Aberrant Kit-Mutated Gastrointestinal Stromal Tumor in Suspected Carney-Stratakis Syndrome
Anam Asif*
VCU School of Medicine Inova Campus, Inova Fairfax Medical Campus 3300 Gallows Road Falls Church, VA, USA
- *Corresponding Author:
- Anam Asif
VCU School of Medicine Inova Campus
Inova Fairfax Medical Campus 3300 Gallows Road Falls Church, VA, 22042-3300, USA
Tel: 7037766699
E-mail: asifa@vcu.edu
Received Date: June 06, 2019; Accepted Date: July 08, 2019; Published Date: July 15, 2019
Citation: Asif A (2019) Aberrant KIT-mutated Gastrointestinal Stromal Tumor in Suspected Carney-Stratakis Syndrome. J Mol Genet Med Vol.3 No. 1:2
Abstract
We present the case of a 33-year Philippines patient with a synchronous presentation of paranganglioma and Gastrointestinal Stromal Tumor (GIST). Germline mutations in SDH subunits are known to predispose to both of these relatively rare tumors called the Carney-Stratakis Syndrome (CSS). Investigations revealed that patient have a germline heterozygous pathogenic SDHB-inactivating mutation. While his paraganglioma did show IHC absence of SDHB, his GIST displayed normal SDHB staining and instead was positive for an exon 9 mutation of KIT. This was confirmed by DNA sequencing. Additionally, prior large series have shown only gastric GISTs in CSS, while this patient’s GIST was in the small intestine. The finding of a KIT-mutated GIST in the setting of a germline SDHB-inactivating mutation in what would otherwise be consistent with Carney-Stratakis Syndrome has not been described previously and highlights the need for better understanding of the function of SDH and its interplay with other tumor suppressors and oncogenes. Specifically, this case demonstrates the need for somatic mutation analysis of tumors even in the presence of known germline SDH mutations since additional actionable mutations such as KIT or PDGFRA can improve prognosis given the high therapeutic index of imatinib mesylate.
Keywords
Carney-Stratakis syndrome; Succinate dehydrogenase; Gastrointestinal stromal tumor; Paraganglioma; Proto-oncogene proteins c-kit
Introduction
Carney-Stratakis Syndrome, or the Carney-Stratakis dyad, is defined by the presence of two primary tumors, a paraganglioma and a Gastrointestinal Stromal Tumor (GIST), resulting from a mutation in one of four genes encoding the subunits of the Succinate Dehydrogenase Complex (SDH) [1]. SDH is present within the inner mitochondrial membrane and is involved in cellular respiration. Specifically, SDH catalyzes the oxidation of succinate to fumarate during the citric acid cycle [2].
Carney-Stratakis Syndrome has an autosomal-dominant inheritance pattern with incomplete penetrance [3]. Causative mutations inactivate one of the SDH genes, which normally act as tumor suppressors, therefore loss of the wild-type allele becomes an oncogenic event [1]. Loss of function of the SDHA, SDHB, SDHC, or SDHD genes (collectively called SDHX) reduces overall activity of the SDH protein complex, thereby increasing intracellular succinate levels [1]. Excess succinate inhibits hydroxylation and inactivation of hypoxia-inducible factor 1- alpha (HIF1a) by prolyl hydroxylase, resulting in persistence of HIF1a within the cytosol which enters the nucleus to induce expression of tumor-promoting genes [4].
Gastrointestinal Stromal Tumors (GISTs) are connective tissue neoplasms of the gastrointestinal tract that are mesenchymal in origin. Theorized to arise from the interstitial cells of Cajal, they form most frequently in the stomach and small intestine [5]. In the United States, the incidence of GISTs is estimated as 3,000 cases annually [6]. Although the majority of GISTs are sporadic, 5% result from hereditary multitumor syndromes [7]. Sporadic GISTs are most commonly the result of somatic gain-of-function mutations in KIT or PDGFRA and have a higher prevalence in males over the age of 50. These are general somatic mutations, though rare reports of germline KIT and PDGFRA mutations have been reported [8,9]. KIT and PDGFRA encode ligand-dependent type III receptor tyrosine kinases and mutations in these genes cause constitutive activation of the receptor tyrosine kinase pathway responsible for signal transduction controlling cellular growth. Resultant continual propagation of signals leads to unchecked proliferation of cells into tumors.
GISTs in Carney-Stratakis Syndrome typically lack KIT or PDGFRA mutations and are referred to as “wild-type”, instead being driven by the characteristic SDHX mutation [10]. There are several differences between KIT/PDGFRA-mutated GISTs and SDHX-mutated GISTs: the latter (i) occur earlier, with a mean age of 23 years at presentation; (ii) are found more often in females; and (iii) are nearly always multifocal and gastric in origin [4]. According to an NIH study of wild-type GISTs, 84 of 84 SDH-deficient GISTs arose in the stomach while 9 of 11 SDH-competent GISTs arose in the small bowel. The SDH-deficient tumors included those with an SDHX mutation (n=63) and an SDHC epimutation (n=21), while the SDH-competent GISTs arose due to an undefined mechanism [11]. These results demonstrate a strong predilection for gastric GIST formation in patients with an SDHX mutation as in Carney-stratakis syndrome.
GISTs are typically treated with surgical resection. If the tumor is inoperable, targeted therapy with imatinib mesylate is used to decrease tumor volume to palliate or with the intent to convert to respectability. Imatinib mesylate is a tyrosine kinase inhibitor which selectively inhibits KIT and PDGFRA thereby hampering the uncontrolled proliferation of the GIST caused by the KIT- and PDGFRA-activating mutations. It is an effective targeted treatment, with one study estimating that patients with metastatic KIT- or PDGFRA-mutated GIST who received treatment with imatinib mesylate had a 2-year survival rate of 80% [12]. In contrast, SDHX-mutated GISTs arise from instability within the SDH protein complex and thereby do not respond to traditional tyrosine kinase inhibitors. However, currently there are no standard treatments for SDHX-mutated GISTs hence there is therapeutic relevance in identifying the driving mutation in a GIST so treatment may be optimized if the patient is a candidate for therapy with imatinib mesylate [13].
Paragangliomas are neuroendocrine tumors arising from sympathetic nerve cells called glomus cells [14]. Derived from the embryonic neural crest, these cells act as chemoreceptors within the sympathetic nervous system so resultant paragangliomas have a propensity for forming along blood vessels and sympathetic paraganglia [14].
Much like sporadic GISTs, most paragangliomas occur in the fourth or fifth decade of life, but 25% have hereditary etiology, including Carney-stratakis syndrome. These present earlier, the mean age at diagnosis being 23 years, and are more likely to be multifocal. They are characterized by SDHX-inactivating mutations and, specifically, SDHB-inactivated mutations have higher rates of malignancy and lymph node metastasis than sporadic paragangliomas [15].
Case Report
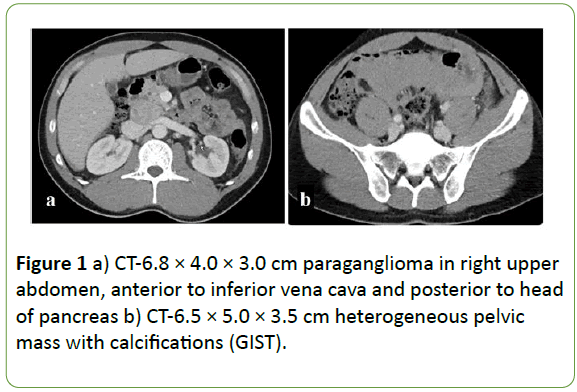
The study presents the case of a 33-year old male of Filipino ancestry with a 5-year history of hypertension, poorly controlled with medication, who presented to the emergency department with severe, diffuse abdominal pain. A CT scan revealed two masses: (i) a 6.8 × 4.0 × 3.0 cm right upper abdomen mass, anterior to the inferior vena cava and posterior to the head of the pancreas (Figure 1a); and (ii) a 6.5 × 5.0 × 3.5 cm heterogeneous pelvic mass with multiple calcifications (Figure 1b). The abdominal mass was biopsied and stained positive for synaptophysin and chromogranin, markers consistent with a neuroendocrine tumor. In addition, the patient's normetanephrine levels were elevated. Coupled with his history of poorly controlled hypertension, a catecholamine-secreting paraganglioma was suspected. He underwent surgery four months later and an encapsulated endocrine tumor was excised, confirming his diagnosis.
Continued surgical exploration of the deep pelvic mass revealed a Gastrointestinal Stromal Tumor (GIST) of the small bowel. It was staged as a low-grade intermediate risk pT3 N0 M0 spindle-cell type GIST with a mitotic rate of less than 5 per 50 HPF.
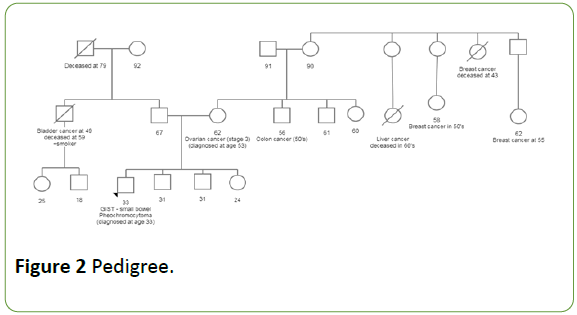
Due to the unique presentation of two primary tumors at a young age, Carney-stratakis syndrome, a familial syndrome characterized by concurrent paraganglioma and GIST resulting from a mutation in the SDH gene, was suspected [10]. The patient's family history of cancer is significant: his mother has a personal history of ovarian cancer diagnosed at age 53 and an extended maternal family history of early-onset breast cancer.
Her brother (patient's maternal uncle) was diagnosed with colon cancer in his 50s. On his paternal side, the patient has an uncle diagnosed with bladder cancer at age 49 in the context of a history of heavy tobacco use (Figure 2). Despite no reported family history of paraganglioma or GIST, Carney-stratkis syndrome was considered due to the possibility of an inherited gene mutation with incomplete penetrance or a de novo mutation.
The patient's germline testing identified a heterozygous pathogenic SDHB-inactivating mutation known as c. 716_719delCTCT. Both tumors underwent Immunohistochemical staining (IHC) to confirm the mutation. As expected, IHC of the paraganglioma demonstrated loss of SDHB expression, consistent with his known germline mutation and Carney- Stratakis Syndrome. Our expectation was the GIST was an SDH-mutant sharing the underlying etiology with the paraganglioma as described by Carney-Stratakis Syndrome. We anticipated KIT (CD117) staining would be negative due to the previous understanding of KIT (CD117) and SDHX mutations being mutually exclusive. Surprisingly, the GIST demonstrated intact SDHB expression despite the germline mutation and instead stained aberrantly as KIT (CD117) positive. Additional KIT mutation analysis of the GIST via PCR revealed a well-characterized exon 9 mutation (c.1509_1510insGCCTAT). This mutation was confirmed on next-generation sequencing, without other mutations of interest being found, resulting in initiation of treatment with imatinib mesylate.
Proband Follow-Up
Following genetic counseling, the patient underwent germline testing performed on a saliva sample by Ambry Genetics that included sequencing and deletion/duplication analysis of the FH, MAX, MEN1, NF1, RET, SDHA2, SDHAF2, SDHB, SDHC, SDHD, TMEM127 and VHL genes. Results were positive for a heterozygous pathogenic germline SDHB-inactivating mutation known as c.716_719delCTCT. No other mutations or variants of uncertain significance were identified.
IHC staining of the paraganglioma demonstrated loss of SDHB expression while the small bowel GIST stained intact for SDHB expression. Given the unexpected CD117 positive staining, KIT mutation analysis was requested on the GIST tissue to guide treatment decision-making. A KIT exon 9 mutation (c. 1509_1510insGCCTAT) was identified and treatment with imatinib mesylate was initiated to which the patient had a positive response.
Normal SDHB expression via IHC and the presence of a KIT exon 9 mutation indicates that the driver of the GIST was not the germline SDHB mutation followed by loss of heterozygosity, but instead an exon 9 KIT mutation. This mutation occurs in approximately 15% of GISTs and is presumed to be acquired as no germline exon 9 mutations are reported in the literature, hence this can be characterized as a sporadic GIST [16]. An error in IHC staining is unlikely due to concordance with tumor KIT mutation status. Furthermore, this patient’s GIST was solitary and in the small bowel, unlike the multifocal gastric GISTs seen in Carney-Stratakis Syndrome.
Discussion
By definition, absence of KIT mutations in GISTs are a requirement for the diagnosis of Carney-Stratakis Syndrome [10]. In one study, 84 of 84 patients with paraganglioma and GIST did not have a KIT mutation [11]. Another study investigating GISTs in both Carney-Stratakis Syndrome and Carney Triad compared to sporadic GISTs found that SDHB immunohistochemistry was a crucial differentiator between their etiologies. Of the ten GISTs obtained from patients with Carney-Stratakis Syndrome and Carney Triad, all ten stained negatives for SDHB immunohistochemically. In contrast, the majority of the 42 sporadic GISTs were SDHB-normal and KIT/ PDGFRA-mutated [17]. This was one of many studies which contributed to the thought that SDHB mutations and KIT mutations in GISTs were mutually exclusive and thereby helped distinguish sporadic GISTs from those in multitumor syndromes. However, this case report joins evidence that this distinction may not be as definite.
One group of researchers reported an unusual extra-gastric mediastinal GIST which stained positive for KIT but also stained only faintly positive for SDHB on IHC [18]. Further DNA analysis revealed a deletion within exon 11 of the KIT gene in addition to a sequence change within exon 1 of the SDHD gene (p.G12S) [18]. Although this SDHD gene variant is classified as likely benign, it was hypothesized to destabilize the SDH complex as a whole for potential downstream pathogenicity. Similarly, another study found an imatinib-sensitizing KIT mutation within a rectal GIST in a patient with a germline SDHD mutation in suspected Carney-Stratakis Syndrome [7]. If these results are considered along with our case report, they may strengthen the argument against SDH variants and KIT/PDGFRA mutations being mutually exclusive and may even allude to a cooperative interplay between the two which instigates tumor progression [18].
Should such an interaction exist, it may potentially improve prognosis in multitumor syndromes by providing additional targets for drug therapy. Inoperable/metastatic GISTs associated with Carney-Stratakis Syndrome generally demonstrate poor prognosis due to primary resistance to therapy with imatinib given the absence of a KIT mutation and a lack of effective therapies targeted to SDHX mutations. In such cases, and those of more advanced metastatic disease, having an additional actionable mutation such as KIT or PDGFRA can provide a valuable tool in the treatment plan.
It is unclear if patients with known SDH mutations who develop GISTs ever are tested for KIT mutations. This case demonstrates the need for somatic KIT mutation analysis even in the presence of a known germline SDHX mutation given the high therapeutic index of imatinib mesylate.
This case was presented to the Molecular Tumor Board at Inova Fairfax Hospital and, after review of the literature, appears to be the first of its kind reported. Although the patient's KIT-positive GIST may truly be sporadic and a coincidental co-occurrence with his paraganglioma, his young age at diagnosis is inconsistent with the typical presentation of sporadic GISTs. This case report demonstrates the need for a better understanding of hereditary multitumor syndromes and their genetic links. The distinctive combination of an SDHB-deficient paraganglioma and KIT-positive GIST in the same patient hints at a potential genetic interaction between the two that is yet to be elucidated. Further study will improve the understanding of Carney-Stratakis Syndrome, or possibly result in the discovery of a novel multitumor syndrome.
Acknowledgement
Ms. Anam Asif researched and reviewed literature on Carney- Stratakis syndrome and its genetics, extrapolated this patient's genetic results to current understanding of cancer genetics, and drafted this manuscript. She is responsible for the overall content of this manuscript as a guarantor.
Dr. Timothy Cannon was the principal investigator for this case report and physician for the patient discussed. He drafted this manuscript, recognized the unique presentation of this patient in regard to the current understanding of cancer genetics, and completed follow-up on the patient to monitor his progress on treatment. He is responsible for the overall content of this manuscript as a guarantor.
Ms. Sarah Ruppert was the clinical geneticist analyzing the case and providing her genetics expertise to help with characterizing the mutations involved and their implications. She also helped draft this manuscript.
Dr. Syed Zaman was the pathologist who helped stain and characterize the mutations present in each of the tumors.
Dr. James Piper was the surgeon who provided the tumors for study and provided information regarding Carney-Stratakis syndrome.
References
- Janeway KA, Kim SY, Lodish M, Nose V, Rustin P, et al. (2011) Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc Natl Acad Sci U S A 108: 314-318.
- Miettinen M, Lasota J (2014) Succinate Dehydrogenase Deficient Gastrointestinal Stromal Tumors (GISTs) - a review. Int J Biochem Cell Biol 53: 514-519.
- Pasini B, McWhinney SR, Bei T, Matyakhina L, Stergiopoulos S, et al. (2007) Clinical and molecular genetics of patients with the Carney-Stratakis syndrome and germline mutations of the genes coding for the succinate dehydrogenase subunits SDHB, SDHC, and SDHD. Eur J Hum Genet 16: 79-88.
- Wang YM, Gu ML, Ji F (2015) Succinate dehydrogenase-deficient gastrointestinal stromal tumors. World Journal of Gastroenterology 21: 2303-2314.
- Call J, Walentas CD, Eickhoff JC, Scherzer N (2012) Survival of gastrointestinal stromal tumor patients in the imatinib era: life raft group observational registry. BMC Cancer 12.
- Rubin JL, Sanon M, Taylor DC, Coombs J, Bollu V, et al. (2011) Epidemiology, survival, and costs of localized gastrointestinal stromal tumors. Int J Gen Med 4: 121-130.
- Gasparotto D, Rossi S, Campagna D, Scavina P, Tiziano FD, et al. (2016) Imatinib-Sensitizing KIT Mutation in a Carney-Stratakis-Associated GI Stromal Tumor. J Clin Oncol 34: e99-e103
- Maeyama H, Hidaka E, Ota H, Minami S, Kajiyama M, et al. (2001) Familial gastrointestinal stromal tumor with hyperpigmentation: association with a germline mutation of the c-kit gene. Gastroenterology 120: 210-215.
- Chompret A, Kannengiesser C, Barrois M, Terrier P, Dahan P, et al. (2004) PDGFRA germline mutation in a family with multiple cases of gastrointestinal stromal tumor. Gastroenterology 126: 318-321.
- Carney JA, Stratakis CA (2002) Familial paraganglioma and gastric stromal sarcoma: a new syndrome distinct from the Carney triad. Am J Med Genet 108: 132-139.
- Boikos SA, Pappo AS, Killian JK, LaQuaglia MP, Weldon CB, et al. (2016) Molecular Subtypes of KIT/PDGFRA Wild-Type Gastrointestinal Stromal TumorsA Report from the National Institutes of Health Gastrointestinal Stromal Tumor Clinic. JAMA Oncol 2: 922-928.
- Din OS, Woll PJ (2008) Treatment of gastrointestinal stromal tumor: focus on imatinib mesylate. Ther Clin Risk Manag 4: 149-162.
- Debiec-Rychter M, Sciot R, Le Cesne A, Schlemmer M, Hohenberger P, et al. (2006) KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer 42: 1093-1103.
- Boedeker CC (2011) Paragangliomas and paraganglioma syndromes. GMS Curr Top Otorhinolaryngol Head Neck Surg 10: 03.
- Neumann HP, Pawlu C, Peczkowska M, Bausch B, McWhinney SR, et al. (2004) Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. JAMA 292: 943-951.
- Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, et al. (2003;) Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 21: 4342-4349.
- Gaal J, Stratakis CA, Carney JA, Ball ER, Korpershoek E, et al. (2011) SDHB immunohistochemistry: a useful tool in the diagnosis of Carney–Stratakis and Carney triad gastrointestinal stromal tumors. Mod Pathol 24: 147-151.
- Ondrej D, Monika S, Magdalena D, Michal M (2012) KIT Mutations and Sequence Changes in Genes Encoding SDH Complex Possibly Need Not be Mutually Exclusive in Gastrointestinal Stromal Tumors. Appl Immunohistochem Mol Morphol 20: 523-524.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences