO as well as analysis of mechanisms of protection by the amino acid taurine [11]. Employing rat neuronal cultures we previously investigated the capacity of DETC-MeSO to protect against cell death in conditions of hypoxia /re-oxygenation. In cultures without drug treatment, exposure to hypoxia/reoxygenation resulted in a decrease in cell survival to approximately 47% of control normoxic levels. In cultures exposed to hypoxia /re-oxygenation and treated with 25 micromolar DETC-MeSO it was demonstrated that drug treatment resulted in large increases in cell survival to approximately 70% of normoxic levels. In analyzing the neuroprotective effects of the amino acid taurine we have previously demonstrated that pre-treatment of primary neuronal cultures for 1 hr followed by hypoxia and reoxygenation exposure resulted in 85% cell survival by comparison to cultures without taurine which demonstrated approximately 49% cell survival relative to normal control levels. To determine the capacity of AEURA to protect against cell stress caused by hypoxia and re-oxygenation we employed PC12 cells cultures with a range of doses of AEURA at 640 ng/ml, 853 ng/ml and 1280 ng/ ml or in the absence of AEURA [11]. Clear high level protection was obtained with 853 ng/ml AEURA with greater than 90% cell survival and with 1280 ng/ml showing survival of 75% compared to cultures without AEURA which showed approximately 59% survival relative to normoxic control cells (Figure 2B).
Conclusion
AEURA a novel homeopathic formula commonly used by individuals suffering from herpes simplex virus infection was found to show antiviral action in PC12 cells in culture. In cells infected with HSV-1 vector or rhinovirus cell death of approximately 50% was observed. However AEURA treatment increased cell viability in these cells back to levels found in control uninfected cells. Furthermore, additional protective functions of AEURA were seen in cells treated with excessive extracellular glutamate or with hypoxia/ re-oxygenation. Under these conditions AEURA pretreatment restored cell viability to high levels (similar to levels in normal unstressed control cells). Our data point to AEURA as a strongly protective agent that shows anti-viral properties in virally infected neuronal cells and is pro-survival in the face of damaging cell stress and toxicity.
References
- Prentice H, Modi JP, Wu JY (2015) Mechanisms of neuronal protection against excitotoxicity, endoplasmic reticulum stress and mitochondrial dysfunction in stroke and neurodegenerative diseases. Oxidative Medicine and Cellular Longevity 2015: 1-8.
- Prentice H, Jigar M, Janet M (2016) Neuroprotective mechanisms of action of DETC-MeSO, GCSF, Sulindac Taurine and AEURA. Scitz Neurology and Neurosciences 1: 1004-1014.
- Han X, Cong H (2017) Enterovirus 71 induces apoptosis by directly modulating the conformational activation of pro-apoptotic protein Bax. J Gen Virol 98: 422-434.
- Kraft RM, Nguyen ML (2006) Caspase 3 activation during herpes simplex virus 1 infection. Virus Res 120: 163-175.
- Michaelis M, Geiler J (2009) Infection of human retinal pigment epithelial cells with influenza A viruses. Invest Ophthalmol Vis Sci 50: 5419-5425.
- Nguyen ML, Kraft RM (2007) p53 and hTERT determine sensitivity to viral apoptosis. J Virol 2007 81: 12985-12995.
- Subramanian T, Vijayalingam S (2007) Evidence for involvement of BH3-only proapoptotic members in adenovirus induced apoptosis. J Virol 81: 10486-10495.
- Mohammad GP, Modi J (2014) Mode of action of S-methyl-N, N-diethylthiocarbamate sulfoxide (DETCMeSO) as a novel therapy for stroke in a rat model. Mol Neuro Biol 50: 655-672.
- Pan C, Prentice H (2012) Beneficial effect of taurine on hypoxia- and glutamate-induced endoplasmic reticulum stress pathways in primary neuronal culture. Amino Acids 43: 845-855.
- Ningaraj NS, Chen W, Schloss JV (2001) S-methyl-N,N-diethylthio-carbamate sulfoxide elicits neuroprotective effect against N-methyl-Daspartate receptor-mediated neurotoxicity. J Biomed Sci 8: 104-113.
- Modi J, Altamimi A (2017) Protective functions of AEURA in cell based model of Stroke and Alzheimer disease. Journal of Neuroscience and Neurological Disorders 1: 17-23.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences