A Case of Infection Related Glomerulonephritis with PLA2R Positive Membranous Nephropathy
Keerthiga N*, Edwin Fernando M, Thirumalvalavan S and Thirumalvalavan K
Department of Nephrology, Government Stanley Medical College, Chennai, India
- *Corresponding Author:
- Keerthiga N
Department of Nephrology, Government Stanley Medical College, Chennai, India
E-mail:keerthinarasimman93@gmail.com
Received date: July 09, 2024, Manuscript No. IPJRM-24-19351; Editor assigned date: July 11, 2024, PreQC No. IPJRM-24-19351 (PQ); Reviewed date: July 25, 2024, QC No. IPJRM-24-19351; Revised date: April 14, 2025, Manuscript No. IPJRM-24-19351 (R); Published date: April 21, 2025, DOI: 10.36648/IPJRM.8.2.56
Citation: Keerthiga N, Fernando ME, Thirumalvalavan S, Thirumalvalavan K (2025) A Case of Infection Related Glomerulonephritis with PLA2R Positive Membranous Nephropathy. J Ren Med Vol.8 No.2
Abstract
We report a case of 44-year-old male who presented with secondary infection of eczema, microscopic hematuria, nephrotic range proteinuria and rapidly progressive renal failure. His biopsy had features of infection related glomerulonephritis with phospholipase A2 receptor positive membranous nephropathy.
Keywords
Infection Related Glomerulonephritis (IRGN); Membranous Nephropathy (MN); Podocyte Phospholipase A2 Receptor Antigen (PLA2R)
Introduction
Primary membranous nephropathy is associated with IgG 4 antibodies against podocyte phospholipase A2 receptor antigen in 70% of cases and serum anti THS7DA antibodies in 5%-10% of cases. Biopsy staining of PLA2R antibodies without presence of serum antibodies is seen in 15% of cases [1]. Infection-Related Glomerulonephritis (IRGN) is an immune complex-mediated glomerulonephritis often preceded by infection with subsequent recovery of renal function after the resolution of the infection. The sites of infection in adult IRGN are more heterogeneous than in children and include upper respiratory tract, skin, lung, heart, urinary tract, teeth/oral mucosa, bone and deep-seated visceral or somatic abscesses. The two most common sites are upper respiratory tract and skin; the former predominates in young adults, whereas the latter is the most frequent site in the elderly population and in diabetics [2]. IRGN encompasses the entities of post infectious glomerulonephritis, IgA dominant IRGN, endocarditis associated glomerulonephritis and shunt nephritis. IRGN has diffuse proliferative, focal segmental proliferative or mesangioproliferative pattern of glomerular injury.
Case Presentation
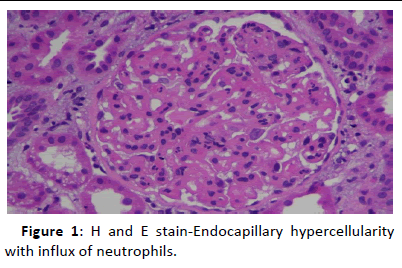
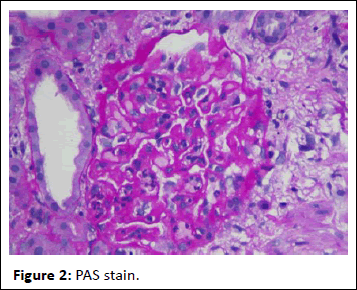
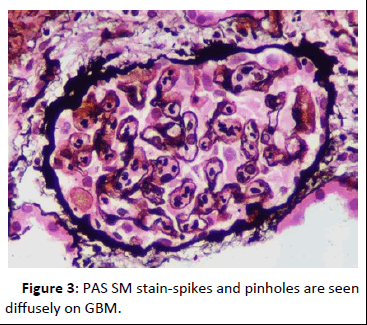
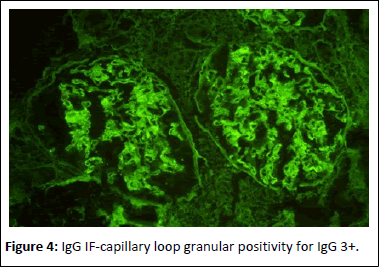
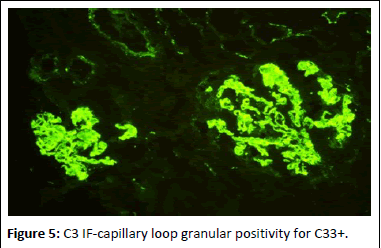
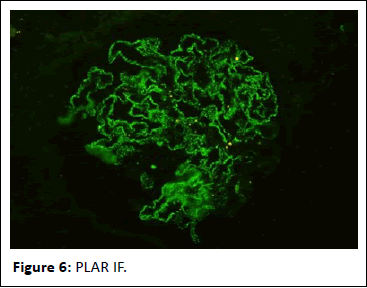
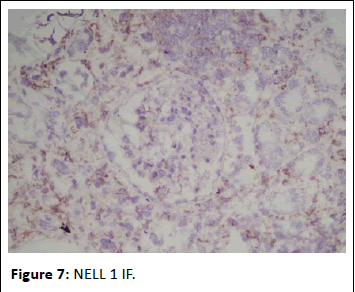
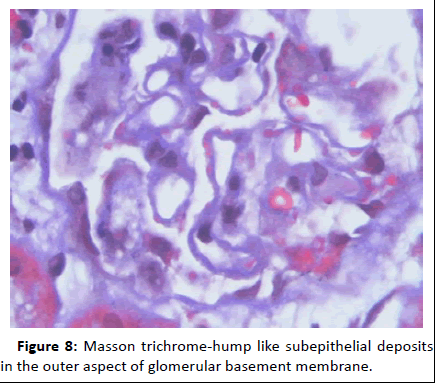
44-year-old gentleman hardware distributor by occupation without any known co-morbidities presented with complaints of chronic eczema for 10 years duration. In September 2022 the lesions had increased in intensity for the past 1 week along with fever for which he took indigenous medications for 4 days, which was followed by oliguria. Basic investigations revealed serum creatinine of 4.8 mg/dl for which nephrology consultation was obtained. Urine was frothy and cola coloured. Examination revealed chronic eczema with impetigious lesions, mild pedal edema, blood pressure of 150/100 mmhg. Investigation revealed urine albumin 1+, blood 1+, urine PCR of 4.1 gram, hemoglobin 10.5 gm/dl, platelet count 3.5 lakhs, serum creatinine 7 mg/dl, serum albumin 2.4 gm/dl with normal sized kidney with normal echoes on ultrasonography. His ANA was negative, C3 was 66 (90-140 mg/dl) and C4 was 14 (10-40 mg/dl). In view of rapidly progressive glomerulonephritis renal biopsy was done. Of the 9 glomeruli none were globally sclerotic. Segmental endocapillary hypercellularity with influx of neutrophils in all glomeruli. Spikes and pinhole lesions are seen diffusely on the glomerular basement membrane on periodic acid schiff-silver stain. Massons trichrome stain reveals occasional large hump like subepithelial deposits in the outer aspect of glomerular basement membrane. No interstitial fibrosis or tubular atrophy. No significant inflammatory infiltrate in the interstitium. Immunofluorescence show capillary loop granular positivity for IgG3+ and C33+. Immuno staining for IgA, IgM and c1q where negative. Immuno staining for phospholipase A2 receptor positive and NELL-1 was negative. The impression was a combination of infection related glomerulonephritis with membranous nephropathy (Figures 1-8).
Figure 1: H and E stain-Endocapillary hypercellularity with influx of neutrophils.
Figure 2: PAS stain.
Figure 3: PAS SM stain-spikes and pinholes are seen diffusely on GBM.
Figure 4: IgG IF-capillary loop granular positivity for IgG 3+.
Figure 5: C3 IF-capillary loop granular positivity for C33+.
Figure 6: PLAR IF.
Figure 7: NELL 1 IF.
Figure 8: Masson trichrome-hump like subepithelial deposits in the outer aspect of glomerular basement membrane.
Results and Discussion
The occurrence of MN with IRGN has been very rare and to the best of our knowledge a combination of MN with IRGN has not been reported. There are few case series and case reports of MN associated with ANCA vasculitis or IgA nephropathy or anti GBM disease [3-7].
MN is a common etiology of nephrotic syndrome in adults. Histologically, it is characterized by a light microscopy pattern of basement membrane thickening with deposition of immunoglobulins and complement proteins. MN can be either idiopathic or associated with an underlying disease such as systemic lupus nephritis, other autoimmune disorders, malignancies or drugs such as nonsteroidal anti-inflammatory drugs.
The association of vasculitic glomerulonephritis with MN is rare, estimated to be <5% of all membranous glomerulonephritis cases and is reported only in a handful of times in the literature [8]. Few case reports and case series of ANCA-associated GN with anti-GBM antibodies. While the etiology of this association is unknown, one hypothesis proposed is that damage induced by ANCA leads secondarily to the development of anti-GBM antibodies.
Many case reports of coexistence of anti-GBM disease and MN. The GBM injury during the course of MN may induce the release of normal or altered GBM matter, thus producing the anti-GBM antibody.
Dual mechanism has been proposed to elucidate the pathological physiology for MN occurring after an anti-GBM disease or concurrent MN and anti-GBM disease. In the first stage, the antibody is combined with antigenic structures anchored on glomerular capillary walls to produce linear deposition of IgG, thus promoting upregulation of basement membrane antigens synthesized and secreted by podocytes. In the second stage, multi-specific antibodies react with these basement membrane components to form immune complexes in the subepithelial areas in situ [9].
Few case reports of coexistence of MN and IgA nephropathy. Superimposition of anti-GBM disease on latent IgA nephropathy can possibly explain the coexistence of anti-GBM disease and IgA nephropathy. It also hypothesized that IgA-related immune complexes may promote immunologic and inflammatory events, causing conformational changes in the GBM antigens and the exposure of GBM antigens and thus the development of the anti-GBM antibody [10].
Worsening renal function in IRGN are due to endocapillary proliferation and crescents. Worsening of renal function in MN are due to ATIN and associated anti GBM disease.
Our patient exhibited PLA2R positive membranous nephropathy with IRGN. Most patients with membranous nephropathy presents with normal or slightly decreased renal function at presentation. Our patient presented with rapidly progressive renal failure. Patients with membranous nephropathy with acute renal insufficiency should prompt for the identification of superimposed condition. In our patient because of superimposed IRGN patient presented with rapidly progressive renal failure. As the patient present with stage 3 membranous nephropathy as per Ehrenreich and Chrug staging for MN, the membranous nephropathy had been present in our patient for longtime and the occurrence of superimposed IRGN maked the patient symptomatic.
Our patient was treated conservatively with intravenous frusemide and antibiotics. Patient renal function improved. After stabilisation of renal function patient was started on T. Enalapril and increased to maximum tolerated dose. Age appropriate malignancy workup was done and found to be negative.
Proteinuria reduced to 900 mg after 6 months of conservative treatment with ACE inhibitor. Currently patient is on T. Enalapril 10 mg per day, proteinuria 900 mg and serum albumin of 3.5 gm/dl. Patient is followed up to one year for any malignancy.
Conclusion
Our case 44-year-old man found to have both infections related glomerulonephritis and membranous nephropathy on kidney biopsy represents a rare dual glomerulopathy. An overlap between infection related glomerulonephritis and membranous nephropathy is rare. Presentation with microscopic hematuria, proteinuria and acute kidney injury should prompt treatment for rapidly progressive glomerulonephritis, to prevent poor clinical outcome.
Declaration of Patient Consent
The authors certify that they have obtained all appropriate patient consent forms. In the forms, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understand that her name and initials will not be published and due efforts will be made to conceal her identity, but anonymity cannot be guaranteed.
Financial Support and Sponsorship
Nil.
Conflict of Interest
There are no conflicts of interest.
References
- James SH, Lien YH, Ruffenach SJ, Wilcox GE (1995) Acute renal failure in membranous glomerulonephropathy: A result of superimposed crescentic glomerulonephritis. J Am Soc Nephrol 6:1541-1546
[Crossref] [Google Scholar] [PubMed]
- Luo C, Tang Z, Chen D, Liu Z (2011) Long-term prognosis for Chinese adult patients with acute postinfectious glomerulonephritis. Clin Nephrol 76:186-194
[Crossref] [Google Scholar] [PubMed]
- Balafa O, Kalaitzidis R, Liapis G, Xiromeriti S, Zarzoulas F, et al. (2015) Crescentic glomerulonephritis and membranous nephropathy: A rare coexistence. Int Urol Nephrol 47:1373-1377
[Crossref] [Google Scholar] [PubMed]
- Nasr SH, Said SM, Valeri AM, Stokes MB, Masani NN, et al. (2009) Membranous glomerulonephritis with ANCA-associated necrotizing and crescentic glomerulonephritis. Clin J Am Soc Nephrol 4:299-308
[Crossref] [Google Scholar] [PubMed]
- Suwabe T, Ubara Y, Tagami T, Sawa N, Hoshino J, et al. (2005) Membranous glomerulopathy induced by myeloperoxidase-anti-neutrophil cytoplasmic antibody-related crescentic glomerulonephritis. Intern Med 44:853-858
[Crossref] [Google Scholar] [PubMed]
- Tse WY, Howie AJ, Adu D, Savage CO, Richards NT, et al. (1997) Association of vasculitic glomerulonephritis with membranous nephropathy: A report of 10 cases. Nephrol Dial Transplant 12:1017-1027
[Crossref] [Google Scholar] [PubMed]
- Kobayashi Y, Fujii K, Hiki Y, Chen XM (1985) Coexistence of IgA nephropathy and membranous nephropathy. Acta Pathol Jpn 35:1293-1299
[Crossref] [Google Scholar] [PubMed]
- Doi T, Kanatsu K, Nagai H, Kohrogi N, Hamashima Y (1983) An overlapping syndrome of IgA nephropathy and membranous nephropathy?. Nephron 35:24-30
[Crossref] [Google Scholar] [PubMed]
- Nasr SH, Ilamathi ME, Markowitz GS, D’Agati VD (2003) A dual pattern of immunofluorescence positivity. Am J Kidney Dis 42:419-426
[Crossref] [Google Scholar] [PubMed]
- Trpkov K, Abdulkareem F, Jim K, Solez K (1998) Recurrence of anti-GBM antibody disease twelve years after transplantation associated with de novo IgA nephropathy. Clin Nephrol 49:124-128
[Google Scholar] [PubMed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences