ISSN : 2347-5447
British Biomedical Bulletin
The PI3K/Akt Signalling Pathway Plays Essential Roles in Mesenchymal Stem Cells
Cuncan Deng and Guihua Liu*
Reproductive Center, The Sixth Affiliated Hospital of Sun Yat-sen University, China
- Corresponding Author:
- Liu G
Reproductive Center
The Sixth Affiliated Hospital of Sun Yat-sen University
China
Tel: +8615918658906
E-mail: lgh99707@163.com
Received Date: April 05, 2017; Accepted Date: April 20, 2017; Published Date: April 22, 2017
Citation: Liu G, Deng C (2017) The PI3K/Akt Signalling Pathway Plays Essential Roles in Mesenchymal Stem Cells. Br Biomed Bull 5:301.
Copyright: © 2017 Liu G et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Mesenchymal stem cells (MSCs) can self-renew and maintain a multi-lineage differentiation potential. The PI3K/Akt signalling pathway is an essential signalling pathway of protein synthesis with complicated downstream activity. This review attempted to explore whether and how the PI3K/Akt signalling pathway influences MSCs. We concluded that MSCs participate in the proliferation, differentiation, mobilization, homing, senescence and apoptosis of mesenchymal stem cells. Mesenchymal stem cells (MSCs) can protect damaged cells through the PI3K/Akt signalling pathway, making it potentially useful in regenerative medicine and chronic diseases, such as ischaemic heart disease, end-stage liver disease, and ischemic stroke. The roles of the PI3K/Akt signalling pathway in MSCs were partly revealed, and more studies are needed. With further understanding of the detailed mechanisms involved, we can treat disease accurately and effectively.
Keywords
PI3K/AKT signal pathway; Mesenchymal stem cell; Differentiation; Senescence; Apoptosis
Introduction
Mesenchymal stem cells (MSCs) can be isolated from a variety of tissues, including bone marrow, umbilical cord, adipose tissue, endometrial polyps, and menses blood, among others [1]. Mesenchymal stem cells are stromal cells that have the ability to self-renew and differentiate along different lineages, making MSCs a possible treatment strategy for inflammatory bowel disease, periodontal regeneration, myocardial ischaemia, systemic lupus erythematosus, end-stage liver disease and ischaemic stroke [2-5].
The phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) signalling pathway is an important signalling pathway of protein synthesis in the body, playing a vital role in the proliferation, differentiation and apoptosis of cancer cells [6-8]. Extensive research has been carried out to explore the mechanisms of the modulation of mesenchymal stem cells, whereas the mechanisms of the PI3K/Akt signalling pathway in physiological activity remain unclear.
This review focused on the PI3K/Akt signalling pathway in mesenchymal stem cells to explore how the PI3K/Akt signalling pathway influences the physiological activity of MSCs and to predict its potential clinical use.
The PI3K/Akt Signalling Pathway
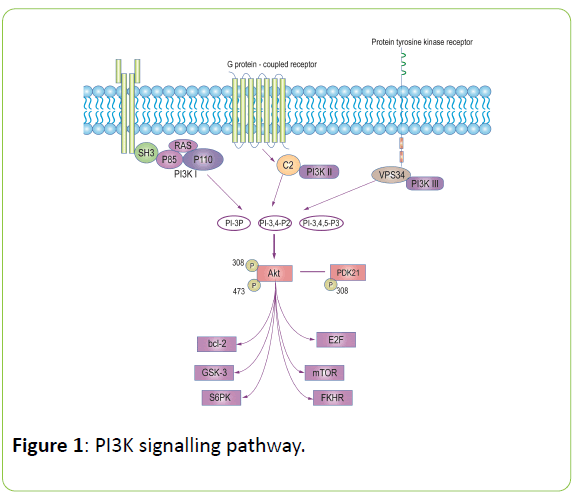
PI3K has a relative molecular mass of 110 × 103 in its P110 catalytic subunit and 85×103 in its P85 regulatory subunit. The amino terminal of P85 contains a SH3 domain and a proline-rich region, which can be combined with the SH3 domain, and its carboxyl terminal contains 2 SH2 domains and 1 region of the P110 binding domain. The P110 subunit of PI3K has homology with protein kinase, which has both Ser/Thr kinase and phosphatidylcholine kinase activity. According to the structural characteristics of P110, the substrates of PI3K can be divided into three subtypes: I, II, and III. Type I is divided into I A and I B subclasses.
The I A subtypes include P110 α, P110 β, and P110 δ, which can form two dimers with p85; the I B subtypes include P110γ, which does not combine with p85, but combines with a joint protein with a relative molecular mass of 101 × 103. This join t protein can be mediated the activation of P110 by G protein’s β and γ subunits [9].
Type PI3K is a PI3K-containing C2 domain; Type III PI3K is a structurally homologous protein found in mammalian cells with the VPS34 molecular structure of yeast. Akt, also known as protein kinase B (PKB), is a serine/threonine protein kinase. There are at least 3 members of the Akt family: Akt1/PKBα, Akt2/PKBβ, and Akt3/PKBγ. PI3K can be activated by G proteincoupled receptors, protein tyrosine kinase receptors, and/or Ras protein.
PI3K promotes the phosphorylation of phosphatidylinositol, including 3-phosphatidylinositol (PI-3P), 3,4-two phosphatidylinositol (PI-3, 4-P2), and phosphatidylinositol-3,4,5- trisphosphate (PI(3, 4, 5)P3), by phosphorylating the 3 hydroxyls of the phosphatidylinositol ring. A variety of grow factors function through the PI3K/Akt signalling pathway [10-12].
Akt/PKB is an important downstream target kinase in the PI3K signalling pathway. PI3K-activated 4-P2, PI-3 and PI-3, 4, 5-P3 combines with the PH region of Akt. This process not only leads to the translocation of Akt from the cytoplasm to the cell membrane but also leads its structural changes, including Ser473 and Thr308 phosphorylation. Phosphorylation of Ser473 or Thr308 sites is necessary for the activation of Akt. Additionally, Akt activation is required for the involvement of the phospholipid dependent protein kinase (PDK).
Activated Akt regulates the cell cycle, mobilization and apoptosis by further activating its downstream factors, such as the Bcl-2 family, mTOR, E2F, glycogen synthesis enzyme 3 (GSK3), fork head transcription factor (FKHR) and S6 protein kinase(S6PK) (Figure 1).
The PI3K/Akt Signalling Pathway in the Proliferation of MSCs
The self-renewal ability of mesenchymal stem cells, which is considered a promising option for regenerative medicine and tissue engineering, has aroused great interest. Several studies have explored how the PI3K/Akt signalling pathway influences mesenchymal stem cell proliferation.
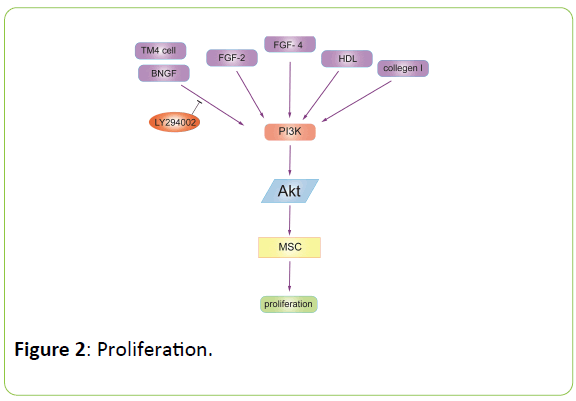
Researchers 10 found that co-culture with the TM4 mouse Sertoli cells, which can produce soluble factors that enhance the growth of bone marrow-derived mesenchymal stromal cells (BM-MSCs) without inhibiting multipotency, accelerated the progress of BM-MSCs from the G1 to the S phase, and the expression of phospho-Akt, mdm2, pho-CDC2, and cyclin D1 were markedly upregulated.
By adding LY294002, an PI3K/Akt inhibitor, the upregulation was inhibited. Epithelial growth factor (EGF) stimulated the proliferation of the BM-MSCs more significantly compared with the other growth factors. The neutralization of EGF via a blocking antibody dramatically limited the promoting growth effect in BMMSCs.
These results suggest that TM4 cells provide a favorable in vitro environment for BM-MSC growth by the involvement of the GF/PI3K/Akt pathway. To explore whether nerve growth factor (NGF) induced the angiogenesis of bone marrow mesenchymal stem cells (MSCs) and the underlying mechanisms, Wen-xia Wang and colleagues [13] collected bone marrow MSCs and treated them with NGF. The results showed that NGF (25, 50, 100 and 200 μg/L) promoted tube formation of MSCs. NGF (50 μg/L) significantly enhanced Akt phosphorylation.
Pre-treatment with the specific PI3K inhibitor LY294002 (10 mol/L) blocked NGF-stimulated Akt phosphorylation, tube formation and angiogenesis. NGF (50 μg/L) markedly increased the proliferation of MSCs.
The research indicated that NGF promoted the proliferation of MSCs and activated the PI3K/Akt signaling pathway, which may be responsible for the NGF induction of MSC angiogenesis. To investigate the change in mRNA expression in rat MSCs after low-level laser irradiation (LLLI) and to reveal the molecular mechanisms, scientists [14] treated Rat MSCs with a laser and used a cDNA microarray to analyse gene expression. Real-time PCR confirmed that the expression levels of v-Akt murine thymoma viral oncogene homologue 1 (Akt1), the cyclin D1 gene (Ccnd1) and the phosphatidylinositol 3-kinase catalytic alpha polypeptide gene (Pik3ca), were upregulated after LLLI, whereas those of protein tyrosine phosphatase non-receptor type 6 (Ptpn6) and serine/threonine kinase 17b (Stk17b) were downregulated.
cDNA microarray analysis revealed that after LLLI, the expression levels of various genes involved in cell proliferation, apoptosis and the cell cycle were affected. Five genes, including Akt1, Ptpn6, Stk17b, Ccnd1 and Pik3ca, were confirmed and the PI3K/Akt/mTOR/eIF4E pathway was identified as possibly playing an important role in mediating the effects of LLLI on the proliferation of MSCs. Jianfeng Xu and colleagues [15] found that the proliferation of MSCs induced by high density lipoprotein cholesterol in a time-and concentration dependent manner, which was triggered with the phosphorylation of Akt and ERK1/2.
The proliferation of MSCs was notably attenuated by the specific inhibitor to the respective pathway. The study suggested that high density lipoprotein protects mesenchymal stem cells from oxidative stress-induced apoptosis via the activation of the PI3K/Akt pathway and the suppression of reactive oxygen species.
The PI3K/Akt signalling pathway also plays a role in the proliferation of mesenchymal stem cells. Kuo-Shu Tsai and colleagues [16] demonstrated that collagen I promotes the proliferation and osteogenesis of hMSCs via the activation of the ERK and Akt pathway. Zhao-jun Wang and colleagues [17] elucidated that suitable treatment with lipopolysaccharides can protect mesenchymal stem cells from oxidative stress-induced apoptosis and promote the proliferation of MSCs through the Toll-like receptor (TLR)-4 and PI3K/Akt signalling pathway. Seung- Cheol Choi and colleagues found that pERK1/2 and pAkt levels were upregulated in the FGF-2-or FGF-4-treated Sca-1+BMMSCs, and this effect is attenuated by special inhibitors. The upregulation of pERK1/2 and pAkt leads to the upregulation of c-Jun in Sca-1+BMMSCs. These data suggested that FGF-2 and FGF-4 promote the proliferation of Sca-1+BM-MSCs by the activation of the ERK1/2 and PI3K-Akt signalling pathway (Figure 2).
As discovered in several studies, the activation of PI3K/Akt signalling pathway is widely involved in the proliferation of MSCs.
The PI3K/Akt Signalling Pathway in the Differentiation of MSCs
In addition to their proliferative ability, mesenchymal stem cells maintain a multi-lineage differentiation potential as well. Human mesenchymal stem cells from bone marrow, adipose tissue and skin exhibit differences in differentiation potential [18]. Different factors lead to the adipocyte differentiation, osteogenic differentiation, endothelial differentiation and neural differentiation of mesenchymal stem cells involving the PI3K/Akt signalling pathway.
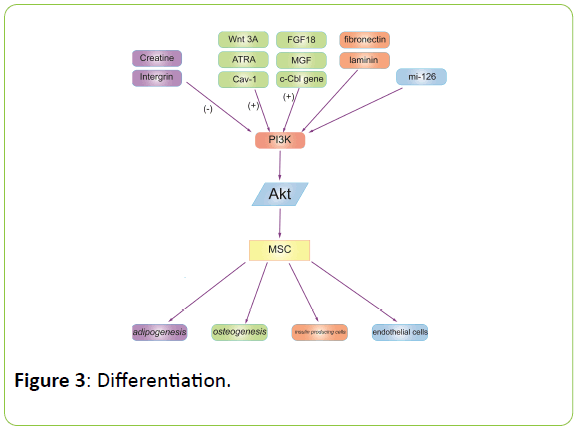
Creatine appears to exert its inhibitory effects on adipogenesis during early differentiation, but not late differentiation, or proliferation stages through the inhibition of the PI3K-Akt-peroxisome proliferator-activated receptor γPPARγ signalling pathway.
In an in vivo model, the administration of creatine to mice resulted in a body mass increase without fat accumulation [19]. Shuang Zhang and colleagues [20] carried out experiments to explore whether Wnt3A and all trans-retinoic acid (ATRA) cooperate in MSC osteogenic differentiation.
ATRA and Wnt3A synergistically promoted Akt phosphorylation, enhancing b-catenin-dependent transcription through GSK3b inhibition or direct b-catenin phosphorylation at Ser552. This event was abolished by LY294002 pretreatment.
This result suggested that ATRA and Wnt3A at least partially enhanced osteogenic differentiation by activating the PI3K/Akt/ GSK3b signalling pathway. Different factors may influence the osteoblast differentiation of MSCs via the PI3K/Akt signalling pathway. Natasha Baker and colleagues [21] incubated MSCs or induced osteogenic differentiation.
They found that LY294002, Akt siRNA and methyl-β- cyclodextrin inhibited MSC osteogenesis, while Cav-1 siRNA and cholesterol oxidase, which displace Cav-1 from caveolae, enhanced Akt signalling induced by osteogenic supplements. They confirmed that PI3K/Akt signalling is an essential pathway for human MSC osteogenesis, and it is likely that the localization of active Akt in non-caveolar and caveolar membrane rafts positively and negatively contribute to osteogenesis, respectively.
c-Cbl is a gene encoding a RING finger E3 ubiquitin ligase and is a direct target of miR-216a. Downregulation of c-Cbl by short hairpin RNAs can mimic the promotion effects of miR-216a and significantly rescue the suppressive effects of dexamethasone on osteogenesis. Pathway analysis showed that miR-216a regulates osteogenic differentiation through the c-Cbl-mediated (PI3K)/Akt pathway.
The recovery effects of miR-216a on the inhibition of osteogenesis by dexamethasone were attenuated after blocking the PI3K pathway. H Li and colleagues [22] demonstrated that miR-216a rescues the dexamethasone suppression of osteogenesis, promotes osteoblast differentiation and promotes bone formation by regulating the c-Cbl-mediated PI3K/Akt pathway.
Yanxiang Tong and colleagues [23] reported that mechanogrowth factor (MGF) further promoted the proliferation and induced the osteogenic differentiation of rabbit mesenchymal stem cells through the PI3K/Akt pathway. Autocrine fibroblast growth factor 18 mediates the dexamethasone-induced osteogenic differentiation of murine mesenchymal stem cells through FGFR1/FGFR2-mediated ERK1/2-MAPKs and PI3K signalling.
The mitogen-activated protein kinase (MAPK) and phosphatidyl inositol-3-kinase (PI3K) pathway were activated in MSCs cultured on poly (lactide-co-glycolide) (PLGA) substrates.
When these signalling pathways were inhibited, the osteogenic differentiation was suppressed. These data suggested that initial extracellular matrix deposition, subsequent matrix remodelling, and corresponding integrin expression profiles play synergistic roles in osteogenesis in MSCs cultured on PLGA at least partially by engaging the MAPK and PI3K signalling pathway [24].
In addition, the PI3K/Akt signalling pathway joins in the progress of differentiation of human mesenchymal stem cells into insulin-producing cells under the stimulation of fibronectin or laminin [25].
By the activation of the PI3K/Akt and MAPK/ERK pathways and the release of paracrine factors, the over-expression of miR-126 promotes the differentiation of mesenchymal stem cells to endothelial cells [26].
Salidroside induces mesenchymal stem cells’ differentiation into hepatocytes by the ERK1/2 and PI3K signalling pathways in vitro [27]. Furthermore, brain-derived neurotrophic factor stimulates the neural differentiation of human umbilical cord blood-derived mesenchymal stem cells with the involvement of MAPK/ERK-dependent p35 upregulation and MAPK/ERKdependent and PI3K/Akt-dependent Bcl2 upregulation (Figure 3) [28].
The PI3K/Akt Signalling Pathway in MSC Mobilization and Homing
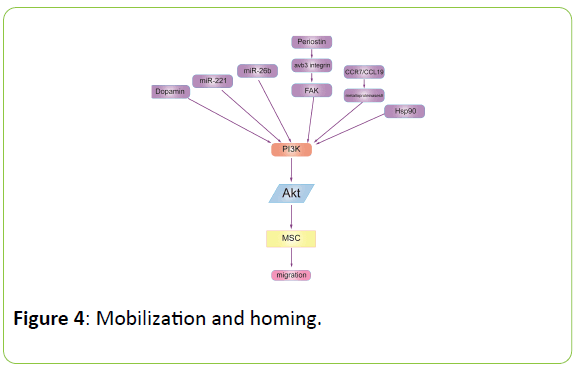
The PI3K-Akt signalling pathway regulates the biological activities of cells, including the homing and mobilization of MSCs. The chemotactic migration of mesenchymal stem cells (MSCs) is fundamental for clinical use in cell-based therapies, but the underlying mechanism remains unknown. Isabel Mirones and colleagues [29] reported that dopamine induced in vitro MPC migration through D2-class receptors and their specific PI3K/Akt pathway. Aisi Zhu and colleagues [30] demonstrate that miR-221 and miR-26b participate in regulating the chemotactic response of MSCs towards HGF through the activation of Akt and FAK. Weidong Bing and colleagues [31] indicated that simvastatin improved the migration of BMSCs via the PI3K/Akt pathway. Periostin expression in the human periodontal ligament promoted the migration of hMSCs through the avb3 integrin/FAK/PI3K/Akt pathway in vitro [32]. Wei Zhang and colleagues [33] suggested that C–C chemokine receptor 7 (CCR7) and chemokine ligand 19 (CCL19) contributes to the migration of BMSCs by upregulating matrix metalloproteinases-9 potentially via the PI3K/Akt pathway. The study performed by Feng Gao and colleagues indicated that Hsp90a promotes MSC migration via the PI3K/Akt and ERK signalling pathways (Figure 4) [34].
Based on this research, we can safely draw the conclusion that the activation and migration of MSCs is somehow achieved through the PI3K/Akt signalling pathway.
The PI3K/Akt Signalling Pathway in MSC Senescence and Apoptosis
Their self-renewal abilities and multi-lineage differentiation potential have made mesenchymal stem cells the most popular target in both scientific research and clinical treatment. However, difficulties in the precise mobilization, senescence and apoptosis of MSCs limit its utilization. The PI3K/Akt signalling pathway is involved in the progress of mesenchymal stem cell senescence and apoptosis.
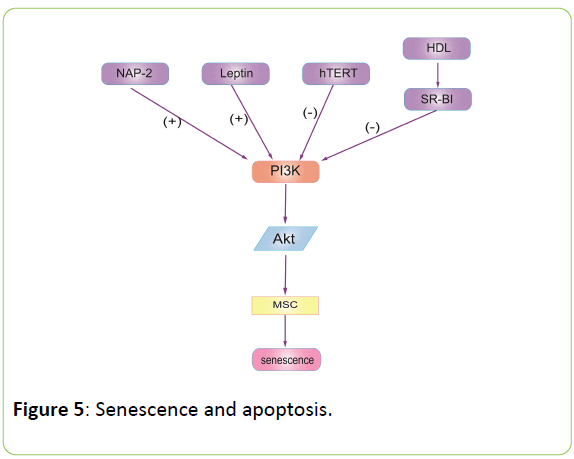
Ihn Han and colleagues [35] demonstrated that the downregulation of the PI3K/Akt signalling pathway and the activated c-jun NH2-terminal kinase participate in apoptosis induction in PC-3 prostate cancer cells of umbilical cord tissuederived mesenchymal stem cells. Haifeng et al [36] showed that leptin and Neutrophil-Activating Peptide 2(NAP-2) act synergistically to promote MSC senescence through the enhancement of the PI3K/Akt signalling pathway in SLE patients. Qiang Zhao and colleagues [37] reported that human telomerase reverse transcriptase (hTERT) mediates the senescence of MSCs through the PI3K/Akt signalling pathway. Jianfeng Xu and colleagues [38] suggested that high-density lipoprotein improves MSC proliferation through the MAPK/ERK1/2 and PI3K/Akt pathway with scavenger receptor-B type I (SR-BI) (Figure 5).
These studies showed that PI3K/Akt signalling pathway is essential in MSC senescence. By activating the PI3K/Akt signalling pathway, MSCs slow down their senescence and apoptosis but improve their proliferation.
The PI3K/Akt Signalling Pathway in the Protection Effect of MSCs
Since MSCs have a self-renewal ability and anti-apoptosis ability, there is great interest in the protective effect of MSCs and the involvement of the PI3K/Akt signalling pathway in its mechanism.
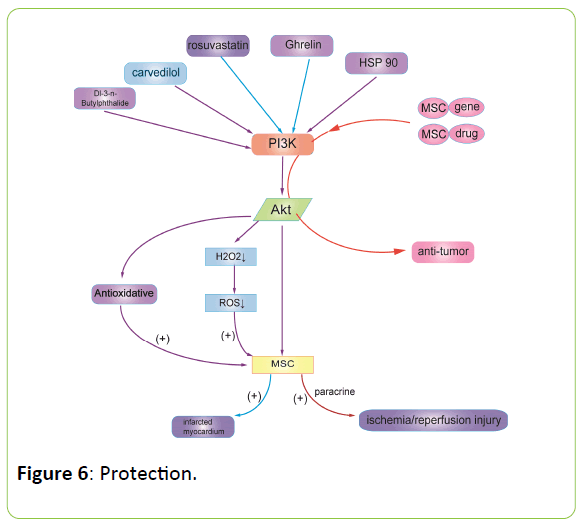
Previous studies have shown that Dl-3-n-Butylphthalide could protect rat bone marrow stem cells against apoptosis due to antioxidative properties and modulation of the PI3K/Akt pathway [39] Meihui Chen and colleagues [40] investigate the effects of carvedilol against hydrogen peroxide (H2O2)-induced bone marrow-derived mesenchymal stem cells (BMSCs) death, which imitate the microenvironment surrounding transplanted cells in the injured spinal cord in vitro. Carvedilol significantly reduced H2O2-induced reactive oxygen species production, apoptosis and subsequent cell death. LY294002, the PI3K inhibitor, blocked the protective effects and upregulation of Akt phosphorylation of carvedilol. The result suggested that carvedilol’s cooperation with BMSCs could be utilized in the treatment of spinal cord injury by improving cell survival and oxidative stress microenvironments through the PI3K/Akt signalling pathway.
Myocardial infarction is a common disease in which the myocardial cells lack blood and oxygen. A study elucidated that rosuvastatin may improve the survival of engrafted adiposederived mesenchymal stem cells after transplantation into infarcted hearts through the PI3K/Akt and MEK/ERK1/2 signalling pathway [41]. Ghrelin improves the efficacy of mesenchymal stem cell-based therapy for cardiac ischaemic disease through the PI3K/Akt pathway as well [42]. The overexpression of phosphoinositide-3-kinase class II alpha promotes mesenchymal stem cell survival in the infarcted myocardium [43]. The activation of PKC, downstream of PI3k, improves the retention, survival and differentiation of transplanted MSCs in the myocardia, which means a better clinical effect in treatment with myocardial ischaemia [44]. Denis Angoulvant and colleagues [45] demonstrated that mesenchymal stem cells (MSC) or their conditioned media MSCCM added at the onset of reperfusion can protect the myocardium from ischaemia/reperfusion injury by the paracrine activation of the PI3K pathway.
The PI3K/Akt signalling pathway takes part in physiological activities, protecting tissues and cells against oxidative damage. MSCs might protect PC12 cells and neurons from ethanolinduced oxidative damage at least partly by means of the upregulation of PI3K/Akt and the modulation of ERK1/2 activation [46]. Both heat shock protein 90 and lovastatin protect mesenchymal stem cells from hypoxia-and serum deprivation-induced apoptosis by the activation of PI3K/Akt and ERK1/2 [34].
If the tumour microenvironment exhibits chronic inflammation, MSCs will detect the tumour tissue around the damage, and endogenous bone marrow MSCs, adipose MSCs or intravenous infusion of MSCs will migrate to the tumour site. Thus, MSCs can be used as vectors for tumour gene therapy to efficiently deliver tumour-treated genes or drugs to tumour sites [47]. MSCs serve as anti-tumour gene carriers that can inhibit tumour activity after transplantation into the body (Figure 6) [48-50].
MSCs can protect the damaged cells in the human body through the PI3K/Akt signalling pathway; thus, MSCs might be a potential treatment in the clinical therapy of chronic diseases. MSCs might protect normal cells in patient with cancer by carrying tumour treated genes or drugs to kill cancers cells accurately.
Future Prospects
In conclusion, the migration and homing of MSCs are influenced by biochemical factors, which then further activate the PI3K/Akt signalling pathway to achieve a biological effect. The PI3K/Akt signalling pathway plays an important role in proliferation, differentiation, mobilization, homing, senescence and apoptosis. Additionally, MSCs can protect tissues and cells from damage via PI3K/Akt.
Although part of the mechanism has been revealed, the complete mechanism of how the PI3K/Akt signalling pathway and its downstream activity influence the physiological process of MSCs remains unclear. Further studies should explore the deeper mechanism of the role of the PI3K/Akt signalling pathway. With a deeper understanding of this mechanism in the future, we might be able to more efficiently grow mesenchymal stem cells and precisely regulate the differentiation of MSCs. We may be able to guide homing and delay senescence and apoptosis.
Mesenchymal stem cells have great therapeutic potential for many diseases, such as ischaemic heart disease, end-stage liver disease, and ischaemic stroke. However, the precise effect of the PI3K/Akt signalling pathway requires further exploration.
With further understanding of the detailed mechanism, we can treat disease accurately and effectively. A more comprehensive understanding of the PI3K/Akt signalling pathway would facilitate its use in both laboratory research and clinical practice.
References
- Ding DC, Shyu WC, Lin SZ (2011) Mesenchymal stem cells. Cell Transplant 20: 5-14.
- Mao F, Tu Q, Wang L, Chu F, Li X, et al. (2017) Mesenchymal stem cells and their therapeutic applications in inflammatory bowel disease. Oncotarget.
- Zorzopulos J, Opal SM, Hernando Insua A (2017) Immunomodulatory oligonucleotide IMT504: Effects on mesenchymal stem cells as a first-in-class immunoprotective/immunoregenerative therapy. World J Stem Cells 9: 45-67.
- Csobonyeiova M, Polak S, Zamborsky R (2017) iPS cell technologies and their prospect for bone regeneration and disease modeling: A mini review. J Adv Res 8: 321-327.
- Cao Y, Gang X, Sun C (2017) Mesenchymal stem cells improve healing of diabetic foot ulcer. J Diabetes Res 9328347.
- Zhang Y, Zhang JW, Lv GY (2012) Effects of STAT3 gene silencing and rapamycin on apoptosis in hepatocarcinoma cells. Int J Med Sci 9: 216-224.
- Bhaskar PT, Hay N (2007) The two TORCs and Akt. Dev Cell 12: 487-502.
- Engelman JA (2009) Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer 9: 550-562.
- Voigt P, Brock C, Nurnberg B (2005) Assigning functional domains within the p101 regulatory subunit of phosphoinositide 3-kinase gamma. J BiolChem 280: 5121-5127.
- Tian H, Guo M, Zhuang Y (2014) Enhanced proliferation of bone marrow mesenchymal stem cells by co-culture with TM4 mouse Sertoli cells: involvement of the EGF/PI3K/AKT pathway. Mol Cell Biochem 393:155-164.
- Yulyana Y, Ho IAW, Sia KC (2015) Paracrine factors of human fetal MSCs inhibit liver cancer growth through reduced activation of IGF-1R/PI3K/Aktsignaling. MolTher 23: 746-756.
- Forte G, Minieri M, Cossa P (2006) Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem cells 24: 23-33.
- Wang WX, Hu XY, Xie XJ (2011) Nerve growth factor induces cord formation of mesenchymal stem cell by promoting proliferation and activating the PI3K/Akt signaling pathway. ActaPharmacol Sin 32: 1483-1490.
- Wu Yh, Wang J, Gong DX (2011) Effects of low-level laser irradiation on mesenchymal stem cell proliferation: a microarray analysis. Lasers Med Sci 27: 509-519.
- Xu J, Qian J, Xie X (2012) High density lipoprotein protects mesenchymal stem cells from oxidative stress-induced apoptosis via activation of the PI3K/Aktpathway and suppression of reactive oxygen species. Int J MolSci 13: 17104-17120.
- Tsai KS, Kao SY, Wang CY (2010) Type I collagen promotes proliferation and osteogenesis of human mesenchymal stem cells via activation of ERK and Akt pathways. J Biomed Mater Res A 94: 673-682.
- Wang ZJ, Zhang FM, Wang LS (2009) Lipopolysaccharides can protect mesenchymal stem cells (MSCs) from oxidative stress-induced apoptosis and enhance proliferation of MSCs via toll-like receptor(TLR)-4 and PI3K/Akt. Cell BiolInt 33: 665-674.
- Al Nbaheen M, Vishnubalaji R, Ali D, (2013) Human stromal (mesenchymal) stem cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential. Stem Cell Rev 9: 32-43.
- Lee N, Kim I, Park S (2015) Creatine inhibits adipogenesis by downregulating insulin-induced activation of the phosphatidylinositol 3-kinase signaling pathway. Stem Cells Dev 24: 983-994.
- Zhang S, Chen X, Hu Y (2016) All-trans retinoic acid modulates Wnt3A-induced osteogenic differentiation of mesenchymal stem cells via activating the PI3K/AKT/GSK3β signalling pathway. Mol Cell Endocrinol 422: 243-253.
- Baker N, Sohn J, Tuan RS (2015) Promotion of human mesenchymal stem cell osteogenesis by PI3-kinase/Akt signaling, and the influence of caveolin-1/cholesterol homeostasis. Stem Cell Res Ther 6: 238.
- Li H, Li T, Fan J (2015) miR-216a rescues dexamethasone suppression of osteogenesis, promotes osteoblast differentiation and enhances bone formation, by regulating c-Cbl-mediated PI3K/AKT pathway. Cell Death Differ 22: 1935-1945.
- Tong Y, Feng W, Wu Y (2015) Mechano-growth factor accelerates the proliferation and osteogenic differentiation of rabbit mesenchymal stem cells through the PI3K/AKT pathway. BMC Biochem 16: 1.
- KunduAK, Khatiwala CB, Putnam AJ (2008) Extracellular matrix remodeling, integrin expression,and downstream signaling pathways influence the osteogenic differentiation of mesenchymal stem cells on poly(lactide-co-glycolide) substrates. Tissue Eng Part A 15: 273-283.
- Lin HY, Tsai CC, Chen LL (2010) Fibronectin and laminin promote differentiation of human mesenchymal stem cells into insulin producing cells through activating Akt and ERK. J Biomed Sci 17: 56.
- Huang F, Fang Zf, Hu Xq (2013) Overexpression of miR-126 promotes the differentiation of mesenchymal stem cells toward endothelial cells via activation of PI3K/Akt and MAPK/ERK pathways and release of paracrine factors. BiolChem 394: 1223-1233.
- Ouyang JF, Lou J, Yan C (2010) In-vitro promoted differentiation of mesenchymal stem cells towards hepatocytes induced by salidroside. J Pharm Pharmacol 62: 530-538.
- Lim JY, Park SI, Oh JH (2008) Brain-derived neurotrophic factor stimulates the neural differentiation of human umbilical cord blood-derived mesenchymal stem cells and survival of differentiated cells through MAPK/ERK and PI3K/Akt-dependent signaling pathways. J Neurosci Res 86: 2168-2178.
- Mirones I, Angel Rodriguez Milla M, Cubillo I (2014) Dopamine mobilizes mesenchymal progenitor cells through D2-class receptors and their PI3K/AKT pathway. Stem cells 32: 2529-2538.
- Zhu A, Kang N, He L (2015) MiR-221 and miR-26b regulate chemotactic migration of MSCs toward HGF through activation of Akt and FAK. J Cell Biochem 117: 1370-1383.
- Bing W, Pang X, Qu Q (2016) Simvastatin improves the homing of BMSCs via the PI3K/AKT/miR-9 pathway. J Cell Mol Med 20: 949-961.
- Matsuzawa M, Arai C, Nomura Y (2015) Periostin of human periodontal ligament fibroblasts promotes migration of human mesenchymal stem cell through the alphavbeta3 integrin/FAK/PI3K/Akt pathway. J Periodont Res 50: 855-863.
- Zhang W, Tu G, Lv C (2014) Matrix metalloproteinase-9 is up-regulated by CCL19/CCR7 interaction via PI3K/Akt pathway and is involved in CCL19-driven BMSCs migration. BiochemBiophys Res Commun 451: 222-228.
- Gao F, Hu X, Xie X (2015) Heat shock protein 90 stimulates rat mesenchymal stem cell migration via PI3K/Akt and ERK1/2 pathways. Cell BiochemBiophys 71: 481-489.
- Han I, Yun M, Kim EO (2014) Umbilical cord tissue-derived mesenchymal stem cells induce apoptosis in PC-3 prostate cancer cells through activation of JNK and downregulation of PI3K/AKT signaling. Stem Cell Res Ther 5: 54.
- Chen H, Shi B, Feng X (2015) Leptin and neutrophil-activating peptide 2 promote mesenchymal stem cell senescence through activation of the phosphatidylinositol 3-kinase/akt pathway in patients with systemic lupus erythematosus. Arthritis Rheumatol 67: 2383–2393.
- Zhao Q, Wang XY, Yu XX (2015) Expression of human telomerase reverse transcriptase mediates the senescence of mesenchymal stem cells through the PI3K/AKT signaling pathway. Int J Mol Med. 36: 857-864.
- Xu J, Qian J, Xie X (2012) High density lipoprotein cholesterol promotes the proliferation of bone-derived mesenchymal stem cells via binding scavenger receptor-B type I and activation of PI3K/Akt, MAPK/ERK1/2 pathways. Mol Cell Biochem 371: 55-64.
- Sun B, Feng M, Tian X (2012) DL-3-n-Butylphthalide protects rat bone marrow stem cells against hydrogen peroxide-induced cell death through antioxidation and activation of PI3K-Akt pathway. Neurosci Lett 516: 247-252.
- Chen M, Chen S, Lin D (2016) Carvedilol protects bone marrow stem cells against hydrogen peroxide-induced cell death via PI3K-AKT pathway. Biomed Pharmacother 78: 257-263.
- Zhang Z, Li S, Cui M (2013) Rosuvastatin enhances the therapeutic efficacy of adipose-derived mesenchymal stem cells for myocardial infarction via PI3K/Akt and MEK/ERK pathways. Basic Res Cardiol 108: 333.
- Han D, Huang W, Ma S (2015) Ghrelin improves functional survival of engrafted adipose-derived mesenchymal stem cells in ischemic heart through PI3K/Akt signaling pathway. Biomed Res Int 2015: 858349.
- Eun LY, Song BW, Cha MJ (2010) Overexpression of phosphoinositide-3-kinase class II alpha enhances mesenchymal stem cell survival in infarcted myocardium. BiochemBiophys Res Commun 402: 272-279.
- He H, Zhao ZH, Han FS (2016) Overexpression of protein kinase C varepsilon improves retention and survival of transplanted mesenchymal stem cells in rat acute myocardial infarction. Cell Death Dis 7: e2056.
- Angoulvant D, Ivanes F, Ferrera R (2011) Mesenchymal stem cell conditioned media attenuates in vitro and ex vivo myocardial reperfusion injury. J Heart Lung Transplant 30: 95-102.
- Liu L, Cao JX, Sun B (2010) Mesenchymal stem cells inhibition of chronic ethanol-induced oxidative damage via upregulation of phosphatidylinositol-3-kinase/Akt and modulation of extracellular signal-regulated kinase 1/2 activation in PC12 cells and neurons. Neuroscience 167: 1115-1124.
- Shah K (2012) Mesenchymal stem cells engineered for cancer therapy. Adv Drug Deliv Rev 64: 739-748.
- Kim SM, Lim JY, Sang IP (2008) Gene therapy using TRAIL-secreting human umbilical cord blood–derived mesenchymal stem cells against intracranial glioma. Cancer Res 68: 9614-9623.
- Studeny M, Marini FC, Dembinski JL (2004) Mesenchymal stem cells: Potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst 96: 1593-1603.
- Hall B, Andreeff M, Marini F (2007) The participation of mesenchymal stem cells in tumor stroma formation and their application as targeted-gene delivery vehicles. HandbExpPharmacol 263-283.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences