ISSN : 2573-4466
Insights in Enzyme Research
Water-Retaining Polymers in Organic Solvent Increase Lipase Activity for Biodiesel Synthesis
Fiamma Ghisleri1*, Pengcheng Fu2 and Francesco Secundo1
1Institute of Chemistry of Molecular Recognition, National Research Council, Via Mario Bianco 9, 20131 Milan, Italy
2College of Life Science and Technology, Beijing University of Chemical Technology, 15 Beisanhuan East Road, Chaoyang, District, Beijing 100029, China
- *Corresponding Author:
- Francesco Secundo
Institute of Chemistry of Molecular Recognition
National Research Council, Via Mario Bianco 9
20131 Milan, Italy
Tel: +390649931
Email: francesco.secundo@icrm.cnr.it
Received Date: February 03, 2017 Accepted Date: March 09, 2017 Published Date: March 12, 2017
Citation: Ghisleri F, Fu P, Secundo F . Water-Retaining Polymers in Organic Solvent Increase Lipase Activity for Biodiesel Synthesis. Insights Enzyme Res. 2017, 1:1. doi: 10.21767/2573-4466.100008
Abstract
Background: Biocatalyzed esterification reactions in non-aqueous media require water removal materials and optimized enzyme formulations to improve reaction rate and conversion. We have tested a lipase biocatalysis process using anionic polymeric chains of polyacrylate as super-absorbent of water in the conversion of methyl oleate from oleic acid and methanol and in toluene as reaction medium. The water removal material that we used also for the enzyme immobilization is commercially named Aquasorb 3005K2 (herein called Aquasorb).
Methods and findings: The synthesis of methyl oleate was catalyzed by Candida rugosa lipase or lipase G from Penicillium camemberti in the presence or absence of Aquasorb. The simple addition of Aquasorb to the reaction media of the lipase (commercial sample) catalyzed reaction allowed an enzyme activity 20% and 40% higher than that in the absence of Aquasorb for lipase from C. rugosa and lipase G, respectively. Furthermore, for the commercial form of lipase G, when used in the presence of Aquasorb, it was obtained a higher conversion of esterification (93% instead of 60%). Aquasorb was used also as support for the adsorption of the enzyme (via hydrogel formation followed by lyophilization) producing a lipase that was 100% and 60% more active than the enzyme dissolved in buffer in the absence of Aquasorb and lyophilized. However, these lipase formulations showed only about 20 % activity compared to when dry Aquasorb was simply added to the non-aqueous reaction medium.
Conclusions: The use of Aquasorb appears beneficial as additive in esterification reactions (e.g., for biodiesel production) catalyzed by lipases in non-aqueous reaction media.
Keywords
Esterification; Candida rugosa lipase; Enzyme formulation; Lipase Penicillum camemberti; Oleic acid
Introduction
Lipases are the group of hydrolases mostly applied in organic synthesis. In particular they have been used in processes such as esterification, transesterification (acidolysis, interesterification, and alcoholysis), aminolysis, oximolysis and thiotransesterification [1]. Lipases have several properties that make them particularly suitable for synthetic purposes. They are stable in organic solvents, do not require cofactors, possess broad substrate specificity and exhibit a high enantioselectivity [2,3].
The synthesis of biodiesel by lipase-catalyzed reactions has attracted increasing interests from numerous research groups in the world [4,5]. Biodiesel is a potential alternative to fossil fuels constituted of monoalkyl fatty acid esters including long chain fatty acids that can be derived from vegetable oil, animal fats, and microbial oil. Furthermore, due to their environmental and economic benefits, lipase-catalyzed biodiesel synthesis is a valid alternative to the conventional chemical catalytic processes [6].
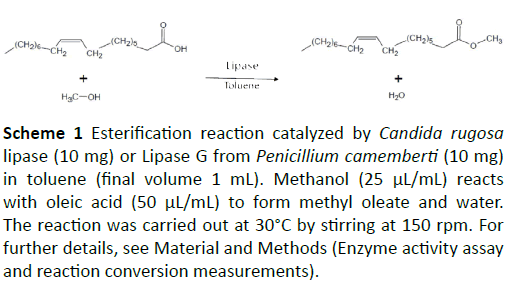
Biodiesel is usually obtained by the conversion of triglycerides from oil and animal fat into fatty acid alkyl esters with the aid of transesterification (also known as alcoholysis) carried out in the presence of short chain alcohols (e.g., methanol or ethanol) as nucleophile. However, the synthetic process that starts from a long chain fatty acid and an alcohol may also occur because of variable percentages of fatty acids that are present in the starting fat. The main drawback in the esterification reaction for the synthesis of the monoalkyl fatty acid ester is the production of water as side product (Scheme 1) that decreases the reaction rate and the yield of esterification.
Scheme 1: FigureEsterification reaction catalyzed by Candida rugosa lipase (10 mg) or Lipase G from Penicillium camemberti (10 mg) in toluene (final volume 1 mL). Methanol (25 µL/mL) reacts with oleic acid (50 µL/mL) to form methyl oleate and water. The reaction was carried out at 30°C by stirring at 150 rpm. For further details, see Material and Methods (Enzyme activity assay and reaction conversion measurements).
Several approaches have been adopted to remove water from the non-aqueous reaction media, including heteroazeotropic distillation, sparging of dry inert gas through the medium, or the application of salt hydrates. The most popular dehydration technologies involve reactive distillation, sorption, and membrane reactors [7]. Concerning the sorption methods, the addition of molecular sieves to reaction media is a simple and appropriate methodology to remove the by-produced water or to dehydrate organic solvents. An advantage of molecular sieves is that although they adsorb both water and the reaction products, they have pores only permeable by water molecules.
Analogously to molecular sieves, water-retaining polymeric material that forms hydrogel [8] could be suitable to remove water from a reaction medium that has to remain dry. Within this kind of materials, Aquasorb is a water retaining polymer (WRP) in the form of beads consisting of super-absorbent anionic polymeric chains of polyacrylate, parallel and regularly crosslinked to each other to form a polymeric network [9]. Aquasorb can form hydrogels that are insoluble in water and in non-aqueous media. It is usually employed in agricultural applications (e.g., to help the root network, or to prevent the cut flowers desiccation during their transportation, etc.). Neverthelss, to the best of our knowledge, it has never been used for capturing water produced in an esterification reaction.
In the present study, we employed Aquasorb 3005 K2 as water removal material in the process of esterification of oleic acid to methyloleate catalyzed by Candida rugosa lipase or lipase G from Penicillum camemberti (Scheme 1). In addition, we have also investigated the possibility to exploit Aquasorb as enzyme support for immobilization, with the purpose to improve the enzyme properties [6].
Methods
Materials
Aquasorb 3005K2 (Aquasorb) (bead diameter was 1-2 mm) used in our study as WRP was kindly donated from SNF Italia in form of lyophilized beads. Oleic acid (95%) was obtained from Alfa Aesar (Germany, Europe). Methanol, toluene and dodecane were obtained from Sigma-Aldrich (St. Louis, MO, USA). Candida rugosa lipase was purchased from Sigma-Aldrich (St. Louis, MO, USA) and Lipase G from Penicillium camemberti was purchased from Amano (Japan). All chemicals were of analytical grade and were used as received without any further purification.
Enzyme preparation
Lipases were used in different forms. They were either tested as untreated commercial product, or were adsorbed on the hydrogel obtained from the Aquasorb and lyophilized. To this aim, 4 mL of C. rugosa lipase or lipase G solution (2.5 mg/mL) in 10 mM phosphate buffer, pH 7 (buffer A) were added to 30 mg of Aquasorb 3005K2 and let to swell until a hydrogel was formed. Then, the lipase containing hydrogel was then frozen at -80°C and lyophilized. As control, lipases without hydrogel (only dissolved in buffer A and lyophilized) were also prepared.
Enzyme activity assay and reaction conversion measurements
Lipase activity was measured by monitoring the esterification of oleic acid (50 μL/mL) with methanol (25 μL/mL) in toluene (final volume 1 mL). In all cases the reaction was carried out using 10 mg of lyophilized enzyme (as described in Enzyme preparation) or untreated commercial lipase. This latter enzyme preparation was tested in the absence or in the presence (30 mg) of Aquasorb. Before use, the enzyme and all reagents were equilibrated for at least 24 h in the presence of a saturated solution of lithium bromide (LiBr) (water activity value of 0.06). The reaction was carried out by stirring at 150 rpm and at 30°C.
The formation of methyl oleate was examined by GC/MS (Chromatograph ThermoQuest-Trace GC (column 30 mx 0.25 mm x 0.25 pM) connected to a Finnigan-Trace DSQ MS system, setting the following parameters: 15°C/min from 150°C for 0.5 min to 230°C for 6 min.
Advancement of the esterification reaction was monitored at scheduled times by a GC-FID system (Agilent 6850), using a polydimethylsiloxane column (30m x 0.32 mm, film thickness 0.25 μM), under a constant flow of 2.7 mL/min. Injector and FID temperature was 300°C, ramp temperature of 15°C/min from 150°C for 0.5 min to 230°C for 6 min. The retention time was 5.6 and 5.9 min for methyl oleate and oleic acid, respectively.
The conversion of oleic acid to methyl oleate was calculated by the ratio of the normalized chromatographic peak area c=Area methyl oleate/(Area oleic acid + Area methyl oleate). The normalized peak area was obtained by dividing the peak area with the molecular weight of the corresponding analyzed compound. Dodecane (2 μL/mL) was used as internal standard to check injection reproducibility.
Results and Discussion
Lipase-catalyzed esterification reactions
Both lipases were able to catalyze the esterification reaction forming methyl oleate from oleic acid, as proved by GC/MS (Figure 1).
Figure 1: GC/MS chromatograms obtained injecting 1 µl of the reaction medium containing oleic acid in toluene (as described in Materials and Methods) before adding the lipase (in red, dashed) and after 4 hours from the addition of C. rugosa lipase (in blue). Mass spectra of the peak at 7.48 min (a) shows the typical fragments of methyl oleate, in particular of molecular mass of 296 [M] +, 264 [M-32]+, 222 [M-74]+, 180 [M-116]+ [10]. The mass spectra of peak at 7.85 min (b) corresponds to that of oleic acid, which shows typical fragments with molecular mass of 282 [M]+, 264 [M-18]+, 222 [M-60]+, 180 [M-102]+ 11. Similar chromatograms were obtained after the addition of lipase G to the reaction media (data not shown).
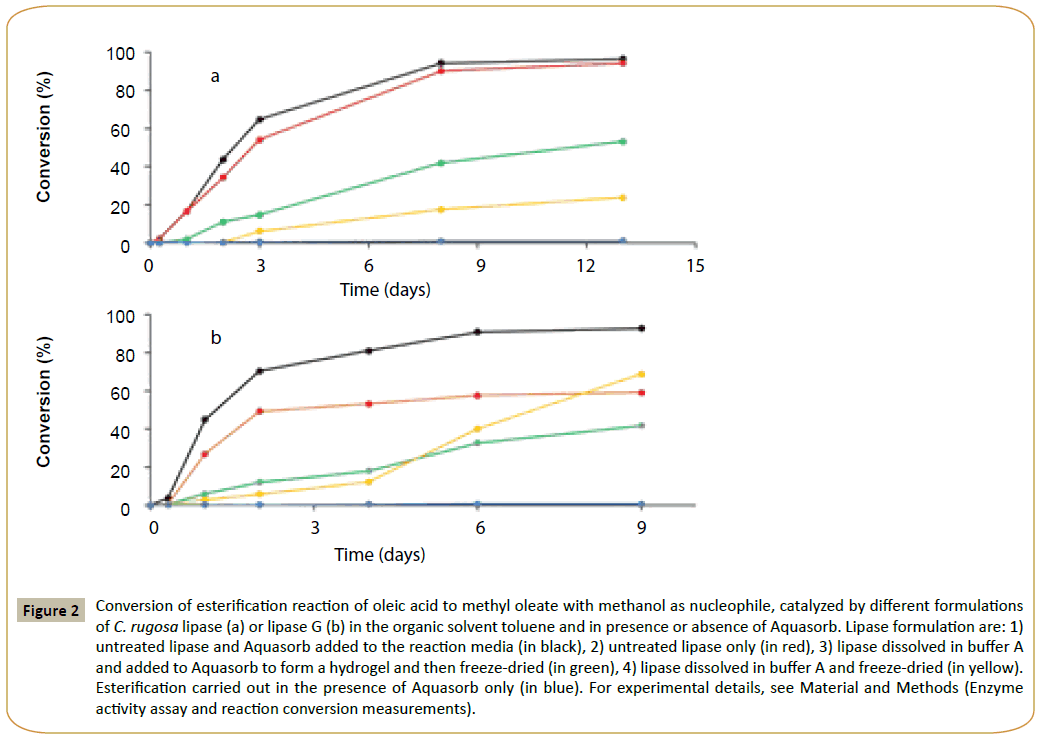
order to determine the enzyme efficiency in a given biocatalyzed reaction, it is important to investigate how a given enzyme preparation affects the reaction conversion (Figure 2) shows that for both lipases, the conversion of oleic acid to methyl oleate markedly depend on the lipase form. It can be seen that the highest conversion was obtained with the untreated lipase and Aquasorb added to the reaction medium. It is important to emphasize that in the study herein presented we could compare only the activity and reaction conversion for different forms of the same enzyme. It was not possible the comparison of the specific activity of the two lipases because the lipase forms herein tested were prepared from the semi-purified commercial enzymes, which are provided without the exact percentage of the lipase protein.
Figure 2: Conversion of esterification reaction of oleic acid to methyl oleate with methanol as nucleophile, catalyzed by different formulations of C. rugosa lipase (a) or lipase G (b) in the organic solvent toluene and in presence or absence of Aquasorb. Lipase formulation are: 1) untreated lipase and Aquasorb added to the reaction media (in black), 2) untreated lipase only (in red), 3) lipase dissolved in buffer A and added to Aquasorb to form a hydrogel and then freeze-dried (in green), 4) lipase dissolved in buffer A and freeze-dried (in yellow). Esterification carried out in the presence of Aquasorb only (in blue). For experimental details, see Material and Methods (Enzyme activity assay and reaction conversion measurements).
In the case of C. rugosa lipase (Figure 2a), the highest final esterification conversion value was about 96% for the untreated lipase used with the water-retaining polymers (in black), and 94% with the untreated enzyme only (curve in red), where the increase seemed minor. The addition of Aquasorb has also increased 20% the initial rate of the reaction catalyzed by the untreated commercial enzyme (Table 1). The reaction conversion with the lyophilized enzyme was lower than for the commercial untreated enzyme being 53% (curve in green) and 24% (in yellow) when lyophilized in presence and absence of Aquasorb, respectively. Furthermore, with the same two forms of C. rugosa lipase the initial reaction rate was 22% and 11% compared to the commercial untreated enzyme used with Aquasorb added to the reaction medium (Table 1).
| Initial rate (mol/h)a | ||
|---|---|---|
| Formulation | C. rugosa lipaseb | Lipase Gb |
| Untreated Lipase and Aquasorbc | 1.62 ± 0.42 (100) | 2.76 ± 0.66 (100) |
| Untreated lipase | 1.38 ± 0.06 (85) | 1.92 ± 0.42 (70) |
| Lipase in buffer with Aquasorb hydrogel and freeze-driedb | 0.36 ± 0.06 (22) | 0.48 ± 0.12 (16) |
| Lipase in buffer and freeze-dried | 0.18 ± 0.06 (11) | 0.3 ± 0.06 (11) |
aInitial rate was calculated multiplying the initial µmoles of the substrate (in the reaction herein tested 160 moles) by the conversion value and considering only the initial linear part of conversion curves reported in Figure 2 (conversion was not higher than 20%). The values refer to a reaction catalyzed by 10 mg of lipase. bIn parenthesis is reported relative activity (%) compared to the untreated enzyme used in the presence of Aquasorb added to the reaction media. cNo activity was observed with only Aquasorb.
Table 1: Initial esterification activity of Candida rugosa lipase and lipase G.
or lipase G from P. camemberti, a more impressive reaction conversion improvement by the water-retaining polymers was possible (Figure 2b), and a conversion of 93 % (black curve in Figure 2b) was possible only when the esterification reaction was catalyzed by the commercial enzyme with Aquasorb added to the reaction medium. Instead, in the absence of Aquasorb the conversion was not higher than 60%. The addition of Aquasorb has also increased 44% the initial rate of the reaction catalyzed by the untreated commercial enzyme (Table 1). The lyophilization of the enzyme, both in the absence or in the presence of Aquasorb, caused a marked decrease of the initial reaction rate (compared to the commercial enzyme used with Aquasorb added to reaction medium) and it was 16% of 11%, respectively (Table 1). The lower activity of the freeze-dried enzyme also resulted in a lower reaction conversion, which was not higher than 42% and 69% when the enzyme was freeze-dried in presence (green curve) and absence (yellow curve) of Aquasorb, respectively .
It is known that lipase formulation modulate enzyme properties [10-13]. The results presented herein show that the lyophilization process has inhibited the lipase functionality, and the presence of Aquasorb hydrogel plays a moderate lyoprotective effect.
Interestingly, Figure 2b indicates a particular behavior of Lipase G lyophilized in the absence of Aquasorb. It can be observed that this enzyme formulation has an increase of activity as a function of time. A possible explanation of this behavior is that the water released as a byproduct from the esterification reaction (Scheme 1) is adsorbed by the enzyme to optimize its functionality (e.g. catalytic activity). In fact, it is known that enzymes require a certain amount of water to function properly in non-aqueous media [13-15]. In the present study this effect is observed only with the lyophilized enzyme. We presume that the lyophilization process subtracts also the water essential for enzyme activity. It should be emphasized that the equilibration with LiBr (water activity 0.06), might not be as efficient as the lyophilization process in the removal of water from the enzyme powder. Thus, when the water activity of the reaction medium increases because of the water formed by the esterification, the enzyme adsorbs water that acts as enzyme “plasticizer”, allowing the increase of enzyme activity and the bioconversion. This agrees with the fact that there is no increase of catalytic activity as a function of time when in presence of Aquasorb, which would compete with the enzyme to take water from the reaction medium. However, in the absence of Aquasorb, the increase of water activity in the reaction medium also cause a higher hydrolytic reaction [16,17], which leads to a lower reaction conversion compared to that obtained with the untreated commercial enzyme used with Aquasorb (Figure 2b, curve in black).
Conclusions
We presented a novel application of Aquasorb in biocatalyzed reactions. In particular, it was used in a form of beads added to the reaction media without any pretreatment or after obtainment of a hydrogel (to allow the adsorption of the enzyme) followed by lyophilization. The advantages showed by Aquasorb for this application are i) the possibility to increase both the reaction conversion and the enzyme activity of enzymes in organic solvent, ii) the low cost per kilo (few dollars) and iii) the high amount of water that can be removed from the reaction medium, being their water absorbing capacity about 400 (w/w), iv) the stability in the organic media (the beads appeared intact after 12 days in organic solvents). Based on the observations and results obtained in this study we envisage Aquasorb might be a possible additive as water removal in the lipase catalyzed esterification of biodiesel or of the numerous esters of industrial interest obtained by lipases [1]. Furthermore, this material could also play a role in the hydration of the enzyme as observed in the comparison of the activity of both immobilized lipases for which Aquasorb had a lyoprotective effect. More investigations are planned for the study of this effect and to confirm the exploitability of these potential applications in other synthetic conditions and the economic feasibility.
Funding
This work was funded by the National Research Council of Italy. It was also partially funded by the Fundamental Research Funds for the Central Universities in China (No: YS0417) and Shaoxing Municipal government "330 overseas talents program 2016" in China.
Conflict of Interest
None.
References
- Bezbradica D, Crovic M, Tanaskovic SJ, Lukovic N, Carevic M, et al. (2017) Enzymatic Syntheses of Esters - Green Chemistry for Valuable Food, Fuel and Fine Chemicals. Curr Org Chem. 2017;21: 104-138.
- Ema T (2004) Mechanism of Enantioselectivity of Lipases and Other Synthetically Useful Hydrolases. Current Organic Chemistry 8: 1009-1025.
- Reetz MT (2002) Lipases as practical biocatalysts. Curr. Opin. Chem. Biol 6: 145-150.
- Pourzolfaghar H, Abnisa F, Mohd W, Wan A (2016) A review of the enzymatic hydro-esterification process for biodiesel production. Renew. Sustain. Energy Rev 61: 245-257.
- Norjannah B, Ong HC, Masjuki HH, Juan JC, Chong WT (2016) enzymatic trans-esterification for biodiesel production: a comprehensive review. RSC Adv 6: 60034-60055.
- Sankaran R, Show PL (2016) Biodiesel production using immobilized lipase: feasibility. Biofuels, Bioprod Bioref 10: 896-916.
- Diban N, Aguayo AT, Bilbao J (2013) Membrane reactors for in situ water removal: a review of applications. Ind Eng Chem Res 52: 10342-10354.
- Ahmed EM (2015) Hydrogel: Preparation, characterization, and applications. J Adv Res 6: 105-121.
- AQUASORBTM (2016) Water retainers for Soils and Substrates. SNF-group brochure at https://www.snf-group.com/images/pdf/Brochures_in_English/Agriculture-AQUASORB.pdf
- Hallgren B, Ryhage R, Stenhagen E (1959) The Mass Spectra of Methyl Oleate, Methyl Linoleate, and Methyl Linolenate. Acta Chemica Scandinavica 13: 845-847.
- National Institute of Standards and Technologies (2017) https://webbook.nist.gov/cgi/cbook.cgi?ID=112-80-1
- Kumar A, Dhar K, Kanwar SS, Arora PK (2016) Lipase catalysis in organic solvents: advantages and applications. Biol Proced Online 18: 1-11.
- Secundo F, Carrea G (2003) Optimization of hydrolase efficiency in organic solvents. Chem-A Eur J 9: 3194-3199.
- Yang L, Dordick JS, Garde S (2004) Hydration of enzyme in non-aqueous media is consistent with solvent dependence of its activity. Biophys J 87: 812-821.
- Zaks A, Klibanov AM (1988) Enzymatic catalysis in non-aqueous solvents. J Biol Chem 263: 3194-3201.
- Kulschewski T, Sasso F, Secundo F, Lotti M, Pleiss J (2013) Molecular mechanism of deactivation of C. antarctica lipase B by methanol. J Biotechnol 168: 462-469.
- Secundo F, Spadaro S (1999) Optimization of Pseudomonas cepacia lipase preparations for catalysis in organic solvents. Biotechnol Bioeng 62: 554-561.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences