Molecular Therapeutic Role Micro-RNA 221 Inhibitor in Hepatocellular Carcinoma
Amal Abou Elfadl, Naglaa I Azzab, Sania K Elwia* and Doaa Shabaan
Faculty of Medicine, Department of Biochemistry, Benha University, Egypt
- *Corresponding Author:
- Sania K Elwia
Faculty of Medicine, Department of Biochemistry, Benha University, Egypt.
Tel: 01062430931
E-mail: Ehaboraby2000@gmail.com
Received Date: July 23, 2018; Accepted Date: August 25, 2018; Published Date: August 31, 2018
Citation: Elfadl AA, Azzab NI, Elwia SK, Shabaan D (2018) Molecular Therapeutic Role Micro-RNA 221 Inhibitor in Hepatocellular Carcinoma. J Mol Cell Biochem Vol.2 No.1:6
Abstract
Background: MicroRNAs (miRNAs) have plays important role not only as posttranscriptional regulation of gene expression also in development and cellular processes. The association between pathogenesis of many diseases and miRNAs very strong, between pathogenesis many diseases and miRNAs very strong, miR-221 is an oncogenic miRNA that plays important role in the initiation and progression of HCC, by affecting pro-oncogenic pathways from that miRNAs is a new class of targets for the development of miRNA-based therapeutic strategy.
Method: The mature miRNA act by competition with cellular target miRNA act by completion with cellular target mRNAs moreover that lead to inhibition of the miRNA and repression of the its direct targets. For assessment effect of anti-miR-221 on HepG2 cells, MTT, cell cycle analysis and apoptosis assay were performed, as regard expression -miR-221 and its target gene were detected by using reverse transcription-polymerase chain reaction.
Results: Our data revealed that the delivery of anti-miR-221 that mediated by ultrasound micro bubble contrast agents. We observed that, cell proliferation inhibited and promoted the apoptosis of cells the expression of miR-221 decreased. Consequently, there was up regulation in the expression of CDKN1B/p27.
Conclusion: The transfection of recombinant plasmids contained anti-miR-221 to suppress oncogenic effect of-miR-221 and cell proliferation was inhibited and cell apoptosis was induced in HepG2 cells. This indicated that effective role miRNA that may be novel gene therapy targets.
Keywords
miRNA; Ultrasound micro bubble; Gene therapy; CDKN1B/p27
List of Abbreviations
HCC: Hepato Cellular Carcinoma; miRISC: miRNA Induced Silencing Complex; MicroRNAs (miRNAs); PTEN: Phosphatase and Tensin Homolog; PDCD4: Programmed Cell Death 4; lncRNA: Interfering Long non-coding RNA; EGFP: Epidermal Growth Factor Plasmid; ER: Endoplasmic Reticulum; CDKN1B/p2: Cyclin Dependent kinase Inhibitors 7; BMF: B-cell lymphoma-2 Modifying Factor
Introduction
Hepatocellular carcinoma (HCC) is one of most common form of primary liver cancer, accounting for 70% to 85% of cases and the third leading cause of death from cancer (an estimated 549,000 deaths per year), HCC accounts for 85% to 90% of all primary liver cancers and ranged as the fifth most prevalent malignancy all over the world Unfortunately the majority of patients are diagnosed with HCC progresses to the middle to late stages.
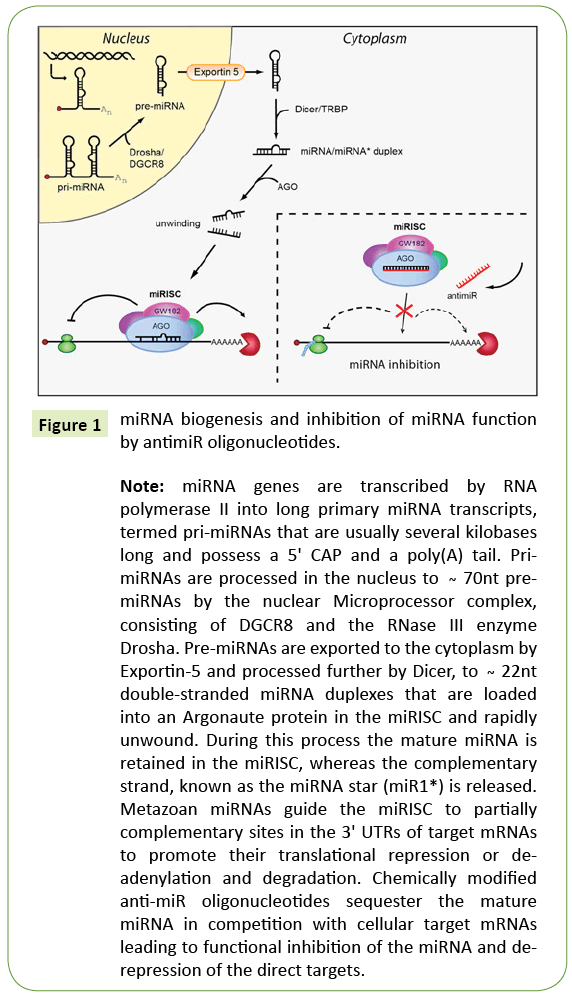
The primary transcripts of miRNA genes are pri-miRNAs, that contain several kilobases long and having a 5’ CAP and a poly(A) tail Pri-miRNAs are processed in the nucleus about 70 nt hairpinstructures, then exported to the cytoplasm by Exportin-5 (Figures 1-5). The miRNA duplexes are included into an Argonaute protein in the miRNA induced silencing complex (miRISC) and unwound rapid. In this process the mature miRNA is regain in to miRISC, and the complementary strand, for realsing the miRNA star (miR*) is released. MiRNA’s play vital roles in normal biological processes as cell proliferation, differentiation, apoptosis, and stress resistance [1-5]. The mis-regulation of genes leads to enhancement development and progression of HCC exert its effect on cellular processes such as cell cycle control, apoptosis, cell migration and invasion. The implication of miRNAs in cancer research by inhibition target gene expression, inhibition of protein translation or mRNA degradation.
Figure 1: miRNA biogenesis and inhibition of miRNA function by antimiR oligonucleotides.
Note: miRNA genes are transcribed by RNA polymerase II into long primary miRNA transcripts, termed pri-miRNAs that are usually several kilobases long and possess a 5' CAP and a poly(A) tail. PrimiRNAs are processed in the nucleus to Ì´ 70nt premiRNAs by the nuclear Microprocessor complex, consisting of DGCR8 and the RNase III enzyme Drosha. Pre-miRNAs are exported to the cytoplasm by Exportin-5 and processed further by Dicer, to Ì´ 22nt double-stranded miRNA duplexes that are loaded into an Argonaute protein in the miRISC and rapidly unwound. During this process the mature miRNA is retained in the miRISC, whereas the complementary strand, known as the miRNA star (miR1*) is released. Metazoan miRNAs guide the miRISC to partially complementary sites in the 3' UTRs of target mRNAs to promote their translational repression or deadenylation and degradation. Chemically modified anti-miR oligonucleotides sequester the mature miRNA in competition with cellular target mRNAs leading to functional inhibition of the miRNA and derepression of the direct targets.
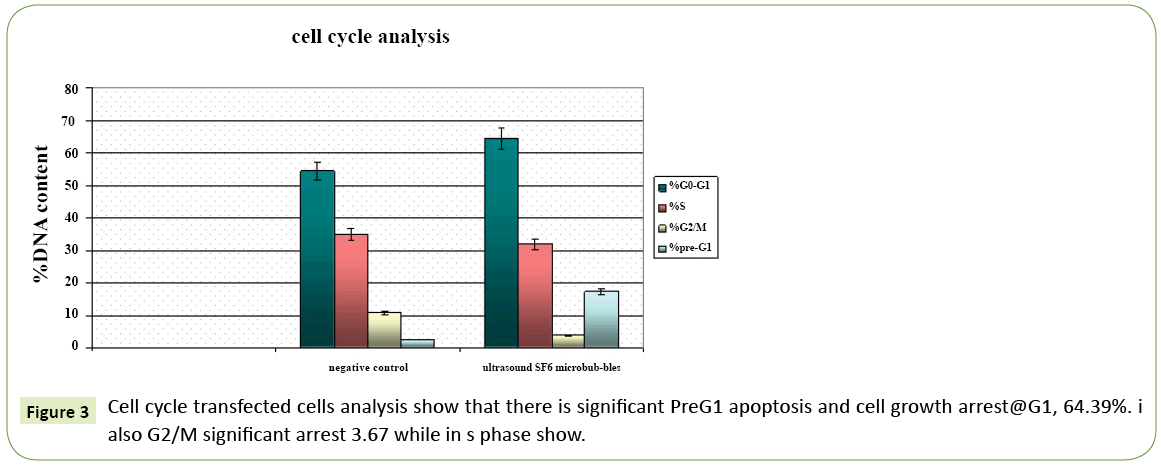
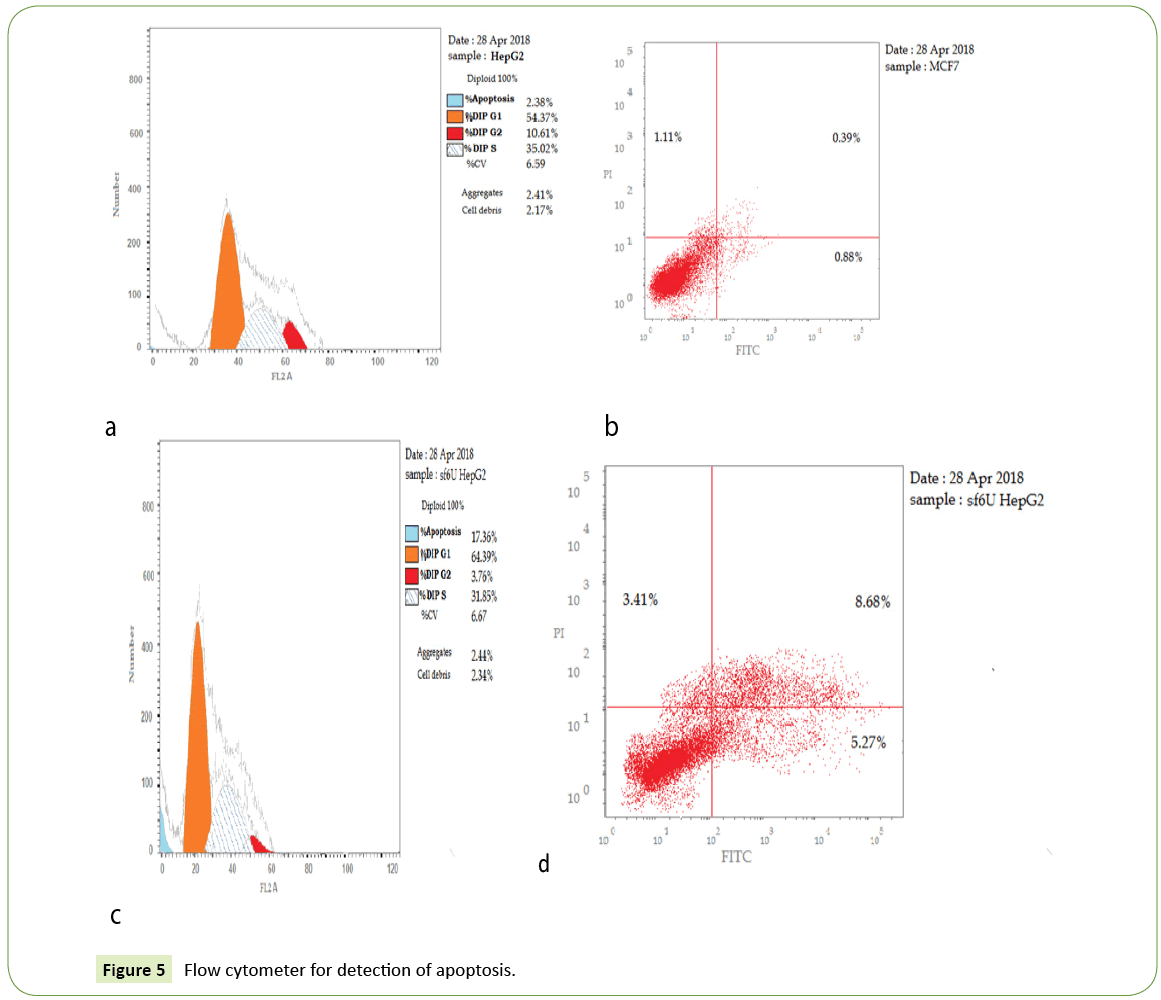
Figure 3: Cell cycle transfected cells analysis show that there is significant PreG1 apoptosis and cell growth arrest@G1, 64.39%. i also G2/M significant arrest 3.67 while in s phase show.
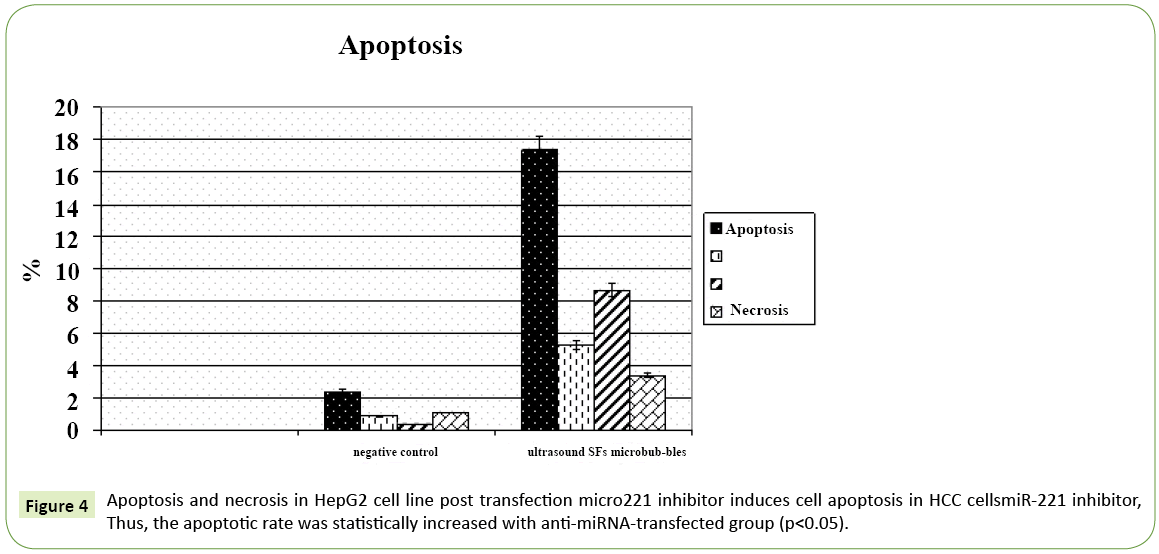
Figure 4: Apoptosis and necrosis in HepG2 cell line post transfection micro221 inhibitor induces cell apoptosis in HCC cellsmiR-221 inhibitor, Thus, the apoptotic rate was statistically increased with anti-miRNA-transfected group (p<0.05).
Also miRNA have an ability to inhibit or promote expression of many related genes, by affecting several cell-signaling pathways that lead to development and progression of cancer, as cell proliferation, differentiation, and apoptosis. Any disturbance by increasing or decreasing of mature miRNAs that make miRNAs may be acritical target therapy, up-regulated miRNAs are considered as oncogenic miRNAs.
The oncogenic miRNAs inhibit the tumor suppressor genes. And exert its anti-apoptotic effect by targeting the tumor suppressors as phosphatase and tensin homolog (PTEN) and programmed cell death 4 (PDCD4) to inhibit mRNA translation through binding. The interaction of intracellular miRNAs to the mRNAs of target genes with its complementary sequences make miRNA act as post-transcriptional regulators of target genes [6-10].
miRNA-based gene therapy is a new evaluation for abnormal miRNA expression correction the strategies of miRNA-based gene therapy treatment as follows: By stopping the function of oncogenic and over-expressed miRNAs, in cancer cells, by applying anti-miRNA oligonucleotides (AMOs), and exert its inhibitory effect on miRNA target genes with its complementary base pairing. Thus this strategy also leads to disturbance of oncogene miRNAs functional that aiming to protection and expression of tumor suppressor genes function that had been lost [11-15].
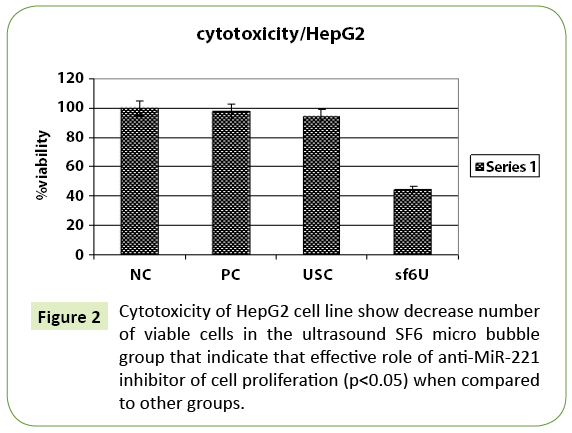
MicroRNA (miRNA) based on gene therapy, as gene delivery systems. With Comparison with other traditional transfection vectors. Ultrasound micro bubbles have been used for gene delivery because of higher safety, stability and higher transfection efficiency also its non-invasive drug gene delivery systems. In this study we investigate. The effects of miR-221 on HepG2 cells were determined by ultrasound micro bubble-mediated gene transfection. The expression of miRNAs was detected using reverse transcription-polymerase chain reaction, MTT cell cycle analysis and apoptosis assays were performed there was significant down regulation of miR-221 (p<0.05). With treatments, with this approach, cell proliferation was inhibited and cell apoptosis was induced.
Materials and Methods
Cell culture
Cell Line cells were obtained from American Type Culture Collection cells were cultured using DMEM (Invitrogen/Life Technologies) that supplemented with 10% FBS (Hyclone) 10 μg/ ml of insulin (Sigma) and 1% penicillin-streptomycin. All of the other chemicals and reagents were from Sigma.
Plate cells (cells density 1.2-1.8 × 10,000 cells/well) in a volume of 100 μl complete growth medium+100 ul of the tested compound per well in a 96-well plate for 24 hours before the MTT assay cells.
The human hepatoma HepG2 cell line (ATCC) was maintained in Dulbecco's modified Eagle's medium (DMEM; HyClone; GE Healthcare Life Sciences, Logan, UT, USA) using 10% fetal bovine serum (FBS; HyClone; GE Healthcare Life Sciences) at 37°C in an atmosphere containing 5% CO2. The cells were routinely passaged every 1‑2 days.
HepG2 cells are highly suitable for invitro study system also for the study of polarized human hepatocytes. With the suitable culture conditions, having higher degree of morphological and functional differentiation.
Inhibition of miR-221 in HCC cells
HCC cells were in 96-well plate (2.5 × 103 cells per well) and incubated at 37°C for 24 hrs. The cells were then transfected with miR-221 inhibitor, miRNA inhibitor negative control.
Design of modified anti-miR oligonucleotides chemically
Sequences of anti‑miR‑221 was synthesized (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and inserted into BamHI and HindIII sites of the GV249 vector [a vector containing enhanced green fluorescent protein (EGFP); Shanghai Jikai Communication Technology Co., Ltd., Shanghai, China]. The recombinant plasmids were named as EGFP‑anti‑miR221. The sequence of primer used for cloning as follow miR‑221 sense, 5'‑AGC TAA AAA AGC TAC ATT GTC TGC TGG GTT TCG‑3' and antisense, 5'‑GAT CCG AAA CCC AGC AGA CAA TGT AGC TTT TTT‑3'.
Cells were divided into 5 groups:
1 Empty control (no treatment)
2 Negative control = Cells+Plasmid
3 Positive control= Cells+Plasmid+Liposome (Lipofectamine® 2000, Invitrogen; Thermo Fisher Scientific, Inc.)
4 Ultrasound control= Cells+Plasmid+Ultrasound exposure
5 Ultrasound SF6 microbubbles= Cells+Plasmid+Ultrasound exposure+SF
Preparation and optimization of the concentration of microbubbles
Transfection efficiency that is mediated by ultrasound micro bubbles is affected by the following. Ultrasound exposure condition; type and concentration of micro bubbles; and cell types thus it is very important to optimize the conditions of ultrasound intensity and concentration of micro bubbles as gene delivery systems that are mediated by ultrasound micro bubbles. The traditional gene transfection is generally mediated by viral vectors or non‑viral vectors. However, due to the shortage of available delivery vectors safety problem with conventional viral vector security of viral vectors and the low transfection efficiency of non-viral vectors, a while non-viral vectors have low transfection efficiency application of these vectors is limited. Ultrasound micro bubbles having a good biological compatibility and stability. The ultrasound images are enhanced with ultrasound contrast agents. Compared with traditional transfection vectors, ultrasound micro bubbles have the advantages of high safety, stability and transfection efficiency non‑invasive gene/drug delivery systems. Also Ultrasound micro bubbles have been used to study the functions of genes and miRNA [15-20].
The ultrasound micro bubble contrast agents, sulfur hexafluoride (SF6; Bracco Suisse SA, Manno, Switzerland) was used in the present study
Micro bubbles were prepared according to the manufacturer's protocol. For preparation of SF6, 5 ml of saline solution was injected into a vial containing freeze‑dried powder. The vial was then agitated until the powder was completely dissolved in the saline solution. The suspension was used within 6 h. A total of about 10,000 cells were seeded onto a 96‑well plate 24 h, Contrast agents was suspended in DMEM containing 10% FBS. The culture was maintained for 48 h at 37°C ultrasound parameters used are 2.0 MHz and MI, 0.28. Prior to treatment, cells were rinsed with DMEM. Ultrasound treatment was then provided the target group for 30 sec. Following transfection, cells were maintained in DMEM. To this medium, 10% FBS was added 6‑8 h post‑transfection. Lipofectamine 2000‑mediated gene transfection was performed according to the manufacturer's protocol. Following 48h transfection, cells were subjected to MTT, RT-PCR, flow cytometry and caspase [3]. Each treatment was performed in 3 wells, and the experiment was performed in triplicate [21-25].
The MTT method of monitoring in vitro cytotoxicity as follow
Each test should include a blank containing complete medium without cells. MTT (20 μl; 5 mg/ml dissolved in PBS; Genview; Sigma‑Aldrich; EMD Millipore, Billerica, MA, USA) was added to the well, and the cell culture was incubated for 4 h at 37°C. The culture medium mixture was then discarded, and the cells were dissolved in 150 μl dimethyl sulfoxide for 10 min. The absorbance of the sample was determined at 490 nm on a Micro plate Spectrophotometer (BioTek Elx 800; BioTek Instruments, Inc., Winooski, VT, USA). Controls were a blank control (no cells) and a negative control (untreated cells). And final cell number should not exceed 106 cells/cm2.
RNA extraction, cDNA synthesis and RT-qPCR
Cells were transfected with the recombinant plasmid (EGFP‑anti‑miR221) and vector plasmid (GV249) through ultrasound microbubble SF6. Cells were then collected 48 h post‑transfection. Cells that were not transfected were set as a blank control. Total RNA was extracted using RN easy RNA extraction kit (Qiagen), according to the manufacturer's protocol.
Ratio of readings at 260 nm and 280 nm (A260/280) provide an estimate of purity of RNA pure RNA had A 280/260 ratio of 1.9- 2.3 (OD260/OD280) RT‑qPCR was performed using the reverse RT‑qPCR kit (BioRad) according to the manufacturer's protocol. Then cDNA was subsequently amplified with BioRad syber green PCR MMX.
The primers for miR‑221, using the internal U6 control (sense, 5'‑TCG CTT CGG CAG CAC A‑3' and antisense, 5'‑AAC GCT TCA CGA ATT TGC GT‑3') and p57 and p27 gene expressions (p27 F 5’-GTCCACCGCAAATGCTTCTA-3’, p27 R 5’TGCTGTCACCTTCACCGTTC-3’) using B Actin as house keeping gene, primers were obtained from sigma. α-actin F 5’-GTGACATCCACACCCAGAGG-3’, β-actin R 5’-ACAGGATGTCAAAACTGCCC-3’.
Using the iScript one-step RT-PCR Kit with SYBR® Green
The miR-221 expression in FFPE experiment and p27 were calculated with the formula 2-Δcq, and the change ratio of miR- 221 in the in vitro experiments was: (1-1/2ΔΔCq) × 100%.
Flow cytometer analysis was performed using FACSCalibur (BD Biosciences, Franklin Lakes, NJ, USA)
This procedure was performed at an excitation wavelength of 488 nm and an emission wavelength of 530±15 nm. Cells were transfected with recombinant plasmids (EGFP‑anti‑miR221) and vector plasmid (GV249) through ultrasound micro bubble SF6. Cells were then collected 48h post‑transfection. Cells that were not subjected to transfection were set as the blank control. Cells were stained with propidiumc iodide and subjected to cell cycle analysis on FACSCalibur. The apoptosis of cells was determined using an Annexin V PE/7 AAD apoptosis detection kit according to the manufacturer's protocol. Flow cytometry was performed using FACSCalibu.
Caspase-3 activity was measured using Invitrogen Ca3 (Active) by using ELISA kit
Performed immediately after the detection of cell viability\on the same wells, according to the instructions of the manufacturer. After incubation at room temperature for 60 min, the fluorescence of each well was measured by using ROBONIK P2000 Elisa reader wl 450 nm.
In this study, we investigate effect of anti‑miR‑221 on HepG2 cell line that was treated with ultrasound contrast agent micro bubbles, first cell proliferation was detected using MTT treatment, then and flow cytometer for detection of cell cycle analysis and apoptosis assay was confirmed by caspase [3] and fluorescent microscope cell. The expression of miRNAs, cell cycle regulatory gene P27 was detected using reverse transcription-polymerase chain reaction RT QPCR (Tables 1-8).
| PCR was performed in a 50 µ reaction | volume |
|---|---|
| 2X SYBR® Green RT-PCR Reaction Mix | 25 µl |
| Forward primer (10 µM) | 1.5 µl |
| Reverse primer (10 µM) | 1.5 µl |
| Nuclease-free H2O | x µl |
| RNA template | (1 pg to 100 ng total RNA) |
Table 1: RT-qPCR was performed using the reverse RT-qPCR kit.
| Reaction | Duration |
|---|---|
| cDNA synthesis | 10 min at 50°C |
| iScript Reverse transcriptase inactivation | 5 min at 95°C |
| PCR cycling and detection (30 to 45 cycles) | 10 sec at 95°C, 30 sec at 55°C to 60°C (Data collection step) |
Table 2: Incubate complete reaction mix in a real-time thermal detection system as follows.
| Types of Cells | % Viability HepG2 |
|---|---|
| Negative control | 100 |
| Positive control | 97.77 |
| Ultrasound control | 94.61 |
| Ultrasound SF6 micro bubbles | 44.26 |
Table 3: Cell viability was reduced in all cell lines tested from 48 hrs posttransfection especially ultrasound SF6 micro bubbles MiR-221 inhibitor inhibits cell growth in HCC cell line.
| Sample | Used conc. µg/ml | %G0-G1 | %S | %G2/M | %Pre-G1 |
|---|---|---|---|---|---|
| Negative control | NC | 54.37 | 35.02 | 10.61 | 2.38 |
| Ultrasound SF6 micro-bubbles | sf6U | 64.39 | 31.85 | 3.76 | 17.36 |
Table 4: Show that there is significant PreG1 apoptosis and cell growth arrest@G1, 64.39%. I also G2/M a significant arrest while in s phase show non-significant change.
| Conc. µg/ml | Apoptosis | |||||
|---|---|---|---|---|---|---|
| Total | Early | Late | Necrosis | |||
| 1 | Negative control | NC | 2.38 | 0.9 | 0.38 | 1.1 |
| 4 | Ultrasound Micro bubbles | SF6 | 17.36 | 5.27 | 8.68 | 3.41 |
Table 5: Apoptosis of transfected cell show that there is increase apoptic rate in early 5.27 and late.68 8 phases with anti microRNA transfected group with ultrasound SF6 micro bubbles while decrease in necrotic tissue 3.41.
| microrna Transfected | Caspase 3 conc. µg/ml | FLD | |
|---|---|---|---|
| Cells+ultrasound+SF6 | sf6U | 424.6 | 9.07 |
| Negative control | NC | 46.8 | 1 |
Table 6: Show that increase caspase 3 with anti microRNA transfected group with ultrasound SF6 micro bubbles. 9.07 as compared to control.
| Fold Change | |||
|---|---|---|---|
| Sample | P27 Fld | miR-221 Fld | |
| Cells+ultrasound +SF6 | sf6U | 6.075251 | 0.388538 |
| Negative control | NC | 1 | 1 |
Table 7: Following transfection, miR‑221 was significantly down regulated with microRNA inhibitor (0.388538) fold associated with up regulated P27 (6.075251) fold change when compared to control.
| miR-221 | Control cells | Transfected cells |
|---|---|---|
| ΔCTC | 3.75 | 3.75 |
| ΔΔ CT | 0 | 1.53 |
| 2^ ΔΔCT | 1 | 0.388538 |
| CDKN1B/p27 | -- | -- |
| ΔCTC | 4.91 | 4.91 |
| ΔΔ CT | 0 | -2.92 |
| 2^ ΔΔCT | 1 | 6.075251 |
Table 8: Following transfection, miR‑221 was significantly down regulated with miR-221 inhibitor consequently, there was up regulation in the expression of CDKN1B/p27 as compared to control.
Discussion
Mi-RNA based on Gene therapy, is a target therapy for HCC miRNAs are included in the occurrence, development and prognosis of cancers thus making them a g target cancer gene therapy. MiRNAs are implicated in the pathogenesis of HCC. The gene delivery with Ultrasound micro bubbles was used. In this study we aimed to investigate the transfection efficiency of anti-miR-221 on HepG2 cells by using ultrasound micro bubbles and assessment effect of anti-miR on HepG2 cells, through MTT, cell cycle analysis and apoptosis assays were performed The expression of miRNA 221 and CDKN P27 were detected by using reverse transcription polymerase chain reaction. The assessment of the functional effects of miRNA inhibitior and its target effects that leads to loss of oncogenic effect of miR-221 that result from interactions between endogenous nucleic acids and antimiR oligonucleotide thus miRNA inhibitior may be a critical target therapy for HCC cell line. Anti-miR221 oligonucleotides as a potential therapeutic for HCC from accumulation in the HCC cancer cells, thus the level of endogenous miR-221 oligonucleotides was reduced by modulating miR-221-related gene levels. After transfection of miR-221 in HepG2 HCC cell lines, the cell growth was inhibited dramatically.
This explained by with liver regeneration the overexpression of miR-221 leads to rapid S-phase entry of hepatocytes. This indicates the relationship between miR-221 and cell proliferation in HCC. And also prove oncogenic role of miR-221 also formation of larger colonies than controls.
These agreements with in this study revealed that treatment with the antimiR-221 induce apoptosis in HCC cell line. Our results in agreement with (30, 28) The cell cycle of transfected cells was analyzed. By performing flow cytometer, the cell cycle was divided into four stages: Cells increase in size in Gap 1. The G1 checkpoint control for preparation to DNA synthesis. DNA replication occurs in S phase in G2 phase the gap between DNA synthesis and mitosis, the cell continue to grow. The G2 checkpoint is control mechanism for preparation to enter the M (mitosis) phase and in M phase Cell growth stops cellular energy is diverted to division into two daughter cells. A checkpoint in the middle of mitosis (Metaphase Checkpoint) to ensure that all cells complete divided.
In this study, cell cycle transfected cells analysis reported that there is significant Pre-G1 apoptos is and Cell growth arrest@ G1, 64.39%. I also G2/M significant arrest 3.67 while in s phase show no significant change. The progression of cell cycle, occur by overexpression of miR-221 thus affecting both CDKN1C/p57, CDKN1B/p27. Reported in HCC cells, stress that occurred in endoplasmic reticulum (ER) induced apoptosis and decreased by miR-221 inhibitor. MiR-221 promoted-apoptosis through ER stress is with p27 (Kip1) that have great effect on cell cycle regulation. Thus, suppression of miR-221 plays important role in the protect HCC cells. From apoptosis that induced by ER stress, p27 and p57are target genes of miR-221 and enhance hepatocarcinogenesis. Thus enhancement of cell proliferation by targeting B-cell lymphoma-2 modifying factor (BMF) thus could inhibit apoptosis.
In the current study, flow cytometer was performed to test the effect of miR-221 on apoptosis in HCC cells. apoptosis of transfected cell shows that there is increase apoptic rate in early 5.27 and late 68.8 phases with anti microRNA transfected group with ultrasound SF6 microbubbles while decrease in necrotic tissue 3.41 apoptosis of HCC cell lines was significantly increased with miR-221 inhibitor.
Our results in agreement is to prove the data of apoptosis, we further detected the caspase-3/7 activity. The result of caspase-3/7 activity was in line with apoptosis. Hence, inhibition of apoptosis by miR-221 in HCC cells lines proved the inverse correlation between miR-221 and activated caspase-3, as a marker of apoptosis. This explained by miR-221 can inhibit apoptosis, thus miR-221 act as posttranscriptional regulator of both necrosis factor related apoptosis. And FAS-induced apoptosis.
The apoptosis of transfected cells was confirmed by performing an Annexin V‑PE/7-AAD double staining assay. Compared with negative controls, the apoptotic rate was significantly increased with anti‑miR‑221/. The miR-221 expression level decreased with miR-221 inhibitor, the biggest ΔΔCq was 1.53 (66% knockdown) for the expression of miR-221 was determined using RT-qPCR. Following transfection, miR‑221 was significantly down regulated; miR-221 was negatively associated with cyclin dependent kinase inhibitors CDKN1B/p27 in HCC cell line. The present data indicated that the delivery of, anti-miR-221 may be mediated by ultrasound micro bubble contrast agents. Leads to, cell proliferation inhibition and cell apoptosis may be induced. These are new target therapy, the higher level of miR-221 in HCC than other liver tissues. Also the overexpression of miR221 in HCC that approve an oncogene role of miRNA and its critical role in the hepatocarcinogenesis.
Conclusion
The strong confirmation of oncogenic role of miR-221 and its vital role in the initiation and progression of human HCC, by affecting multiple pro-oncogenic pathways. The use of synthetic inhibitor of miR-221 might be a target therapy in HCC cell line. The transfection of recombinant plasmids, which contained anti‑miR221 in HepG2 cells by using Ultrasound micro bubbles as gene delivery system. Finally, it was identified that anti miR-221 inhibited cell proliferation and induced cell apoptosis in HepG2 cells. This indicated critical role of miRNAs to be target novel gene therapy.
Acknowledgements
We gratfully thank to Vaccines; VACSERA, Giza, Egypt.
References
- Stewart BW, Wild CP (2014) World cancer report. World Health Organization.
- Suzuki R, Oda Y, Utoguchi N, Maruyama K (2011) Progress in the development of ultrasound-mediated gene delivery systems utilizing nano and micro bubbles. J Control Release 149: 36-41.
- Winter J, Diederichs S (2011) MicroRNA biogenesis and cancer. Methods Mol Biol 676: 3-22.
- Gibb EA, Brown CJ, Lam WL (2011) The functional role of long non-coding RNA in human carcinomas. Mol Cancer 10: 38.
- Kudo M (2011) Hepatocellular carcinoma in 2011 and beyond: From the pathogenesis to molecular 5-targeted therapy. Oncology 81: 1-10.
- Budhu A, Ji J, Wang XW (2010) The clinical potential of microRNAs. J Hematol Oncol 3: 37.
- Maltby S, Plank M, Ptaschinski C, Mattes J, Foster PS (2015) MicroRNA function in mast cell biology: Protocols to characterize and modulate microRNA expression. Methods Mol Biol 1220: 287-304.
- Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, et al. (2007) MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 133: 647-658.
- Kota J, Chivukula RR, O’Donnell KA, Wentzel EA, Montgomery CL, et al. (2009) Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell 137: 1005-1017.
- Majid S, Dahiya R (2014) MicroRNA based therapeutic strategies for cancer: Emphasis on advances in renal cell carcinoma. MicroRNA Targeted Cancer Therapy pp: 175-188.
- Moscato S, Ronca F, Campani D, Danti S (2015) Poly (vinyl alcohol)/gelatin Hydrogels Cultured with HepG2 Cells as a 3D Model of Hepatocellular Carcinoma: A morphological study. J Funct Biomater 6: 16-32.
- Raisch J, Darfeuille-Michaud A, Nguyen HT (2013) Role of microRNAs in the immune system, inflammation and cancer. World J Gastroenterol 19: 2985-2996.
- Hung CH, Chiu YC, Chen CH, Hu TH (2014) MicroRNAs in hepatocellular carcinoma: Carcinogenesis, progression, and therapeutic target. Biomed Res Int 2014: 1-4.
- Tomanin R, Scarpa M (2004) Why do we need new gene therapy viral vectors? Characteristics, limitations and future perspectives of viral vector transduction. Curr Gene Ther 4: 357-372.
- Dai R, Li J, Liu Y, Yan D, Chen S, et al. (2010) miR-221/222 suppression protects against endoplasmic reticulum stress-induced apoptosis via p27 (Kip1)- and MEK/ERK-mediated cell cycle regulation. Biol Chem 391: 791-801.
- Gramantieri L, Fornari F, Ferracin M, Veronese A, Sabbioni S, et al. (2009) MicroRNA-221 targets Bmf in hepatocellular carcinoma and correlates with tumor multifocality. Clin Cancer Res 15: 5073-5081.
- Fornari F, Gramantieri L, Ferracin M, Veronese A, Sabbioni S, et al. (2008) MiR-221 controls CDKN1C/p57 and CDKN1B/p27 expression in human hepatocellular carcinoma. Oncogene 27: 5651-5661.
- Yan C, Zhu D, Huang D, Xia G (2015) Role of ultrasound and microbubble-mediated heat shock protein 72 siRNA on ischemia-reperfusion liver injury in rat. Int J Clin Exp Med 8: 5746-5752.
- Brayman AA, Chen L, Miao CH (2008) Ultrasound with microbubbles enhances gene expression of plasmid DNA in the liver via intraportal delivery. Gene Ther 15: 1147-1155.
- Krol J, Loedige I, Filipowicz W (2010) The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 11: 597-610.
- Carthew RW, Sontheimer EJ (2009) Origins and mechanisms of miRNAs and siRNAs. Cell 136: 642-655.
- Guo X, Guo S, Pan L, Ruan L, Gu Y, et al. (2017) Anti-microRNA-21/221 and microRNA-199a transfected by ultrasound micro bubbles induces the apoptosis of human hepatoma HepG2 cells. Onco Lett 13: 3669-3675.
- Park JK, Kogure T, Nuovo GJ, Jiang J, He L, et al. (2011) miR-221 silencing blocks hepatocellular carcinoma and promotes survival. Cancer Res 71: 7608-7616.
- Yuan Q, Loya K, Rani B, Mobus S, Balakrishnan A, et al. (2013) MicroRNA-221 overexpression accelerates hepatocyte proliferation during liver regeneration. Hepatology 57: 299-310.
- Turato C, Simonato D, Quarta S, Gatta A, Pontisso P (2014) MicroRNAs and Serpin B3 in hepatocellular carcinoma. Life Sci 100: 9-17.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences