ISSN : 2348-9502
American Journal of Ethnomedicine
Evaluation of the Nutritional Compositions and Analgesic Effects of the Flavonoid Fraction of Eugenia uniflora Ripe Fruit Pulp
Department of Biochemistry, University of Nigeria, Nsukka, Enugu State, Nigeria, Nsukka, Nigeria
- *Corresponding Author:
- Nwanneka N Onwudiwe
Department of Biochemistry
University of Nigeria, Nsukka, Enugu State, Nigeria
Tel: +2348032745063
E-mail: diego.onwudiwe25@gmail.com,
parker.Joshua@unn.edu.ng,
martinso568@gmail.com
Received Date: July 01, 2018; Accepted Date: August 20, 2018; Published Date: August 22, 2018
Citation: Onwudiwe NN, Njoku OU, Nwodo OFC, Parker JE, Ogugofor MO (2018) Evaluation of the Nutritional Compositions and Analgesic Effects of the Flavonoid Fraction of Eugenia uniflora Ripe Fruit Pulp. Am J Ethnomed Vol.5 No.1:7
DOI: 10.21767/2348-9502.10007
Abstract
Objectives: This present study was carried out to determine the phytochemical compositions, proximate compositions, anti-nutrients content and analgesic activities of the flavonoid fraction from the ripe fruit pulp of Eugenia uniflora.
Method: The phytochemical, proximate and anti nutrient compositions of the ripe fruit pulp of Eugenia uniflora and flavonoid extraction were determined using standard methods. The analgesic effect of the flavonoid fraction was evaluated using acetic acid-induced writhing and tail immersion test in mice. A dose 50 mg/ kg body weight of the flavonoid fraction was orally administered using cannula. The activity was compared with a standard reference drug 10 mg/kg diclofenac and normal control.
Results: The different phytoconstituents of the fruit pulp include the alkaloids, flavonoids, glycosides, reducing sugar, tannins, terpenoids, saponins and steroids. The proximate showed moisture (90.35%), carbohydrate (4.68%), ash (3.05%), protein (1.31%), fat (0.63%) and fiber (0.11%) contents. The anti-nutrients present included oxalate (34.08%) and cyanogenic glycoside (0.16%). The fraction and the standard drug significantly lowered (p>0.05) the total number of writhes induced by acetic acid. The percentage inhibition decreased from 0% in the normal control to 57.78% at 50 mg/kg of the fraction. The fraction significantly increased (p<0.05) the pain reaction time (PRT) induced by tail immersion test compared to the standard drug and control groups.
Conclusion: The fruit has adequate dietary nutrients and the concentration of the anti-nutrients will not be harmful to prevent the consumption of the fruit. It also suggests that E. uniflora fruit pulp possesses effective analgesic activity possibly mediated via both peripheral and central mechanism.
Keywords
Eugenia uniflora, Phytochemicals, Proximate, Anti-nutrients, Flavonoids fraction, Analgesic and pain
Introduction
The contribution of fruits and its constituent to human nutrition cannot be overstated. They are complemented with foods to ensure balance diet and some serve as raw materials to industries. They also serve as a source of vitamins, minerals and organic compounds which are required in small amount to make the body function properly. Also, they contain good quality of water, carbohydrates and proteins [1,2]. Eugenia uniflora is widely regarded as one of the best tasting of Eugenia Species. It is mostly grown for its edible fruits which are consumed fresh or made into jam and relish [3]. Besides its dietary importance, it is also a source of nutrients. The rise in the nutritional importance of Eugenia uniflora fruit also has been stimulated by a range of degenerative disease [4]. It also contains some anti-nutritional factors such as oxalate, phenol, cyanogenic and cardiac glycosides. Anti-nutrients are substances that interfere with the absorption of nutrients [5]. Oxalate has a harmful effect on human nutrition and health, because it reduces calcium absorption and aids the formation of kidney stone [6]. Cyanogenic glycosides are group of plant toxins known as cyanogens [7]. They are present in fruit and wilting leaves of many members of the rose family including cherries. Cynogenic levels vary widely with cultivar, climatic conditions, plant part and degree of processing. Cardiac glycosides are organic compounds containing a glycoside (sugar) that act on the contractile force of the cardiac muscle [8] because of their potency in disrupting the function of the heart, most are extremely toxic. They also protect plant from insects or other animals that feed on them. The phenolic compounds contribute towards color and sensory characteristics of fruits and vegetable [9], and also play role in providing against pathogens and predators [10]. They also play a role as anti-nutrients due to their ability to reduce digestibility of proteins either by direct precipitation or by inhibition of enzyme activity [11]. Phytochemicals are naturally and biologically active plant compounds that provide health benefits. They are found in plant foods and they work together with nutrients and dietary fiber to protect against disease [12]. They exhibit a wide range of biological activities, arising mainly from their antioxidant properties, anti-inflammatory and analgesic strengths, and ability to boost, the body’s natural detoxification system [13].
Pain is often interpreted as a suffering that results from the perception of painful stimuli. It is a disabling accompaniment of many medical conditions and is also a specific sensation with its own peripheral and central mechanisms independent of other five senses [14]. Pain itself is not a disease; rather it is perceived as an indication of ill health [15]. Pain is defined as an unpleasant sensory or emotional experience associated with actual or potential tissue damage. It is usually a warning signal and often causes a lot of discomfort [16]. Pain control is one of the most important therapeutic approaches. Drugs which alter pain sensitivity or remove pain are referred to as analgesics. The classical analgesic drugs such as the opiate and many nonsteroidal anti-inflammatory drugs have their origin from natural products [17]; many developed synthetic compounds that act by similar mechanisms are not entirely free of side effects like ulceration, gastrointestinal bleeding and respiratory distress and nausea. Based on these, great attention has been paid to plant based drugs and this has attracted research attention for the search of bioactive compounds from medicinal plants [18]. Eugenia uniflora which belongs to the family myrtaceae is an ever green shrub that can thrive in a variety of habitats both in its native and introduced state [19]. The plant has been considered very effective in treating many ailments including fever, diarrhea, diabetes, blood pressure, pain, and inflammation [20]. Earlier studies on the plant have shown that flavonoids are one of the most bioactive compounds found in the Eugenia uniflora fruit pulp [21,22]. Flavonoids are a large and diverse group of compounds that are widely distributed in all foods of plants origin. There has been increasing interest in dietary flavonoids, due to the growing evidence of their versatile health benefits [23]. Flavonoids have been found to possess antioxidant, analgesic, anti-allergic, anti-hepatotoxic, anti-inflammatory, anticancer and anti-viral activities [24]. The present study was conducted to determine some nutritional constituents, and the analgesic activity of flavonoids extracted from Eugenia uniflora fruit pulp.
Materials and Methods
Plant collection and identification
The ripe fruit pulp of Eugenia uniflora was sourced within the University of Nigeria Nsukka, and authenticated by a taxonomist from the Department of Plant Science and Biotechnology Institute, deposited in the Herbarium number 3, Nsukka, Enugu State.
Preparation of plant material
The fresh pulps of Eugenia uniflora were carefully separated from the seed and the pulps ground using mortar and pestle. A quantity, 50 g of the sample was kept in labeled air tight container and used immediately for proximate and anti-nutritional analysis using the method of AOAC [25]. Also another quantity, 310 g of the sample was macerated in 3 volumes (w:v) absolute methanol for 72 h. Thereafter, the extractive was filtered out with muslin cloth and Whatman No. 4 filter paper. The extractive was left to stand at room temperature for 4 days to obtain a semi-solid material. The extract was subjected to phytochemical analysis using the method of Sofowora [26], Trease and Evans [27].
Extraction of the flavonoid fraction
A known quantity 7.76 kg of the fresh fruit pulp was macerated twice in 1 liter of 80% ethanol for 72 h at room temperature and, then filtered using Whatman No.4 filter paper. The alcoholic extract was subjected to solvent-solvent extraction technique according to the method described by Harborne [28]. The filtrate was mixed thoroughly with equal volume of chloroform to partition the aqueous-ethanol extract. The two layers were drawn out separately and the chloroform fraction was dried in vacuo, weighed and designated the chloroform fraction (CLF). The main aqueous (portion) was measured and extracted three times with an equal volume of ethyl acetate for 48 h at room temperature. The ethyl acetate soluble extract were pulled together and dried in vacuo and weighed. The total flavonoid content of the different fraction was determined in percentage.
Total percentage content of flavonoid fractions
A quantity, 1 g each of the chloroform and ethyl acetate fractions was dissolved in 25 ml of 80% methanol. Thereafter 0.5 ml of both the chloroform and ethyl acetate were treated separately with 0.1 ml of 10% aluminum chloride followed by addition of 0.1 ml of distilled water and it was then incubated at room temperature for 30 minutes. The absorbance of the reaction mixture was measured at 415 nm with UV/VIS spectrophotometer. The amount of 10% aluminum chloride was substituted by the same amount of distilled water and used for the blank. Quantification was done using rutin standard equivalent. Results were expressed as percentage flavonoid content (%w/w)=RE × V × D × 106 × 100/W, where RE=rutin equivalent (ug/ml). V=total volume of sample (ml), D=dilution factor, W=sample weight (g).
Determination of flavonoid on the fractions
The presence of flavonoid in the ethyl acetate fraction was determined by the method described by Siddiqui [29]. Four mililiters of the fraction was treated with 15 ml of 50% methanol solution, warmed for few minutes and metal magnesium added to the solution. This was followed by addition 5-6 drops of concentrated HCl to observe a colour change from grey to orange.
Proximate composition determination
The moisture, ash, crude fiber, protein, fat and carbohydrate composition of the sample were determined using the method of AOAC.
Determination of moisture content: The sample (2 g) was weighed into a previously weighed crucible and placed into an oven set at 100°C to dry to a constant weight. It was removed from the oven and transferred to desiccators, cooled for 10 minutes and weighed. The moisture content was calculated as loss in weight of the original sample and expressed as percentage moisture content.
Determination of ash content: two grams (2 g) of the sample was placed into the previously weighed porcelain crucible and reweighed. It was first ignited and then transferred into a furnace which was set at 500°C. The sample was left in the furnace for 8 h to ensure proper ash. The crucible containing the ash was then removed, cooled in a desiccator and weighed at room temperature to get weight of the ash.
Determination of crude fiber: two grams (2 g) of the sample and 150 ml of preheated dilute H2SO4 were heated to boil for about 30 minutes and filtered. The left over residue was washed 3 times with hot water and returned into the beaker. A known volume 150 ml preheated KOH was added and the mixture heated to boil. This was followed by addition of 2 drops of antifoaming agent and was boiled for a further 30 minutes and filtered. It was then washed 3 times with hot water and acetone. This was then dried at 130°C for 1 h after which the content was incinerated to ashes at 500°C in a furnace for 3 h, cooled in a desiccator and reweighed. The percentage fiber was calculated as the loss in weight on incineration.
Determination of crude protein: Microkjeldahl method involving digestion, distillation and titration was used for crude protein determination. A quantity, 0.5 g of the sample was weighed into microkieldahl flask. This was followed by addition of 15 ml of concentrated H2SO4 and 1 g of Kjeldahl catalyst. The flask was set and heated cautiously in a digested rack under fume cupboard. The heating increased until content of the flask was completely digested to give a clear solution. The digest was allowed to cool. 10 ml distilled water was added to avoid caking. The digested sample solution was transferred into Kjehdahl distillation apparatus and 15 ml of 40% w/v of NaOH added. The digested sample solution was then steam distilled into a 50 ml receiver flask containing 5 ml boric acid plus mixed indicator solution. The pink colour solution was titrated against 0.1 M Hcl solution. The percentage nitrogen was calculated and multiplied by 6.25 to obtain the value of the crude protein.
Determination of fat: A weighed quantity, 0.5 g of the sample was weighed and transferred into a thimble and its content into the soxhlet extractor. The boiling flask was filled with 100 ml of petroleum ether; the thimble was covered slightly with cotton wool. It was then left to stand for 3 h. The thimble was removed and the solvent distilled off from the flask. The flask was then disconnected and placed in an oven at 60°C for 2 h, cooled in a desiccator and weighed. The percentage fat was calculated as the weight of fat over the sample weight.
Determination of carbohydrate: The sum of the percentage moisture, ash, crude protein, crude fat, crude fiber, was subtracted from 100.
Determination of phenol: the sample (5 g) was weighed using electric weighing balance. The weighed sample was soaked 100 ml of 2 M HCl acid and placed inside the oven for 3 h at 20-30°C. It was then allowed to cool and the filtered. The filtrate was poured into a separating funnel 30 ml of diethyl ether was added for washing. The mixture was shaken and two distinct layers formed. The second layer formed a clear solution and the first layer was poured back into the separating funnel and washed again with 20 ml of diethyl ether which separated into two layers. The first layer was discarded and the second weighed. The layer was poured into a weighed beaker and heated to dryness in a water bath. The beaker containing the dried sample was removed and cooled in a desiccator and weighed. The percentage content of phenol was determined Harborne [30,31].
Determination of oxalate: Oxalate concentration was determined by the method described by Ukpabi and Ejidoh [32]. The principle involves digestion, oxalate precipitation and permanganate titration.
A weighed quantity, 20 g of the sample was suspended in a 190 ml of distilled water in a 250 ml volumetric flask. 10 ml of 6 M HCl was added and then suspended to be digested at 100°C for 1 h. It was then cooled and made up to 250 ml in a volumetric flask with distilled water for titration. The 125 ml duplicate portion of the filtrate was measured and 4 drops of (0.1 g methyl red in 100 g ethanol) indicator was added wisely. The pH of the filtrate was adjusted (4-4.5) with concentrated NH4OH solution in drops until the test solution change from salmon pink colour to faint yellow colour. Thereafter, the solution was heated at 90°C, cooled and filtered to remove precipitate containing ferrous ion. The filtrate was treated with 10 ml of 5% calcium chloride (CaCl2) solution and heated in water bath at 90°C and stirred constantly, after which it was allowed to cool and left overnight at 5°C. The suspension was then centrifuged at 2500 rmp for 5 minutes. The supernatant was decanted and precipitated completely dissolved in 10 ml of 20% (v/v) H2SO4. The total filtrate resulting from digested of 20 g the sample was made up to 300 ml. An aliquot of 125 ml of the filtrate was heated until near boiling point and then titrated against 0.05 M standard KMnO4 until a faint pink colour appeared that persisted for 30 seconds. The oxalate concentration was calculated from the titration value.
Determination of cardiac glycoside: twenty grams (20 g) of the sample was soaked in 10 ml of 70% ethanol for 2 h and then filtered. Extract obtained was then purified using lead acetate and NaHPO4 solution before the addition of freshly prepared buljet’s reagent (containing 10 ml aqueous plus 5 ml of 10% aqueous NaOH). The difference between the intensity of colours of the experimental and blank (distilled water and buljet’s reagent) samples gives the absorbance and is proportional to the concentration of the glycosides, El-Olemy [33].
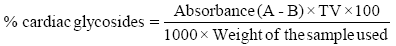
Absorbance of filtrate (test sample) is A
Absorbance of blank (buljet’s reagent) is B
Total volume of extract is TV.
Determination of cyanogenic glycoside
The sample (20 g) in four different conical flasks was added 100 ml of distilled water in each flask containing the samples. The samples were then covered with foil and placed in a vibrator to be agitated for 3 h. After which, the samples were filtered using a filter paper and the total volume of the extract was measured and recorded. 2 ml of the extract was dispensed into different test tubes and 2 ml of 10% DNS solution added. The mixture was boiled in a thermostatically controlled heating mantle for 20 minutes at room temperature. It was cooled and 10 ml of distilled water was introduced for dilution to take place. The solution of the extract was read with a UV visible spectrophotometer at 540 nm.
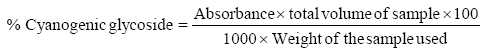
Animals
A total of thirty Wistar albino mice of average weight between 18-28 g purchased from the animal house of Veterinary Medicine, University of Nigeria Nsukka, Enugu State were used for the experiment. The animals were housed in separate cages and acclimatized to laboratory conditions for seven days, fed with balanced commercial pellet diet and had free access to water ad libitum. They were divided into six groups of five mice each. They were given oral dose of water, a standard drug diclofenac and the dose fraction.
Median lethality (LD50) test
The method of Lorke [34] was used for the acute toxicity study. Eighteen (18) albino mice of either sex were randomly grouped into three groups of three mice each and dosed orally with different graduated doses 10-5000 mg/kg body weight of the extract. In the first phase, three groups of mice were administered with the fraction doses of 10, 100, 1000 mg/kg body weight orally by means of a cannula under laboratory conditions. They were observed for 24 h for signs of toxicity. Based on the percentage survival rate, further increased doses of 1600, 2900, 5000 mg/ kg body weight were administered respectively in the phase two of second set of three groups of mice. The mice were observed for another 24 h for mortality and signs of toxicity. The LD50 was calculated as the geometric mean of the highest dose that survived and the lowest lethal dose that killed the mice.
Assay of analgesic effect
Study of the analgesic activity of the flavonoid fraction of Eugenia uniflora fruit pulp was carried out using acetic acid-induced writhing and tail immersion test. The dose of 50 mg/kg of flavonoid fraction and 10 mg/kg of the standard drug diclofenac were used in this study.
Acetic acid-induced writhing test
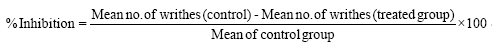
This study was carried out using the method of Zakaria et al. [35]. A total of fifteen Wistar albino mice randomly divided into three groups of five (5) mice each were used in this phase. The animals were fasted for 12 h and treated with 1 ml/kg of distilled water, 10 mg/kg of the standard drug and 50 mg/kg dose of the flavonoid fraction respectively. Acetic acid (1% v/v) was administered intraperitoneally (i.p) to all the groups at a dose of 1 ml/kg body weight one hour after the administration of the flavonoid fraction and standard drug to induce pain characterized by abdominal constrictions of writhes. Anti-nociception was observed and recorded by counting the number of writhes after injection of acetic acid for 30 munites; stretching movements consisting of arching of the back, elongation of body and extension of hind limbs were counted. The percentage inhibition of abdominal writhing was used to assess the degree of analgesia and was calculated using the formula.
Tail immersion test
The procedure is based on the observation that morphine – like drugs are selectively capable of prolonging the reaction time of the typical tail-withdrawal reflex in rats by immersing the end of the tail in warm water at 55°C.
This was carried out using the method of Uma-Devi et al. A total of fifteen Wistar albino mice randomly divided into three groups of five (5) mice per group were used in this phase. The animals were fasted for 12 h and treated with 1 ml/kg of distilled water for Group 1 (control), 10 mg/kg of the standard drug and 50 mg/ kg dose of the flavonoid fraction respectively. One hour after the administration of the extract, about 2-3 cm of the tail of each of the mice was immersed in the water bath at exactly 55°C. Within a few seconds the mice would react by withdrawing and flicking the tail from the warm water. The pain reaction time (PRT) was recorded for all the animals at 0.5 s unit using a stopwatch. The cut off time was put at 15 seconds.
Statistical analysis
The data were expressed as mean ± SD and test of statistical significance was carried out using one way ANOVA. The difference between the mean was tested with Post Hoc Duncan and t-test and values of p ˂0.05 were considered statistically significant.
Results and Discussion
The quantitative phytochemical analysis is very useful in the evaluation of some active biological constituents of plants. The phytochemical compounds were extracted using 80% methanol and crude extract. The quantitative phytochemicals analysis conducted revealed the quantity of different phyto-constituents alkaloids, glycosides, flavonoids, reducing sugar, saponins, steroids, tannins and terpenoids contents in the fruit pulp.
The data in Table 1, revealed that E. uniflora fruit pulp had flavonoids, reducing sugar, alkaloids, tannins, terpenoids, glycosides, saponins and steroids. The flavonoids were found to be high with the reducing sugar. This result agreed with the work of Amita [36] on the genus Eugenia (mrytaceae). The flavonoids and tritepenoids are generally associated with the mrytaceae. The phytochemical compounds exhibit numerous pharmacological activities like analgesic, antipyretic, anti-fungal, anti-microbial, anti-oxidant, antidiabetic, anti-inflammatory, hepatoprotective, antioxidant activity. Studies have also shown that flavonoids are one of the most active compounds of E. uniflora, and polyphenolic compound have been found to have many biological and pharmacological activities. Flavonoids are known to inhibit oxidative damage [37]. They protect against UV damage that can affect tropical fruits growing under severe conditions [38]. They have health promoting effects like anti-allergic, anti-oxidant, anti-inflammatory, anticancer, analgesic and anti-viral effects. Alkaloids are essential in medicine and constitute a seeable proportion of the valuable drugs; they are used as basic medicinal agents for their analgesic, antispasmodic and bactericidal effects [39]. Fruits that have tannins as their components are astringent in nature and are used for treatment of intestinal disorders such as diarrhoea, dysentery as well as the treatment of swollen tissues and in the prevention of cancer [40,41].
Table 1: Quantitative phytochemical constituents of extract.
| Phytochemicals | Mean ± SD (mg/g) |
|---|---|
| Reducing sugar | 315.36 ± 9.14 |
| Flavonoids | 280.74 ± 2.79 |
| Alkaloids | 75.20 ± 8.62 |
| Terpenoids | 61.30 ± 0.13 |
| Tannins | 28.70 ± 0.75 |
| Glycosides | 6.79 ± 0.15 |
| Saponins | 2.55 ± 0.11 |
| Steroids | 2.06 ± 0.07 |
Data are presented as mean ± SD.
The proximate composition of food is the estimation of its nutritive value. Eugenia uniflora fruit pulp contained high moisture value 90.35%. This is not surprising as many fleshy fruits have high percentage of moisture. The moisture content of any food is an index of its water activity and it is used as a measure of stability and the susceptibility to microbial contamination (Table 2).
Table 2: Proximate compositions of Eugenia uniflora fruit pulp.
| Parameters | (%) Mean ± SD |
|---|---|
| Moisture | 90.35 ± 0.07 |
| Ash | 3.05 ± 0.07 |
| Crude fiber | 0.11 ± 0.01 |
| Crude protein | 1.31 ± 0.00 |
| Fat | 0.63 ± 0.05 |
| Carbohydrate | 4.68 ± 0.02 |
Values are expressed in % mean ± SD.
The ash content is a reflection of mineral contents preserved in the plants. The ash content of E. uniflora fruit pulp showed value of 3.05% higher than fiber, protein and fat. This suggests high deposit of mineral elements in the fruit pulp. Fruit generally are not good sources of protein and fat. Dietary fat increases the palatability of food by absorbing and retaining flavours [4]. Food rich in dietary fiber contributes to the prevention of various diseases such as constipation, haemorrhoids, colon cancer, excess cholesterol and diabetes [23]. The carbohydrate content showed a value of 4.687%. The carbohydrate content contributes to the energy value in the E. uniflora fruit pulp and is essential for the maintenance of life in both plants and animals. It is use as a raw material for many industries [12]. The result obtained indicates that Eugenia uniflora fruit pulp is a fairly good source of carbohydrate and protein.
For anti-nutritional factor of the E. uniflora fruit pulps, oxalate was the highest in concentration followed by phenol. The level of cardiac and cyanogenic glycosides was low. Oxalate has a harmful effect on human nutrition and health, because it reduces calcium absorption and aids the formation of kidney stones (Table 3).
Table 3: Anti nutritional content in Eugenia uniflorafruit pulp.
| Anti nutrients | (%) Mean ± SD |
|---|---|
| Oxalate | 34.08 ± 0.67 |
| Phenol | 10.98 ± 0.02 |
| Cardiac glycoside | 0.29 ± 0.00 |
| Cyanogenic glycoside | 0.16 ± 0.00 |
| Phytate | ND |
Values are expressed in % mean ± SD.
Many fruits including cherries are considered with low oxalate and the relative amounts of oxalate formed in plants foods depend both on the species and its nitrogen status. Oxalates may not be harmful if consumed in moderate amount [42,43]. Therefore, the result of the oxalate content in the E. uniflora fruit pulp could have a positive impact on the health of consumers to enhance the bioavailability of essential dietary minerals of the E. uniflora fruit pulp. It has been reported that higher intake of cyanides could result in the development of neurological disease in human. The amounts of cyanides produced in plants that accumulate more than 50-200 mg are considered to be dangerous [44]. However, smaller amount of cyanides could have several long-term adverse effects on human health. Cyanogenic glycosides account for approximately 90% of wide group of plant toxins known as cyanogens. The amount of cyanogenic glycosides in plant is usually reported as the level of releasable free hydrogen cyanide [45]. Cherry fruits have been reported to contain 4.6 mg/ kg amount of cyanogenic glycosides [46]. These results showed that E. uniflora fruit pulp is safe with regards to cyanide poisoning due to the fact that the cyanogenic glycosides level was far below the detrimental level 4.6 mg/kg. However, in terms of nutritional composition, Eugenia uniflora fruit pulp was fairly adequate with low amounts of anti-nutrients.
Acute toxicity tests provide data on the relative toxicity likely to arise from a single brief exposure of any substance at different levels of bioactive compounds inherent in the plant [47]. The result of the toxicity study of the flavonoid fraction of E. uniflora fruit pulp produces no mortality or signs of toxicity after 48 h observation period even at the dose of 5000 mg/kg. This showed that in a single dose, there were no adverse effects and hence the extract is generally regarded as safe (Table 4).
Table 4: Results of the acute toxicity (LD50) test.
| Dose (mg/kg body weight) | No of animals before Administration | No of deaths after Administration |
|---|---|---|
| Phase one | ||
| 10 | 3 | - |
| 100 | 3 | - |
| 1000 | 3 | - |
| Phase two | ||
| 1600 | 3 | - |
| 2900 | 3 | - |
| 5000 | 3 |
The abdominal constriction response induced by acetic acid is a sensitive procedure to evaluate peripherally acting analgesics [48]. Acetic acid induced writhing test was used for detecting both central and peripheral analgesia, whereas tail flick immersion test is the most sensitive to centrally acting analgesic [49]. The acetic acid induced writhing test was selected because of several advantages including the ability to mimic human clinical pain conditions, sensitive to mild analgesics, production of tonic stimulus and sensitivity to non-steriodal anti-inflammatory drugs (Table 5) [50,51].
Table 5: Effect of flavonoid fraction of E. uniflorafruit pulp on acetic acid induced writhing reflex in mice.
| Group | Treatment | Mean number of writhing | % Inhibition |
|---|---|---|---|
| Control | Distilled water | 236.00 ± 62.47b | 0 |
| Group II | 10 mg/kg | 97.20 ± 55.75a | 58.92 |
| Group III | 50 mg/kg | 100.00 ± 15.37a | 57.78 |
Mean ± SD, n=5. The same superscript shows non- significant (p >0.05) difference between the means, different superscript shows significant (p <0.05) difference.
The result showed that the 50 mg/kg of the flavonoid fraction of E. uniflora fruit pulp and the 10 mg/kg of the standard drugs diclofenac significantly reduced (p<0.05) pain, the abdominal constriction and stretching of hind limb in the test and standard drug groups compared to the control group. The pain induced by acetic acid in the group treated with the standard drug (10 mg/kg of diclofenac) was significantly reduced (p<0.05) than the 50 mg/kg of the fraction compared to the control group. The standard drug exhibited a writhing inhibition percentage of 58.92% and test 57.78% when compared to the control. A nonsignificant (p>0.05) difference was observed in the reduction of pain between the standard drug (10 mg/kg) and 50 mg/kg of the fraction. The analgesic effect of the 50 mg/kg of E. uniflora fruit pulp flavonoid fraction and 10 mg/kg of the standard drug seen in this experiment may be mediated through peripheral pain mechanism involved in the inhibition of prostaglandin synthesis [52]. Pain sensation in acetic acid induced writhing/ abdominal constriction method is elicited by triggering localized inflammatory response resulting to the release of free arachidonic acid from tissue phospholipid [53] via cyclooxygenase (COX) and prostaglandin synthesis [54]. Local peritoneal receptors are postulated to be involved in the abdominal constrictions response. This method has been associated with increased levels of PGE2 and PGF2α in peritoneal fluids as well as lipoxygenase products [55]. The increase in prostaglandin levels with the peritoneal cavity then enhances inflammatory pain by increasing capillary permeability [56]. It has been observed that any agent that decreases the number of writhing will render analgesic effect preferably by inhibition of prostaglandin synthesis, a peripheral mechanism of pain inhibition.
The tail immersion test is based on the observation that morphine-like drugs selectively prolongs the reaction time of the typical tail withdrawal reflex in mice [57]. In this model increase in pain reaction time (PRT) period indicates the level of analgesia of drugs (Table 6) [58].
Table 6: Effect of flavonoid fraction of E.uniflora fruit pulp on acetic acid induced writhing test in mice.
| Group | Treatment | Mean PRT (seconds) ± SD |
|---|---|---|
| Control | Distilled water | 3.20 ± 0.44a |
| Group II | 10 mg/kg | 4.00 ± 0.70b |
| Group III | 50 mg/kg | 4.80 ± 0.44c |
Mean ± SD, n=5. The same superscript shows non- significant (p>0.05) difference between the means, different superscript shows significant (p<0.05) difference.
The dose 50 mg/kg of the flavonoid fraction and 10 mg/kg of the standard drug significantly (p< 0.05) increased the pain reaction time relative to the control group. The 50 mg/kg of the flavonoid fraction significantly (p< 0.05) increased the pain reaction time compared to 10 mg/kg of the standard drug. Treatment with 50 mg/kg of the flavonoid fraction produced a better analgesic effect than that of the 10 mg/kg of standard drug in alleviating central pain.
Conclusion
E. uniflora fruit pulps showed a fairly adequate nutrient composition and they need little or no processing before they are consumed. Thus, the fruit pulps could form a more stable source of various nutrients. The analgesic effects of flavonoid fraction E. uniflora fruit pulps could be mediated through central and peripheral pain inhibition and thus could be useful in the management of pain.
Acknowledgements
The authors acknowledge Prof. O.U. Njoku of the Department of Biochemistry, University of Nigeria for supervision and contributions towards this research and Kola Olawole of Project Research and Development Institute Enugu who freely availed me his services in the laboratory towards this work.
Conflict of Interest
No conflict of interest is associated with the publication of this work.
References
- Oliveira AL, Lopes RB, Cabral FA, Eberlin MN (2006) Volatile compounds from pitanga fruits (Eugenia uniflora L.). Food Chemistry 99: 1-5.
- Wenkam A (1990) Utilization and Processing of Fruits. Macmillian Press, London 5: 388-506.
- Okoh E, Rosemary U, Suleman JH, Thomas SA (2011) Proximate and phytochemical analysis of leaf, stem and root of Eugenia uniflora (Surinam or Pitanga cherry). Journal of Natural Product Plant Resource 1: 1-4.
- Venkatesh S, Madhava RB, Dayanand, RR, Ramesh M (2003) Antipyretic activity of Rumex nepalensis roots. Journal Natural Products and Medicine 7: 53-55.
- Beecher GR (2003) Overview of dietary flavonoids; nomenclature, occurrence and intake. Journal Nutrition. 133: 3248-545.
- Mutalik S, Paridhavi K, Mallikarijuna RC, Udupa N (2003) Antipyretic and analgesic effect of leaves of Solanum melongena Linn in rodents. Indian journal of pharmacology 35: 312-315.
- Conn E (1995) The world of phytochemicals. Proceeding of the 10th Annual Penn state Symposium in Plant Physiology 1: 1-14.
- Simeonova FP, Fishbein L (2004) Hydrogen cyanide and cyanides: Human health aspect. Geneva: world health organization. Concise international chemical assessment document, p: 61.
- Alasalvar JM, Grigor D, Zhang PCQ, Shahidi F (2001) Comparison of volatiles, phenolics, sugars, antioxidant vitamins and sensory quality of different colored carrot varieties. Journal of Agricultural and Food Chemistry 49: 1410-1416.
- Bravo L (1998) Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutrition Review 56: 317-333.
- Ferdous M, Rouf R, Shilpi JA, Uddin SJ (2008) Anti-nociceptive activity of the ethanolic extract of Ficus racemosa (Lin). Oriental Pharmacy and Experimental Medicine 8: 93-96.
- Polk M (1996) Feast on Phytochemicals. AICR Newsletter 54: 532-40.
- Fahey J, Talalay P (1995) The role of crucifers in cancer chemo protection in phytochemicals and health. Gustine DL, Flores HE (eds.) Rock Ville MD: American Society of Plant Physiologists 89: 10377-10389.
- Ranadran K, Basinath L (1986) A critical analysis of the experimental evaluation of nociceptive reactions in animals. Pharmaceutical Research 3: 253-270.
- Debono DJ, Hoekesma LJ, Hobbs RD (2013) Caring for patients with chronic pain: pear and pitfalls. Journal of the American Osteopathic Association 113: 620-627.
- Rang HP, Dale MM, Ritter JM, Moore PK (2003) Pharmacology (5thedn), New Delhi India; Churchill Livingstone, pp: 430
- Montgomery RD (1980) Cyanogens. In: Liener IE (ed.) Toxic constituents of plant foodstuffs New York, Academic Press, pp: 149-160.
- Ibanga OI, Okon DE (2009) Journal of Food Science Technology 7: 106-110.
- Ferguson LR (2001) Role of plant polyphenols in genomic stability. Mutation Research 475: 89-111.
- Noonan SC, Savage GP (1999) Oxalic acid and its effects on humans. Asia pacific Journal of Clinical Nutrition 8: 64-74.
- Olusanyan JO (2008) Essential of food and nutrition (1stedn), Apex Books Limited Lagos 36: 77.
- Raquibul SM, Hossain MM, Aktar R, Jamila M, Mazumder MEH et al. (2010) Analgesic activity of different fractions of the Aerial perts of commenila Benghalensis linn. International Journal of Pharmacology 6: 63-67.
- Woolfe G, MacDonald AD (1994) The evaluation of analgesic action pethidine hydrochloride. Journal of Pharmacology and Experimental Therapeutics 80: 300.
- Uma-Devi P, Ganasounder IA, Roa SB, Srivasan KK (1999) In Vitro Radioprotection by Ocimum flavonoids, Survivalm of Mice. Radiation Research 151: 74-78.
- Association of Official Analytical Chemists (AOAC) (1990) Official method of analysis, (15thedn), Inc Virginia, pp: 220-224.
- Singh B, Rastogi RP (1997) Cardenolides-glycosides and genins. Phytochemical 9: 315-331.
- Toma WO, Graciosa JS, Hiruma CA, Andrade FDP, Vilegas W, et al. (2003) Evaluation of the analgesic and anti-edamatogenic activities of Quassia mara bark extract. Journal of Ethnopharmacology 85: 19-23.
- Habtamu F, Fekadu B Gullelat D (2014) Evaluation of bioavailability and sensory preference of processed Anchote (Coccinia Abyssinica) tubers in Eastern Wollega, Ethiopia. Journal of Food and Nutrition Sciences 2: 1-12.
- Siddiqui AA, Ali M (1997) Pratical pharmaceutical chemistry, (1stedn), CBS publishers and distributors, New Delhi, pp: 126-131.
- Harborne JB (1995) The Flavonoids: Advances in Research. Journal of Chemical Education 72: 72-73.
- Harborne JB (1973) Phytochemical methods. Chapman and Hall ltd, London, pp: 49-98.
- Udeme JO, Kpobari NN, Akaninwor JO, Uwakwe AA (2013) Proximate, phytochemical and mineral elements compositions of some edible fruits grown in oil producing community of rivers state, Nigeria. Journal of Environmental Science Toxicology and Food Technology 5: 38-48.
- Edeoga HO, Eriata DO (2001) Alkaloid, Tannin and Sapnin contents of some medicinal plants. Journal of Medicinal Aromatic Plant Sciences 23: 344-349.
- Lorke D (1983) A new approach for practical acute toxicity testing. Archives of Toxicology 55: 275-285.
- Zakaria ZA, Abdul GZF, Raden M, Nor RNS, Gopalan HK, et al. (2008) Antinociceptive, anti-inflammatory and antipyretic properties of an aqueous extract of Dicranopteris linearis leaves in experimental animal models. Journal of Natural Medicine 62: 179-187.
- Amita G, Neetu K, Mahabeer PD, Sharma MC (2014) Biological importance of phytochemical constituents of isolated from genus Eugenia. Journal- Indian Chemical Society 91: 1539-1553.
- Tuncel G, Nout MJ, Brimer L Goktan D (1990) Toxicological, nutritional and microbiological evaluation of tempe fermentation with rhizopus pligosporus of bitter and sweet apricot seeds. International Journal of Food Microbiology 11: 337-344.
- FLEPPC (2005) Identification and biology of non-native plants in Florida’s natural area Eugenia uniflora.
- Ebun-Oluwa PO, Alade AS (2007) Nutritional potential of Belandiern Nettle spurge Jatropha cathatica seed. Pakistan Journal of Nutrition 6: 345-348.
- Bajai AM (2001) Effect of Natural extract of pineapple on disstibility, performance traits and nitrogen balance of broiler chicks. Australian Journal of Basic and Applied Sciences 5: 10-30.
- Kumara NK (2001) Identification of strategies to improve research on medicinal plants used in Sri Lanka. In: WHO Symposium. University of Ruhunna, Galle, Sri Lanka.
- Antia BS, Akpan EJ, Okon A, Umoren IU (2006) Nutritive and Anti-Nutritive Evaluation of Sweet Potatoes (Ipomoea batatas) Leaves. Pakistan Journal of Nutrition 5: 166-168.
- Saha P, Mazumber UK, Halder PK, Islam A, Kumar SRB (2011) Evaluation of acute and subchronic toxicity of Legenaria siceraria aerial part. International Journal of Pharmacetical Science Research 2: 1507–1512.
- Gene RM, Segura I, Adzet T (1989) Heterothecainuloides: Anti-inflammatory and analgesic effects. Journal of Ethnopharmacology 60: 157.
- Trease GE, Evans WC (2002) Pharmacognosy, (15thedn), Saunders London, pp. 214-393.
- Hassan LG, Usman BB, Kamba AS, Hassan SW (2009) Nutritional compositions of vegetable spaghetti. Nigeria food Journal 27: 41-49.
- Reynertson KA, Margaret JB, Edward JK (2005) Antioxidant Potentials of Seven Myrtaceous Fruits. Ethnobotany Research and Application 3: 25-35.
- Galeotti F, Barile E, Curir P, Dolci M, Lanzotti V (2008) Flavonoids from carnation (dianthus caryophyllus) and their antifungal activity. Phytochemisrty Letters 1: 44.
- Sanchez-alonso F, Lachica M (1988) Oxalate salts in the leaves of plum (Prunus salicina L) and cherry (P. Avium. L). New Phytologist 108: 505-508.
- Sofowora A (1993) Medicinal plant and traditional medicine in Africa. Published by Wiley and Sons Limited Chichester, p: 256.
- Wills RBH (1998) Post harvest. An introduction to physiology of handling fruits, vegetable and omamentals, (4thedn), Publication University of New Wales Press Limited, p: 560.
- El-Olemy MM, Al-Muhtadi FJ, Afifi AFA (1994) Experimental Phytochemistry: A Laboratory Manual, King Saud University Press, Saudi Arabia, pp: 21-27.
- Ahmed F, Hossan MH, Rahman AA, Shahid IZ (2006) Antinociceptive and sedative effects of bark of Cerberaodollam Gaertn. Oriental Pharmacy and Experimental Medicine 6: 344-348.
- Deraedt R, Joughney S, Delevakee F, Falhour M (1980) Release of prostaglandin E and F in an algogenic reaction and its inhibition. European Journal Pharmacology 51: 17-24.
- Davis RH (1991) Cyanogens, in toxic substances in crop plant. In: Mello JPF, Duffus CM, Duffus JH (eds.) The royal society of chemistry, Cambridge pp: 202-225.
- Yao LH (2004) Flavonoids in food and their health benefits. Plant Food Human Nutrition summer 59: 113-22.
- Tjolsen A, Berge O, Hunskaar S, Rosland JH, Hole K (1992) The formalin test; an evaluation of the method. Pain 51: 5-17.
- Onwudiwe NN, Njoku OU, Joshua PE (2010) Phytochemical analysis and acute toxicity/lethality study of ethanol extract of Eugenia uniflora pulp. Research Journal of Pharmacognosy and Phytochemistry 2: 336-339.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences
