Multipath Natural Product Supplement Suppresses Dementia Symptoms in Amyloid-ÃÆà ½Ãâò and Tau Transgenic Drosophila
Kennedy Matsagas1, Cristina Rizza1, Ben Goertzel1, Gregory Benford1,2 and Bryant Villeponteau3*
1Genescient Inc., Fountain Valley, California, USA
2University of California, Irvine, California, USA
3Centagen, Inc., Boulder, Colorado, USA
- *Corresponding Author:
- Bryant Villeponteau
Centagen, Inc., Boulder, Colorado, USA
Tel: +1 303-395-2660
E-mail: bvillepo@san.rr.com
Received Date: 17 December 2017; Accepted Date: 1 Jan 2018; Published Date: 10 Jan 2018
Citation: Matsagas K, Rizza C, Goertzel B, Benford G, Villeponteau B (2017) Multipath Natural Product Supplement Suppresses Dementia Symptoms in Amyloid-β and Tau Transgenic Drosophila . J Biol Med Res. Vol.2 No.1:2.
Abstract
Background: Numerous reports on Alzheimer’s Disease (AD) indicate that AD has many biochemical pathways. However, most AD treatments have targeted a single pathway such as beta amyloid or phosphorylated tau and have not been very successful in stopping AD progression. To address the multipath nature of AD, we have used multiple natural products that target critical aging and independent AD pathways to test whether a multipath approach might be more effective than the single pathway approaches typically used.
Methods and findings: We have constructed Drosophila melanogaster models of AD with inducible transgenic human Amyloid-β and tau mutations. Induction of either AD mutant gene in the transgenic flies after emergence from pupal stage leads to the early onset of slowed mobility that was tracked by assaying crawl times as a function of age. The transgenic Amyloid-β and tau Drosophila assays were utilized to test various natural products that target separate biological pathways involved in brain aging and dementia. Screening a library of natural products, we identified a group of 7 natural substances (MX100A) that synergistically prevented nearly all early mobility difficulties and reverses the lifeshortening effects of Amyloid-β and tau. The favorable results on AD symptoms in Drosophila with MX100A were observed in animals treated from youth and from later adult ages. MX100A had no observed negative side effects.
Conclusion: Treatment of transgenic AD Drosophila with the multipath MX100A dietary supplement prevents nearly all early mobility difficulties and reverses the lifeshortening effects of Amyloid-β and tau. In patients with AD, progressively more severe mobility difficulties and heightened risks of mortality are common symptoms of the later stages of AD. While successful animal results have not typically translated into effective treatments for AD, the multipath MX100A treatment results are promising enough to warrant further testing.
Keywords
Dementia; Drosophila ; Tau Transgenic Drosophila
Abbreviations
AD: Alzheimer’s Disease; Amyloid-β: Betaamyloid; ptau: Phosphorylated tau; MAPT: Microtubule- Associated Protein Tau
Introduction
Alzheimer’s Disease (AD) is a complex neurodegenerative disease that causes most of all cases of human dementia. Most of the drugs that are currently being tested to treat Alzheimer’s Disease in FDA clinical trials target beta amyloid (Amyloid-β) or phosphorylated tau (ptau), but not both simultaneously.
The currently available drugs also can cause serious adverse events, such as amyloid related imaging abnormalities (ARIA) [1,2] which sometimes lead to life threatening cerebral hemorrhages. As these single-path therapeutics have disappointed in clinical trials, interest in multi-target approaches to AD has grown [3,4].
While ptau and Amyloid-β have been the dominant focus of research on AD pathophysiology, other mechanisms are clearly involved. The risk of AD increases exponentially with age [5,6]. Thus, age-related processes like mitochondrial dysfunction, neural vascular aging, chronic inflammation, and astrocyte aging are other potential factors in the complex changes in brain function that occur over the decades preceding actual AD diagnosis [7-14]. This apparent complex etiology of AD suggests that many biochemical pathways should be altered simultaneously for a more successful intervention into AD.
In devising our AD intervention strategy, our overall aim was to test the hypothesis that AD progression can be significantly slowed by acting on multiple longevity genes while boosting mitochondrial function and stress resistance.
To carry out this strategy, we first conducted genetic and machine learning work on age-related databases for Drosophila melanogaster and humans. Using genetic databases from these two very divergent species, we were able to identify many common neural-specific genetic and biochemical pathways involved in longevity. We also added gene targets known to be important to human AD, such as Amyloid-β and tau.
To identify potential botanical drugs, we initially screened natural products that act on identified longevity and ADspecific pathways. We next screened both individual and combinations of natural products in both Amyloid-β and ptau transgenic Drosophila models of AD. We then identified the combinations that provided the best protection against the development of neural dysfunction in the flies, as assayed by slowed mobility in our crawl tests and early death.
Our screening led to a seven-component botanical drug, MX100A, that was effective in preventing neural motor dysfunction in our transgenic Drosophila AD models.
Materials and Methods
MX100A treatment
The 7 natural products in MX100A are Astragalus membranaceus extract, Berberine HCl, Pterocarpus marsupium extract, L-Theanine, Genistein, Lithium Orotate (1.5 mg Lithium), and Selenium Glycinate (70 mcg Selenium). MX100A is protected by US Patent 9744204 [15]. The formulation is available for testing from Genescient, Inc. Please contact Genescient at science@genesceint.com.
Transgenic Drosophila models
We first screened individual botanical components and optimized combinations for their potential in slowing AD motor dysfunction in transgenic flies expressing mutant human AD-inducing beta-amyloid or tau genes that were obtained from human patients with early onset Alzheimer’s disease. The transgenic Drosophila constructs were created by crossing a nervous system specific gene-switch driver line of flies (Elav-GS) [16] with upstream activation sequence (UAS) promoter fly lines for both Amyloid-β and tau (UAS-Amyloid-β 42 and UAS-MAPT, respectively).
AD motor dysfunction symptoms were then inducible in the resulting flies with the administration of RU486 (Mifepristone) [16]. The detailed animal testing of the 7 component MX100A used the same specially engineered Amyloid-β and tau Drosophila animal models.
In each treatment group, 100 adult transgenic flies were placed in male-female pairs in 50 vials immediately after emerging from their pupae. Each vial contained 1.5 g of banana-agar food media, and the drug treatment solutions were painted on top of the food and allowed to dry. Four days after the flies were placed in vials, gene expression was induced in the transgenic flies using 0.16 mg of RU486 in 50 microliters of 100% ethanol per vial to over-express either mutant human beta-amyloid (Amyloid-β) or tau. Two days after mutant Amyloid-β or tau induction, 150 mL of a deionized water solution containing 0.0164 mg of MX100A was added to each vial of the early MX100A treatment groups.
In delayed treatment groups, MX100A was given 8 days after Amyloid-β or tau induction. The flies were moved into fresh vials every day. As a measurement of the progression of neural dysfunction, the flies were knocked to the bottom of their vials and then timed on how fast they could crawl back up to the top of the vial. These crawl tests are metrics of mobility, which in both humans and animals declines with age and with the progression of AD in humans. The crawl tests were performed three times per week until the flies died or reached a crawl time greater than one minute.
Fly media
All studies employed transgenic lines of D. melanogaster that were maintained in vials containing a banana medium at about 25°C.
The banana medium (BM) is a low protein diet containing a boiled mixture of (i) 100 g agar in 6.6 L distilled water, (ii) 900 g peeled bananas, 165 mL Eden barley malt syrup, 110 mL light and 110 mL dark Karo corn syrup blended into 400 mL distilled water, and (iii) 240 g Fleischmann’s instant dry yeast and 160 mL 95% ethanol blended into 460 mL water. After cooling to 48°C, 15.6 g methyl-4-hydroxybenzoic acid (10% w/v in 95% ethanol) was added as an antifungal agent. Life spans on this medium are nearly as long as on dietary restriction diets [17].
Fly longevity assays – Vials
Flies were housed in small vials with 1 male and 1 female flies/vial and enough vials to have 50 flies of each sex. The vials were changed every 2-3 days or 3 times per week, with flies combined into new vials to preserve the 2 flies per vial as flies died. Sexual selection (1 of each sex or 2 of one sex if not enough of each sex) was preserved until all flies were dead.
Statistical analysis
All statistical analyses were carried out using Microsoft Excel.
Results
MX100A is composed of 7 components
Our AD transgenic fly screening of herbal extracts and purified natural products led us to a botanical mix of 7 components that target multiple AD and longevity pathways, which included inflammation, acetylcholinesterase, AMPK, ptau, Amyloid-β, mitochondria and metabolic dysfunction, genomic instability, adrenergic receptors, NMDA receptors, GABA receptors, autophagy (mTOR), and epigenetic factors. The list of 7 herbal extracts and natural products in MX100A is given in Table 1 along with the known actives and targets [18-57].
| Components | Known Active(s) | Targets | References |
|---|---|---|---|
| Astragalusmembranaceus (extract) |
Astrogalosides I-VII Flavenoids, HDTICs |
Telomerase, Mitochondria, ptau, mTOR, TNF-α, ERK, AMPK |
[18-24] |
| Berberine HCL | Berberine (98%) | Acetylcholinesterase, AMPK, α-adrenergic receptors, β-Amyloid | [25-30] |
| Vaccinium uliginosum or Pterocarpusmarsupium(extracts) |
Resveratrol Analogs | PPARα, PGE2, AMPK, phosphodiesterase, Mitochondria | [31-35] |
| L-Theanine | L-Theanine (98%) | NMDA receptors, EAATs, GABA receptors, eNOS, mitochondria | [36-42] |
| Genistein | Genistein (98%) | ERα, AMPK, p450c21 | [43-45] |
| Lithium Orotate | Lithium | NCS-1/Frequenin, Amyloid-β, NMDA, GSK3B, ptau | [46-54] |
| Selenium Glycinate | Selenium | PRPF8, ERCC1, Selenoproteins | [55-57] |
Table 1: Composition, known actives, and targets of MX100A.
MX100A prevents nearly all the early mobility and mortality effects from beta-amyloid or tau expression in our AD Drosophila model
The detailed testing of our botanical drug MX100A used the same genetically engineered transgenic AD Drosophila model that we developed for our preliminary screens testing the individual botanical components and optimized combinations of the best components for their potential in slowing the progression of motor dysfunction in the transgenic flies. The transgenic flies were induced to express either mutant human beta-amyloid (Amyloid-β) or tau and then tested for lifespan and how fast they could crawl up the side of their housing vial, which is a mobility assay.
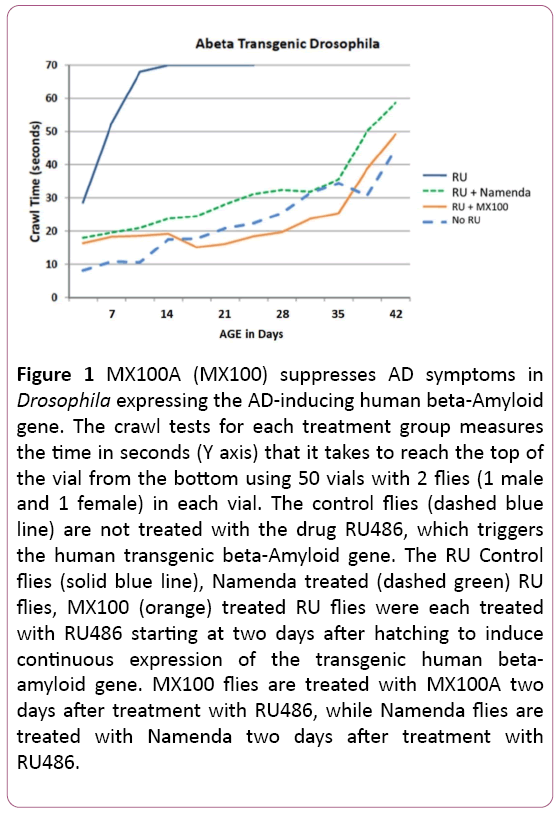
Figure 1 shows our typical Drosophila fly results with the botanical supplement MX100A (labeled MX100 in Figure 1) on the Amyloid-β Transgenic Drosophila model using 100 flies for each group housed in 50 vials with one male and one female in each vial. The Figure 1 assay is a climbing or crawling test measuring the time in seconds (Y axis) that it takes each fly to climb to the top of the vial from the bottom. With age (X axis), the flies naturally take longer to climb or crawl to the top of the vial. The Control flies (dashed blue line) have not been induced to express the beta-Amyloid gene and represent the natural progression of longer crawling times with advancing age. The RU Control flies (solid blue line) are induced with the drug RU486 to express the beta-amyloid gene, which greatly increases the crawling time at every age and induces early death. In contrast, MX100A flies expressing the beta-amyloid gene and treated with MX100A (solid orange line) have crawling times with age that are like those of control flies and slightly better than those flies expressing the beta-amyloid gene and treated with the drug Namenda (dashed green line), which is an existing FDA-approved AD pharmaceutical. The MX100A-treated Amyloid-β -expressing Drosophila also reverts to the lifespan of Control flies.
Figure 1: MX100A (MX100) suppresses AD symptoms in Drosophila expressing the AD-inducing human beta-Amyloid gene. The crawl tests for each treatment group measures the time in seconds (Y axis) that it takes to reach the top of the vial from the bottom using 50 vials with 2 flies (1 male and 1 female) in each vial. The control flies (dashed blue line) are not treated with the drug RU486, which triggers the human transgenic beta-Amyloid gene. The RU Control flies (solid blue line), Namenda treated (dashed green) RU flies, MX100 (orange) treated RU flies were each treated with RU486 starting at two days after hatching to induce continuous expression of the transgenic human betaamyloid gene. MX100 flies are treated with MX100A two days after treatment with RU486, while Namenda flies are treated with Namenda two days after treatment with RU486.
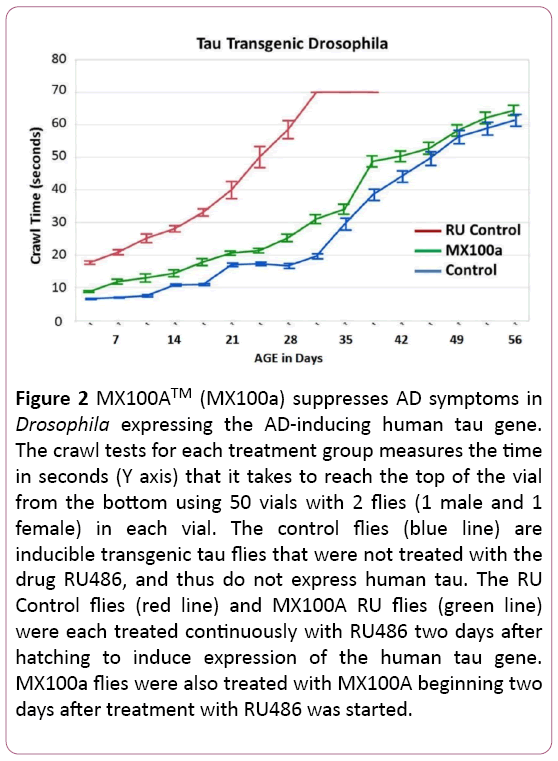
Figure 2 shows similar results for MX100A treatment of transgenic Drosophila mutants that have an inducible human mutant tau gene. As with Figure 1, the Control flies (blue line) carry the mutant gene, but have not been treated with RU486 to induce expression of tau. These flies demonstrate the normal slow increase in climbing time with age.
Figure 2: MX100ATM (MX100a) suppresses AD symptoms in Drosophila expressing the AD-inducing human tau gene. The crawl tests for each treatment group measures the time in seconds (Y axis) that it takes to reach the top of the vial from the bottom using 50 vials with 2 flies (1 male and 1 female) in each vial. The control flies (blue line) are inducible transgenic tau flies that were not treated with the drug RU486, and thus do not express human tau. The RU Control flies (red line) and MX100A RU flies (green line) were each treated continuously with RU486 two days after hatching to induce expression of the human tau gene. MX100a flies were also treated with MX100A beginning two days after treatment with RU486 was started.
The RU flies (red line) were treated with RU486 to express the transgenic human tau gene, which greatly increases the crawling time beginning at a very early age. In contrast, RU flies treated with MX100A (RU + M100a - green line) have crawling times that are similar to flies not treated with RU (Control flies - blue line). As before, the MX100A treated RU flies also revert to the lifespan of the non-induced Control flies. In the Figure 2 graph, we also show the 95% confidence error bar for the mean climbing times for each treatment group of 100 flies.
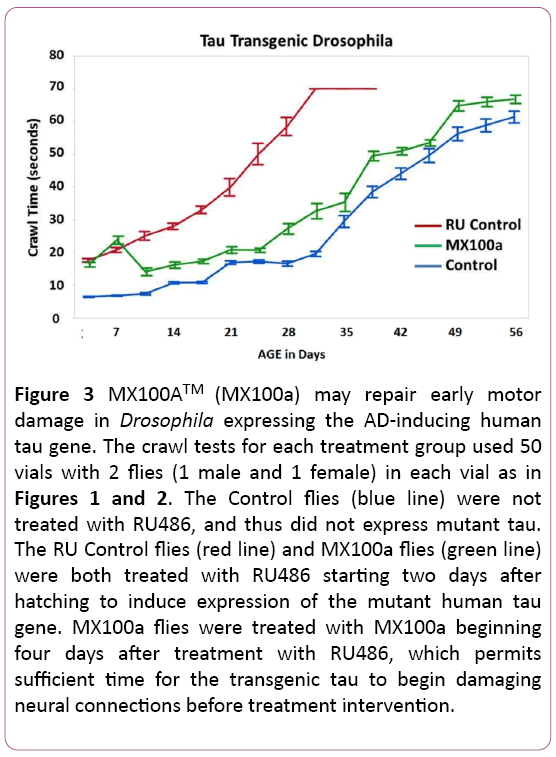
MX100A may reverse some early damage in transgenic Drosophila carrying a human tau gene
Another question that we wanted to explore with the transgenic tau flies was whether early damage from tau expression in our animal model could be repaired. Figure 3 addresses this issue by delaying the MX100A treatment of mutant tau transgenic flies for 4 days after RU486 induction of tau. The delayed start of treatment by MX100A (green line) does lead to poorer initial function of the MX100A treated flies in Figure 3 (compare to Figure 2), but crawl function then reverts to the typical progression of MX100A treated flies after MX100A treatment was added at 7 days. The delayed-start MX100A treated RU flies in Figure 3 also revert to the lifespan of the non-induced Control flies and do not appear to have any shortened lifespan due to the late start of MX100A treatment.
Figure 3: MX100ATM (MX100a) may repair early motor damage in Drosophila expressing the AD-inducing human tau gene. The crawl tests for each treatment group used 50 vials with 2 flies (1 male and 1 female) in each vial as in Figures 1 and 2. The Control flies (blue line) were not treated with RU486, and thus did not express mutant tau. The RU Control flies (red line) and MX100a flies (green line) were both treated with RU486 starting two days after hatching to induce expression of the mutant human tau gene. MX100a flies were treated with MX100a beginning four days after treatment with RU486, which permits sufficient time for the transgenic tau to begin damaging neural connections before treatment intervention.
Discussion
We have constructed two Drosophila models of Alzheimer’s Disease (AD) using flies with inducible transgenic mutant Amyloid-β or tau genes taken from human patients that died from early onset AD. With the use of these two inducible transgenic Drosophila models of AD, we screened many purified natural products and herbal extracts for their efficacy in reversing the apparent mobility dysfunction symptoms of AD in both the Amyloid-β and tau models.
Using these transgenic screens, we were able to identify a seven-component natural product supplement MX100A that reverses the AD gene-induced symptoms (i.e., mobility loss and shortened lifespan) in both the Amyloid-β (Figure 1) and tau (Figure 2) transgenic AD flies. Therefore, this AD treatment is multipath and works with high efficacy on both the Amyloid- β and tau pathways.
We also note that MX100A may act on many other longevity pathways (Table 1) such as inducers of: telomerase, mitochondria efficiency (e.g. AMPK and PPAR), and autophagy (e.g. mTOR). MX100A also helps reduce inflammation (e.g. TNFα) and stress (e.g. NMDA and GABA receptors in brain neurons).
Figure 3 suggests that MX100A may be able to reverse some of the early neural motor damage in tau transgenic flies, as MX100A can be added 4 days after the induction of tau and is able to reverse the early tau damage. Since Alzheimer ’s disease is usually diagnosed after extensive brain damage has already occurred, the potential ability of MX100A to reverse some of the neural damage is another hopeful sign that MX100A may be useful in the treatment of Alzheimer’s Disease patients.
Conclusion
Drosophila models of AD, as described here, are far less expensive than mammal models and can yield results faster with larger N values. The use of Drosophila animal models in drug discovery could be a valuable tool for identifying and testing multipath natural product mixes of 7 to 20 compounds. Since Alzheimer’s Disease, like most age-related diseases and disorders, is likely caused by multiple factors [7-14], the Drosophila models of AD could be a game changer for identifying effective multipath drugs for Alzheimer’s and other neurodegenerative diseases.
Acknowledgement
We thank Dr. John Tower at the University of Southern California for providing us Drosophila with inducible promoters, Dr. Marc Horwitz for his dosing of botanical mixtures and Dr. Vincent Simmons for his critiques of the final manuscript.
Funding
Supplies and services for the Drosophila work and article processing charges are funded by Genescient Corporation. KM was paid by Genescient for her role in the study design, data collection, data analysis, and preparation of the manuscript.
References
- Ketter N, Brashear HR, Bogert J, Di J, Miaux Y, et al. (2017) Central review of amyloid-related imaging abnormalities in two phase III clinical trials of bapineuzumab in mild-to-moderate alzheimer's disease patients. J Alzheimers Dis 57:557-573.
- Sperling RA, Jack CR Jr, Black SE, Frosch MP, Greenberg SM, et al. (2011) Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: Recommendations from the Alzheimer's Association Research Roundtable Work-group. Alzheimers Dement 7: 367-385.
- Dias KS, Viegas C Jr (2014) Multi-target directed drugs: A modern approach for design of new drugs for the treatment of Alzheimer's Disease. CurrNeuropharmacol 12: 239-255.
- Wang Y, Wang H, Chen HZ (2016) AChE inhibition-based multi-target-directed ligands, a novel pharmacological approach for the symptomatic and disease-modifying therapy of Alzheimer's disease. CurrNeuropharmacol 14:364-375.
- Rocca WA, Hofman A, Brayne C, Breteler MM, Clarke M, et al. (1991) Frequency and distribution of Alzheimer's disease in Europe: A collaborative study of 1980-1990 prevalence findings. The EURODEM-Prevalence Research Group. Ann Neurol 30: 381-390.
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA (2003) Alzheimer disease in the US population: Prevalence estimates using the 2000 census. Arch Neurol 60: 1119-1122.
- Derungs R, Camici GG, Spescha RD, Welt T, Tackenberg C, et al. (2017) Genetic ablation of the p66(Shc) adaptor protein reverses cognitive deficits and improves mitochondrial function in an APP transgenic mouse model of Alzheimer's disease. Mol Psychiatry 22: 605-614.
- Grimm A, Friedland K, Eckert A (2016) Mitochondrial dysfunction: the missing link between aging and sporadic Alzheimer's disease. Biogerontology 17: 281-296.
- Lourenco CF, Ledo A, Barbosa RM, Laranjinha J (2017) Neurovascular uncoupling in the triple transgenic model of Alzheimer's disease: Impaired cerebral blood flow response to neuronal-derived nitric oxide signaling ExpNeurol 291: 36-43.
- Luo H, Han G, Wang J, Zeng F, Li Y, et al. (2016) Common aging signature in the peripheral blood of vascular dementia and Alzheimer's disease. MolNeurobiol 53: 3596-3605.
- Rodriguez-Arellano JJ, Parpura V, Zorec R, Verkhratsky A (2016) Astrocytes in physiological aging and Alzheimer's disease. Neuroscience 323: 170-182.
- Verkhratsky A, Marutle A, Rodriguez-Arellano JJ, Nordberg A (2015) Glial asthenia and functional paralysis: A new perspective on neurodegeneration and Alzheimer's disease. Neuroscientist 21: 552-568.
- Verkhratsky A, Rodriguez-Arellano JJ, Parpura V, Zorec R (2017) Astroglial calcium signalling in Alzheimer's disease. BiochemBiophys Res Commun 483: 1005-1012.
- Huang W, Li Z, Zhao L, Zhao W (2017) Simvastatin ameliorate memory deficits and inflammation in clinical and mouse model of Alzheimer's disease via modulating the expression of miR-106b. Biomed Pharmacother 92: 46-57.
- Villeponteau B, Rizza C (2017) Inventorsmultipath nutritional supplement for memory, cognition, and coordination US patent publication number 9,744,204 B1.
- Ford D, Hoe N, Landis GN, Tozer K, Luu A, et al. (2007) Alteration of Drosophila life span using conditional, tissue-specific expression of transgenes triggered by doxycyline or RU486/Mifepristone. ExpGerontol 42: 483-497.
- Mair W, Piper MD, Partridge L (2005) Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS biology 3: e223.
- Aldarmaa J, Liu Z, Long J, Mo X, Ma J, et al. (2010) Anti-convulsant effect and mechanism of Astragalusmongholicus extract in vitro and in vivo: protection against oxidative damage and mitochondrial dysfunction. Neurochemical research 35: 33-41.
- Auyeung KK, Mok NL, Wong CM, Cho CH, Ko JK (2010) Astragalussaponins modulate mTOR and ERK signaling to promote apoptosis through the extrinsic pathway in HT-29 colon cancer cells. Int J Mol Med 26: 341-349.
- Li XT, Zhang YK, Kuang HX, Jin FX, Liu DW, et al. (2012) Mitochondrial protection and anti-aging activity of Astragalus polysaccharides and their potential mechanism. Int J MolSci 13: 1747-1761.
- Liu J, Zhang JF, Lu JZ, Zhang DL, Li K, et al. (2013) Astragaluspolysaccharide stimulates glucose uptake in L6 myotubes through AMPK activation and AS160/TBC1D4 phosphorylation. ActapharmacologicaSinica 34: 137-145.
- Liu Y, Liu F, Yang Y, Li D, Lv J, et al. (2014) Astragalus polysaccharide ameliorates ionizing radiation-induced oxidative stress in mice. Int J BiolMacromol 68: 209-214.
- Zhang L, Yang Y, Wang Y, Gao X (2011) Astragalusmembranaceus extract promotes neovascularisation by VEGF pathway in rat model of ischemic injury. Die Pharmazie 66:144-150.
- Zou F, Mao XQ, Wang N, Liu J, Ou-Yang JP (2009) Astragalus polysaccharides alleviates glucose toxicity and restores glucose homeostasis in diabetic states via activation of AMPK. ActapharmacologicaSinica. 30: 1607-1615.
- Durairajan SS, Liu LF, Lu JH, Chen LL, Yuan Q, et al. (2012) Berberine ameliorates beta-amyloid pathology, gliosis, and cognitive impairment in an Alzheimer's disease transgenic mouse model. Neurobiol Aging 33: 2903-2919.
- Habtemariam S (2011) The therapeutic potential of Berberisdarwinii stem-bark: Quantification of berberine and in vitro evidence for Alzheimer's disease therapy. Nat Prod Commun 6: 1089-1090.
- Ji HF, Shen L (2011) Berberine: A potential multipotent natural product to combat Alzheimer's disease. Molecules 16: 6732-6740.
- Ji HF, Shen L (2012) Molecular basis of inhibitory activities of berberine against pathogenic enzymes in Alzheimer's disease. ScientificWorldJournal 2012: 823201.
- Panahi N, Mahmoudian M, Mortazavi P, Hashjin GS (2013) Effects of berberine on beta-secretase activity in a rabbit model of Alzheimer's diseaseArch Med Sci 9: 146-150.
- Bonesi M, Loizzo MR, Conforti F, Passalacqua NG, Saab A, et al. (2013) Berberisaetnensis and B. libanotica: A comparative study on the chemical composition, inhibitory effect on key enzymes linked to Alzheimer's disease and antioxidant activity. J Pharm Pharmacol 65: 1726-1735.
- Chang J, Rimando A, Pallas M, Camins A, Porquet D, et al. (2012) Low-dose pterostilbene, but not resveratrol, is a potent neuromodulator in aging and Alzheimer's disease. Neurobiol 33: 2062-2071.
- Joseph JA, Fisher DR, Cheng V, Rimando AM, Shukitt-Hale B (2008) Cellular and behavioral effects of stilbene resveratrol analogues: implications for reducing the deleterious effects of aging. J Agric Food Chem 56: 10544-10551.
- Robb EL, Stuart JA (2014) The stilbenes resveratrol, pterostilbene and piceid affect growth and stress resistance in mammalian cells via a mechanism requiring estrogen receptor beta and the induction of Mn-superoxide dismutase. Phytochemistry 98: 164-173.
- Sato D, Shimizu N, Shimizu Y, Akagi M, Eshita Y, et al. (2014) Synthesis of glycosides of resveratrol, pterostilbene, and piceatannol, and their anti-oxidant, anti-allergic, and neuroprotective activities. Bioscience, biotechnology, and biochemistry 78: 1123-1128.
- Lin VC, Tsai YC, Lin JN, Fan LL, Pan MH, et al. (2012) Activation of AMPK by pterostilbene suppresses lipogenesis and cell-cycle progression in p53 positive and negative human prostate cancer cells. J Agric Food Chem 60: 6399-6407.
- Cho HS, Kim S, Lee SY, Park JA, Kim SJ, et al. (2008) Protective effect of the green tea component, L-theanine on environmental toxins-induced neuronal cell death. Neurotoxicology 29: 656-662.
- Di X, Yan J, Zhao Y, Zhang J, Shi Z, et al. (2010) L-theanine protects the APP (Swedish mutation) transgenic SH-SY5Y cell against glutamate-induced excitotoxicity via inhibition of the NMDA receptor pathway. Neuroscience 168: 778-786.
- Egashira N, Hayakawa K, Osajima M, Mishima K, Iwasaki K, et al. (2007) Involvement of GABA(A) receptors in the neuroprotective effect of theanine on focal cerebral ischemia in mice. J PharmacolSci 105: 211-214.
- Siamwala JH, Dias PM, Majumder S, Joshi MK, Sinkar VP, et al. (2013) L-theanine promotes nitric oxide production in endothelial cells through eNOS phosphorylation. J NutrBiochem 24: 595-605.
- Takeda A, Sakamoto K, Tamano H, Fukura K, Inui N, et al. (2011 Facilitated neurogenesis in the developing hippocampus after intake of theanine, an amino acid in tea leaves, and object recognition memory. Cell MolNeurobiol 31:1079-1088.
- Unno K, Fujitani K, Takamori N, Takabayashi F, Maeda K, et al. (2011) Theanine intake improves the shortened lifespan, cognitive dysfunction and behavioural depression that are induced by chronic psychosocial stress in mice. Free Radic Res 45: 966-974.
- Wu Z, Zhu Y, Cao X, Sun S, Zhao B (2014) Mitochondrial toxic effects of Amyloid-β through mitofusins in the early pathogenesis of Alzheimer's disease. Molecular neurobiology 50: 986-996.
- Kaminska B, Ciereszko R, Kiezun M, Dusza L (2013) In vitro effects of genistein and daidzein on the activity of adrenocortical steroidogenic enzymes in mature female pigs. J PhysiolPharmacol 64: 103-108.
- Li Y, Chen H, Hardy TM, Tollefsbol TO (2013) Epigenetic regulation of multiple tumor-related genes leads to suppression of breast tumorigenesis by dietary genistein. PloS one 8: e54369.
- Palacios-Gonzalez B, Zarain-Herzberg A, Flores-Galicia I, Noriega LG, Aleman-Escondrillas G, et al. (2014) Genistein stimulates fatty acid oxidation in a leptin receptor-independent manner through the JAK2-mediated phosphorylation and activation of AMPK in skeletal muscle. BiochimBiophysActa 1841: 132-140.
- Forlenza OV, de Paula VJ, Machado-Vieira R, Diniz BS, Gattaz WF (2012) Does lithium prevent Alzheimer's disease? Drugs Aging 29: 335-342.
- Sudduth TL, Wilson JG, Everhart A, Colton CA, Wilcock DM (2012) Lithium treatment of APPSwDI/NOS2-/- mice leads to reduced hyperphosphorylated tau, increased amyloid deposition and altered inflammatory phenotype. PloS one 7: e31993.
- Nunes MA, Viel TA, Buck HS (2013) Microdose lithium treatment stabilized cognitive impairment in patients with Alzheimer's disease. Curr Alzheimer Res 10:104-107.
- Trujillo-Estrada L, Jimenez S, De Castro V, Torres M, Baglietto-Vargas D, et al. (2013) In vivo modification of Amyloid-β plaque toxicity as a novel neuroprotective lithium-mediated therapy for Alzheimer's disease pathology. ActaNeuropatholCommun 1: 73.
- Forlenza OV, De-Paula VJ, Diniz BS (2014) Neuroprotective effects of lithium: implications for the treatment of Alzheimer's disease and related neurodegenerative disorders. ACS ChemNeurosci 5: 443-450.
- Nery LR, Eltz NS, Hackman C, Fonseca R, Altenhofen S, et al. (2014) Brain intraventricular injection of Amyloid-β in zebrafish embryo impairs cognition and increases tau phosphorylation, effects reversed by lithium. PloS one 9: e105862.
- Sofola-Adesakin O, Castillo-Quan JI, Rallis C, Tain LS, Bjedov I, et al. (2014) Lithium suppresses Amyloid-β pathology by inhibiting translation in an adult Drosophila model of Alzheimer's disease. Front Aging Neurosci 6: 190.
- Wallace J (2014) Calcium dysregulation, and lithium treatment to forestall Alzheimer's disease - A merging of hypotheses. Cell calcium 55: 175-181.
- Zhao L, Gong N, Liu M, Pan X, Sang S, et al. (2014) Beneficial synergistic effects of microdose lithium with pyrroloquinolinequinone in an Alzheimer's disease mouse model. Neurobiol Aging 35: 2736-2745.
- Ishrat T, Parveen K, Khan MM, Khuwaja G, Khan MB, et al. (2009) Selenium prevents cognitive decline and oxidative damage in rat model of streptozotocin-induced experimental dementia of Alzheimer's type. Brain Res 1281: 117-127.
- Cardoso BR, Ong TP, Jacob-Filho W, Jaluul O, Freitas MI (2010) Nutritional status of selenium in Alzheimer's disease patients. Br J Nutr 103: 803-806.
- Lakshmi BV, Sudhakar M, Prakash KS (2015) Protective effect of selenium against aluminum chloride-induced Alzheimer's disease: behavioral and biochemical alterations in rats. Biol Trace Elem Res 165: 67-74.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences