ISSN : 2393-8862
American Journal of Pharmacology and Pharmacotherapeutics
Translating the Role of Vitamin D Supplementation in Chronic Liver Disease: A Systematic Review and Meta-analysis of Randomized Controlled Trials
Petrana Martinekova1, Mahmoud Obeidat1, Mihaela Topala1, Szilard Vancsa1, Daniel Sandor Veres2, Adam Zolcsak2, Miheller Pal3, Laszlo Foldvari-Nagy4, Peter Banovcin5, Balint Eross1, Peter Hegyi1 and
Krisztina Hagymasi3*
1Department of Translational Medicine, Semmelweis University, Budapest, Hungary
2Department of Biophysics and Radiation Biology, Semmelweis University, Budapest, Hungary
3Department of Surgery, Transplantation and Gastroenterology, Semmelweis University, Budapest, Hungary
4Department of Morphology and Physiology, Faculty of Health Sciences, Semmelweis University, Budapest, Hungary
5Department of Gastroenterology, University Hospital in Martin, Martin, Slovak Republic
- *Corresponding Author:
- Krisztina Hagymasi
Department of Surgery, Transplantation and Gastroenterology, Semmelweis University, Budapest, Hungary
E-mail: hagymasi.krisztina@med.semmelweis-univ.hu
Received date: November 19, 2023, Manuscript No. IPAPP-23-18139; Editor assigned date: November 21, 2023, PreQC No. IPAPP-23-18139 (PQ); Reviewed date: December 05, 2023, QC No. IPAPP-23-18139; Revised date: March 12, 2025, Manuscript No. IPAPP-23-18139 (R); Published date: March 20, 2025, DOI: 10.36648/2393-8862.12.1.201
Citation: Martinekova P, Obeidat M, Topala M, Vancsa S, Veres DS, et al. (2025) Translating the Role of Vitamin D Supplementation in Chronic Liver Disease: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Am J Pharmacol Pharmacother Vol:12 No:1
Abstract
Background: Vitamin D (VD) deficiency is highly prevalent in Chronic Liver Disease (CLD). Although international societies recommend supplementation in cases of proven deficiency, its impact on CLD remains uncertain. Our aim was to evaluate the effect of VD supplementation in CLD by conducting a systematic review and meta-analysis of Randomized Controlled Trials (RCTs).
Methods: We systematically searched three databases on 8th November 2022 (PROSPERO: CRD42022370312). Our primary outcomes involved survival, Controlled Attenuation Parameter (CAP), Liver Stiffness Measurement (LSM), and effects on changes in liver enzymes. Secondary outcomes included lipid profile and Homeostasis Model Assessment of Insulin Resistance (HOMA-IR), among others. Pooled Risk Ratio (RR), Mean Difference (MD), and corresponding 95%Confidence Intervals (CI) were calculated using the random-effects model.
Findings: Forty-one RCTs were included, comprising 3,562 patients. When comparing the VD group with the control, the overall survival RR was 1.14 (CI: 0.85; 1.54; 4 RCTs) at 6 months and 0.99 (CI: 0.83; 1.17; 4 RCTs) at 12 months. VD supplementation resulted in non-significant lower CAP (3 RCTs, MD: -23.50 dB/m; CI: -81.72, 34.72). The MD for LSM was -0.65 kPa (3 RCTs; CI: 1.98; 0.68). A significant reduction in HOMA-IR was observed in the VD group (12 RCTs; MD: 0.44; CI: -0.87; -0.01). A summary forest plot showed that alanine aminotransferase (20 RCTs; MD: -3.26 IU/L; CI: -6.37, -0.16) and gamma-glutamyl transferase (10 RCTs; MD: 5.15 IU/L; CI: 9.05; -1.25) were significantly reduced in the supplemented patients.
Conclusions: Our results showed significant differences for ALT, GGT, and HOMA-IR in the VD group. In addition, there were no differences in survival, CAP, and LSM. Further RCTs with adequate power and appropriate sample size are warranted to clarify these results.
Keywords
Vitamin D; Micronutrient; Liver cirrhosis; Chronic liver disease
Key Points
• Basic science suggested a possible protective role of vitamin D in hepatic fibrogenesis. The beneficial effect of vitamin D supplementation on liver steatosis and fibrosis was not shown based on the result of our study.
• Insulin resistance, determined by HOMA-IR, was significantly reduced after vitamin D supplementation. Prolonged supplementation may offer a more profound effect. While insulin levels were greatly reduced in vitamin D patients, there was no evidence of difference in fasting plasma glucose levels.
• There was a significant reduction in ALT and GGT in supplemented patients. The inclusion of eligible participants with liver enzymes above the normal range may be warranted to ascertain this effect.
Introduction
The burden of Chronic Liver Diseases (CLD) is a global concern, leading to significant mortality. Ye, et al. demonstrated an increasing trend in global cirrhosis-related mortality by 47.15% from 1990 to 2017 [1]. The most pronounced increase was detected in countries with middle or high sociodemographic index and Eastern Europe, where unfavorable trends in cirrhosis-related mortality were driven by lifestyle-modifiable etiologies such as Alcohol-Related Liver Disease (ALD) or Non-Alcoholic Fatty Liver Disease (NAFLD).
The nuclear Vitamin D Receptor (VDR) has been detected in various tissues and cells, involving skeletal muscle, liver, pancreas, or immune cells [2]. The circulating form of vitamin D, calcifediol (25-hydroxycholecalciferol, 25(OH)D), can be used by many cells to locally produce its active metabolite owing to the presence of a 1α-hydroxylase enzyme (CYP27B1) [3]. Due to the potential of vitamin D to regulate the expression of numerous genes, intensive research on its extra-skeletal properties has been conducted over the last two decades.
Vitamin D (VD) deficiency is common in CLD, despite its endogenous cutaneous production [4]. There are multifactorial causes, including patient lifestyle (sedentarism, lack of optimal nutritional intake) and the effect of a disease (intestinal edema in portal hypertension, hepatic dysfunction, maldigestion, malabsorption, hypercatabolic state) [5,6]. Association of VD deficiency with liver dysfunction, complications, and mortality, including its role in hepatic fibrogenesis, have been previously described [7-11].
The European Association for the Study of Liver (EASL), the American Association for the Study of Liver Diseases (AASLD), and the European Society for Clinical Nutrition and Metabolism (ESPEN) recommend VD supplementation in patients with CLD when a deficiency is detected to reach the desired levels of calcifediol (above 30 ng/ml or 75 nmol/l) [12-14]. Nonetheless, it remains unclear whether VD supplementation in deficient CLD patients yields any impact, as demonstrated by previously published meta-analyses [15]. However, additional Randomized Controlled Trials (RCTs) have been published. Furthermore, a quantitative summary of the effect on liver steatosis or fibrosis has not been meta-analyzed beforehand. On the basis of the recommendations of international societies and the inconsistent results of VD supplementation in this population, we aimed to assess and clarify the role of calciferol in managing CLD.
Materials and Methods
This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guideline (Supplementary Table S1) and the Cochrane Handbook [16,17]. The pre-established protocol was registered in advance on PROSPERO (CRD42022370312), and we fully adhered to it [18].
Eligibility criteria
We followed the subsequent inclusion criteria: 1) Human studies; 2) Randomized controlled trials; and 3) Fitting the PICO framework (Table 1) [19]. To increase comprehensiveness and decrease publication bias, we also included conference abstracts, as recommended by Scherer, et al.
| P | Adult patients (≥ 18 years) with CLD, irrespective of stage and etiology. |
| I | Standard of care+vitamin D supplementation in any form, dose, duration, route of administration, administered as monotherapy or in combination with calcium. |
| C | Standard of care with or without placebo. |
| O | Primary: Survival, Controlled Attenuation Parameter (CAP), Liver Stiffness Measurement (LSM), Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), Gamma-Glutamyl Transferase (GGT), Alkaline Phosphatase (ALP), C-Reactive Protein (CRP), and Interleukin-6 (IL-6). |
| Secondary: Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), insulin, Fasting Plasma Glucose (FPG), Total Cholesterol (TC), Total Triglycerides (TG), Low-Density Lipoprotein cholesterol (LDL), High-Density Lipoprotein cholesterol (HDL), adiponectin, International Normalized Ratio (INR), albumin, and bilirubin. | |
| Surrogate: Virologic response, Bone Mineral Density (BMD), and sarcopenia. |
Table 1: Detailed PICO framework.
Information sources and search strategy
A literature search was conducted on the 8th of November 2022 in three electronic databases: MEDLINE (via PubMed), Embase, and Cochrane Central Register of Controlled Trials (CENTRAL), with no restrictions or filtering options. Forward and backward citation chasing of included articles was performed on the 5th of January 2023, to identify further relevant studies. For a detailed search strategy, (Supplementary Table S2).
Selection and data collection process
After automatic and manual duplicate removal, two independent review authors (PM and MT) conducted a selection to identify studies potentially eligible for further assessment. Cohenâ??s kappa was used to describe the agreement during the selection. A third independent reviewer (MO) resolved disagreements in case of any discrepancy. Data from eligible studies were manually extracted and cross-checked by two independent investigators (PM and MT). Differences in extracted data were resolved by discussion
Data items
We collected the following data items: Study characteristics (first author, year, geographical location, number of participating centers, study period, and sample size); demographics (etiology of CLD, age, sex, and Body Mass Index (BMI)); details about the intervention and control groups separately (number of participants, age, sex, BMI, baseline VD level, and the standard of care applied); intervention specifics (a form of VD supplements, dosage, brand, duration, monotherapy or in combination with calcium); and outcome measures as reported in each article. Primary and secondary outcomes were assessed at the end of the trial period, and the change (after treatment baseline values) was extracted if data for change were available.
Study risk of bias assessment
Two authors (PM and MT) performed the risk of bias assessment independently using the Cochrane collaboration RoB2 tool with disagreements resolved by consensus [20]. The subsequent domains were critically evaluated: Randomization process, deviation from the intended interventions, missing outcomes, outcome measurement, and selection of reported results. Each domain and each outcome were judged to have a high (red), unclear (yellow), or low (green) risk of bias.
Quality of evidence
Each finding was evaluated according to the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) framework using the GRADEpro tool (software) to estimate the level of evidence.
Synthesis method
The minimum number of studies required to perform a metaanalysis was set at three. As we assumed considerable betweenstudy heterogeneity in all cases, a random-effects model was used to pool effect sizes. When there were two treatment or control arms, we combined them to create a single pairwise comparison, as recommended by the Cochrane Handbook. We calculated the Risk Ratio (RR) with a 95% Confidence Interval (CI) for dichotomous variables and Mean Differences (MD) for continuous variables. Results were considered statistically significant if the pooled CI did not contain the null or one value for MDs and RRs, respectively. We summarized the findings of the meta-analysis in forest plots. In addition to the betweenstudy variance (Ï?2), between-study heterogeneity was described by Higgins and Thompsonâ??s I2 statistics. Further details on the statistical analysis can be found in Supplementary File S1, Synthesis methods and search key.
Results
Search and selection
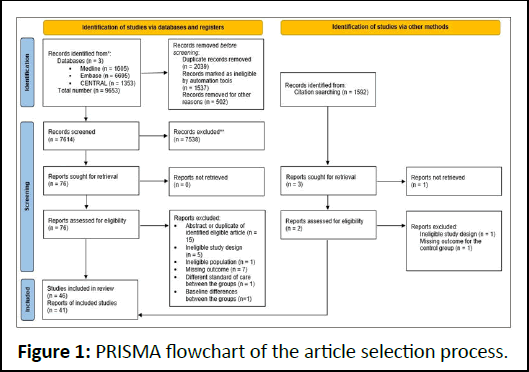
Altogether, 9,653 studies were identified. After duplicate removal, 7,614 articles remained; titles and abstracts were reviewed for these. Seventy-six studies were found eligible, 46 of which were after full-text selection (Figure 1). Two RCTs were published only as abstracts, and one abstract was a post hoc analysis.
Figure 1: PRISMA flowchart of the article selection process.
Baseline characteristics of included studies
Supplementary Table S3 yields a summary of relevant studies. Thirty-three of the included studies were performed in Asia, five in Europe, two in Africa, and one in the USA. The most commonly used form of VD supplementation was vitamin D3 (cholecalciferol, n=24), followed by calcitriol (n=5), vitamin D2 (ergocalciferol, n=5), and calcifediol (n=1). Six studies did not specify the form and dose of VD.
In 17 RCTs, patients suffered from NAFLD, 13 RCTs involved patients with chronic viral Hepatitis C (CHC), one trial was performed on patients with Chronic Hepatitis B (CHB), and one trial included both CHC and CHB. Eight studies involved patients with liver cirrhosis regardless of etiology, and one involved liver transplant recipients.
Survival
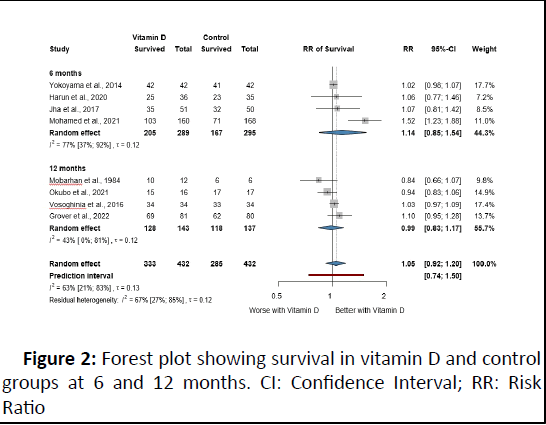
Eight RCTs were included in the survival analysis divided into six and twelve-month periods. We found insufficient evidence for improved or worse survival in patients with VD supplementation with overall RR 1.14 (95% CI: 0.85; 1.54) and 0.99 (95% CI: 0.83; 1.17) at six and twelve months, respectively (Figure 2).
Figure 2: Forest plot showing survival in vitamin D and control groups at 6 and 12 months. CI: Confidence Interval; RR: Risk Ratio
Liver steatosis (CAP) and liver fibrosis (LSM)
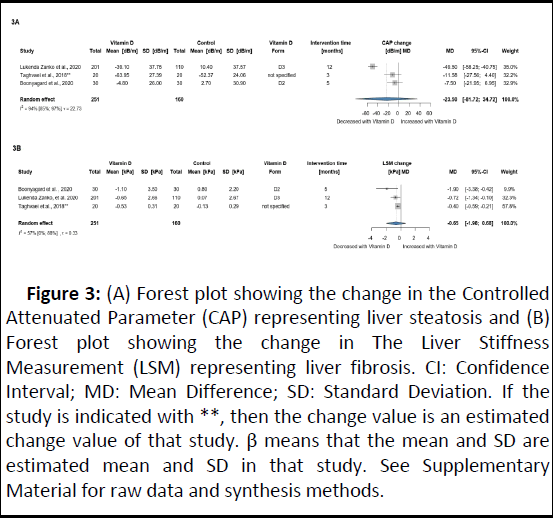
Three RCTs with 411 NAFLD participants were included in this analysis. Patients receiving VD showed a potentially relevant but not significant effect on steatosis assessed by CAP (MD: 23.50 dB/m; 95% CI: -87.72; 34.72), whereas no significant or relevant difference was found for LSM (MD: -0.65 kPa; 95% CI: 1.98; 0.68) (Figure 3). A summary of additional findings on liver fibrosis and steatosis is presented in Table 2.
Figure 3: (A) Forest plot showing the change in the Controlled Attenuated Parameter (CAP) representing liver steatosis and (B) Forest plot showing the change in The Liver Stiffness Measurement (LSM) representing liver fibrosis. CI: Confidence Interval; MD: Mean Difference; SD: Standard Deviation. If the study is indicated with **, then the change value is an estimated change value of that study. β means that the mean and SD are estimated mean and SD in that study. See Supplementary Material for raw data and synthesis methods.
| Author and year | Findings |
| Afsordeh, et al. 2019 | Eight weeks of aerobic training statistically improved the grade of fatty liver in both the AT+VD group and AT group; meanwhile, VD supplementation of 50,000 IU/week alone did not improve the grade. |
| Barchetta, et al. 2016 | Cholecalciferol for 24 weeks did not result in significant changes between the HFF, P3NP, and FLI groups. |
| Boonyagard, et al. 2020 | No significant differences were observed in steatosis grade and fibrosis stage assessed by Fibroscan® after 5 months of D2 supplementation. |
| Geier, et al. 2018 | Disease activity evaluated by NAFLD Activity Score (NAS) improved in 3/3 VD and 3/4 placebo patients. Absolute reduction of steatosis by at least 20% was observed in 2/3 VD patients but in none of the placebo patients. |
| Hoseini, et al. 2020 | Although the grade of fatty liver was reduced in AT+VD, AT, and VD groups, the most pronounced reduction was observed when aerobic training was combined with VD supplementation. Moreover, the grade of fatty liver significantly increased in the control group. |
| Hosseini, et al. 2018 | A single intramuscular dose of cholecalciferol+vitamin E 400 IU/day reduced the severity of liver steatosis by one grade in 8/37 (21.6%). The same standard of care+vitamin E 400 IU without VD caused a grade reduction only in 1/38 (2.6%). |
| Komolmit, et al. 2017 | Six weeks of D2 supplementation significantly increased anti-fibrogenic MMP-2 and MMP-9 levels while reducing profibrogenic TGFâ??β. |
| Pilz, et al. 2016 | Treatment with 2800 IU/day of cholecalciferol for eight weeks showed no difference in ELF score and levels of hyaluronic acid between the groups. |
| Sharifi, et al. 2014 | No significant differences in the grade of hepatic steatosis were observed between the VD-supplemented and placebo arms. |
| Sriphoosanaphan, et al. 2021 | Six weeks of VD in CHC patients after curative treatment with DAA does not improve serum fibrogenesis markers (MMP-9, P3NP, TGF-β) and may not facilitate the healing process of the remaining hepatic fibrosis. |
| Yaghooti, et al. 2021 | Treatment with calcitriol 0.25 μg/day for 17 weeks did not result in better HSI or APRI when compared to the placebo group. |
| Note: APRI: AST to Platelet Ratio Index; AT: Aerobic Training; D2: Ergocalciferol; DAA: Direct Antiviral Agents; ELF: Enhanced Liver Fibrosis; FLI: Fatty Liver Index; HFF: Hepatic Fat Fraction; IU: International Units; MMP: Matrix Metalloproteinase; P3NP: Procollagen 3 N-Terminal Peptide; TGF-β: Tumor Growth Factor β; VD: Vitamin D. | |
Table 2: Summary of further findings on liver fibrosis and steatosis.
Liver enzymes
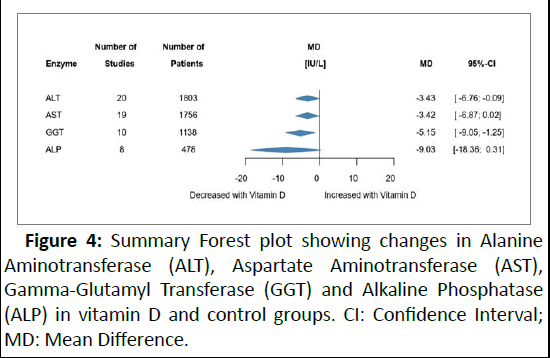
The effect of VD supplementation on liver enzymes is illustrated in Figure 4. In the vitamin D group, a significant decrease in ALT was observed with a small overall change of MD -3.26 IU/L (95% CI: -6.37; -0.16). A similar result was found for GGT (MD 5.15 IU/L; 95% CI: -9.05; 1.25). No difference between the groups was observed for AST and ALP (Supplementary file S2).
Figure 4: Summary Forest plot showing changes in Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), Gamma-Glutamyl Transferase (GGT) and Alkaline Phosphatase (ALP) in vitamin D and control groups. CI: Confidence Interval; MD: Mean Difference.
Glucose and lipid metabolism
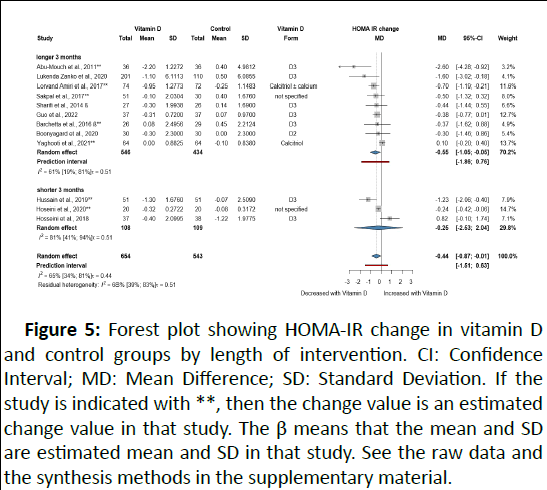
Twelve RCTs with 1,197 participants investigated the effect of VD on HOMA-IR. All but one study involved only patients with NAFLD. VD-supplemented patients showed decreased HOMA-IR with MD -0.44 (95% CI: -0.87; -0.01), indicating an improvement (Figure 5).
Figure 5: Forest plot showing HOMA-IR change in vitamin D and control groups by length of intervention. CI: Confidence Interval; MD: Mean Difference; SD: Standard Deviation. If the study is indicated with **, then the change value is an estimated change value in that study. The β means that the mean and SD are estimated mean and SD in that study. See the raw data and the synthesis methods in the supplementary material.
Fasting insulin levels decreased modestly in patients with VD (MD 0.91 μIU/L; 95% CI: -1.59; -0.22), whereas FPG did not decrease significantly between the groups. No difference was observed in adiponectin, LDL, HDL, TC, and TG (Supplementary File S3).
Additional outcomes
Inflammatory markers: We observed a small decrease in CRP with MD -0.58 mg/L (95% CI: -0.83; -0.33) in the VD group. Overall, there was no significant difference in IL-6 between the groups with an MD change of 1.42 pg/mL (95% CI: -4.51; 1.69) (Supplementary File S4).
Bone mineral density and sarcopenia: Due to insufficient data and differences in reporting BMD, we were unable to metaanalyze this outcome. Four RCTs evaluated the effect of VD on BMD, and two studies investigated the effect on skeletal muscles (Supplementary File S4).
Virologic response: The meta-analysis showed a higher point estimate for sustained virologic response in supplemented patients but without statistical significance (RR=1.29; 95% CI: 0.96; 1.74) (Supplementary file S5). In addition, Wang, et al. detected no differences in quantitative HBs antigen or HBV DNA levels in D3 and control groups in CHB patients.
INR, albumin, bilirubin: No significant differences were observed for INR (MD: -0.08; 95% CI: -0.59; 0.43), albumin (MD: -0.07; 95% CI: -0.27; 0.13 g/dL), and bilirubin (MD: -0.09; 95% CI: -0.62; 0.44 mg/dL) (Supplementary File S4).
Adverse events: Details of adverse events are provided in Supplementary Table S4.
Risk of bias and certainty of evidence
Most studies resulted in a moderate risk of bias. The high risk of bias was mainly due to deviations from the intended intervention and measurement of the outcome (Supplementary File S6). The quality of evidence was very low and low based on the GRADE assessment (Supplementary Table S5).
Publication bias and heterogeneity
No publication bias was detected for GGT, FPG, TG, LDL, HDL, TC, HOMA-IR, and AST. Eggerâ??s tests suggested a potential publication bias for ALT (Supplementary File S7).
Discussion
We comprehensively assessed the role of VD in CLD. Current evidence showed no statistical difference between VD supplementation and standard of care in terms of liver steatosis and fibrosis. Moreover, clinically negligible but statistically significant reductions in ALT, GGT, CRP, insulin, and insulin resistance were observed in the VD group. On the contrary, we did not find sufficient evidence that supplementation affects survival, IL-6, lipid profile (LDL, HDL, TC, TG), adiponectin, FPG, or liver function tests (INR, albumin, bilirubin).
Vitamin D deficiency, especially severe VD deficiency, has been associated with increased mortality in patients with CLD. Furthermore, a recent meta-analysis by Yang, et al. confirmed the association between severe VD deficiency (25O HD<10 ng/mL) and mortality risk in patients with LC (RR 1.79, 95% CI: 1.44; 2.22). Mayr, et al. reported that cirrhotic ICU patients with VD levels >20 ng/mL at admission had better survival than those with moderate or severe (25O HD<10 ng/mL) deficiency. Nonetheless, our study failed to demonstrate prolonged survival in the VD-supplemented group either for 6 or 12 months. Our analysis recognized the study of Mohamed, et al. as an outliner, where the supplemented group showed better survival. Interestingly, only patients with decompensated Liver Cirrhosis (LC) and Spontaneous Bacterial Peritonitis (SBP) were included in this trial. Further research may help to explain the role of cholecalciferol in immunity and ascertain its effect on decompensated cirrhotic patients with SBP. Cathelicidin is recognized as one of the factors responsible for the antimicrobial effects of VD. As infections contribute to the high mortality observed in liver diseases, further translational insight into the VD-cathelicidin axis is warranted.
Emerging evidence suggests a protective effect of VD against liver fibrogenesis. Although the mechanisms of action on hepatic fibrogenesis remain uncertain, VD has shown antifibrotic properties based on its regulation of HSCs via the VDR. Quiescent HSCs are activated upon stimulation by growth factors and cytokines, consequently playing an important profibrogenic role. Komolmit, et al. detected reduced levels of profibrotic TGF-β, while some antifibrotic Metalloproteinases (MMP) were significantly increased compared with those in the placebo arm. On the other hand, neither 6 weeks nor 4 months of VD supplementation resulted in statistical differences between the groups in the remaining RCTs. VD has been shown to alleviate hepatic fibrosis in cholecalciferol-treated mice through its effect on histidine-rich calcium-binding protein. A study by Ding, et al. demonstrated that VDR knockout mice develop spontaneous liver fibrosis. In addition, it has been shown that a non-calcemic VD derivate, calcipotriol, could prevent thioacetamide-induced liver cirrhosis in a mouse model. This meta-analysis suggests that VD may have modest beneficial effects on the reduction of steatosis and fibrosis measured noninvasively by CAP and LSM in NAFLD. Given the modest effect and the fact that the current EASL guideline recommends liver biopsy as a reference to evaluate NASH and liver fibrosis resolution, these findings must be interpreted with caution.
Insulin resistance is an important pathophysiological factor contributing to the development and progression of NAFLD. In addition, it has been found to influence the course of disease in patients with CHC. Our meta-analysis of 12 RCTs with 1197 participants showed a modest effect of VD on insulin resistance measured by HOMA-IR. This finding is concurrent with a previously published meta-analysis where VD improved insulin resistance with the pooled MD -1.06 (95% CI: -1.66; -0.45; p=0.0006). Interestingly, a double-blind trial of 54 participants with NAFLD and concomitant VD deficiency/insufficiency compared the effect of calcitriol (1 μg/day) and cholecalciferol (50000 IU/week) and found that calcitriol reduced HOMA-IR 1.8 times more than cholecalciferol. Due to data limitations, we were unable to compare different forms of VD. Similarly, the present data indicate that insulin levels were significantly reduced in the supplemented group. While the dogma implied hyperinsulinemia as a secondary consequence of insulin resistance in the past, current evidence has questioned this belief. Nevertheless, we found no difference in adiponectin levels, another contributing factor of insulin resistance described in the literature.
In populations at risk for VD deficiency, recommended doses vary widely. The majority of the included RCTs used cholecalciferol as the preferred form, although higher absorption rates of calcifediol have been described in the literature. Recent guidelines recommend against ergocalciferol supplementation, which was chosen in five of the studies included in our analysis. Indeed, a tailored approach to VD supplementation is needed, based on the specific mechanisms underlying the deficiency. Pludowski, et al. recommended using calcifediol in patients with CLD. However, data on its effect are scarce, as this study shows that only one RCT used this form. Interestingly, it has been proposed that the oral route of administration may be preferable for VD deficiency in the loading phase, whereas both oral and parenteral routes may be equivalent in maintenance treatment. This is contradicted by conclusions of two other studies, in which the intramuscular route was more effective in restoring VD levels. It remains unanswered how much these findings reflect the needs of CLD.
Strengths and limitations
Several strengths of the present study must be acknowledged. A rigorous methodology was strictly applied at each step. We included only RCTs to reduce confounding and increase the level of evidence. In addition, we performed a subgroup analysis for the length of the intervention, whereas subgrouping by etiological factor or form of vitamin D was not possible due to the low number of studies in different groups.
On the other hand, limitations should also be considered. The results are based on the limited number of trials performed mainly in Asia (80.5%); therefore, the conclusions might not be extrapolatable to the general population. Furthermore, differences in the applied interventions in terms of duration, doses, and follow-up also contributed to the high heterogeneity. Despite the fact that patients with ALD had significantly lower VD serum concentrations than individuals with other causes of liver cirrhosis, only one study included solely ALD patients in the trial.
Implications for practice and research
Rapid application of scientific results is paramount. As the burden of liver diseases indicates progressive trends in lifestylemodifiable diseases and liver disorders steadily contribute to premature deaths, it is necessary to translate mechanistic knowledge into bedside care. As VD is recommended to patients by individual societies, this meta-analysis provides a bridge to understanding the role of VD in the selected population. VD may be a cost-effective and simple tool to improve some outcomes in patients with CLD. However, further RCTs with adequate power and appropriate sample size are warranted to clarify the topic, especially to evaluate the effects of prolonged VD supplementation (â?¥ 6 months) on liver steatosis and fibrosis in CLD.
Conclusion
This meta-analysis showed that additional use of VD may result in modest improvements in HOMA-IR, ALT, GGT, CRP, and insulin. However, it did not lead to significant differences in survival, CRP, IL-6, lipid profile, adiponectin, FPG, INR, albumin, and bilirubin. VD neither improved nor worsened CAP and LSM, and therefore its effect on liver steatosis or fibrosis should be further investigated.
Conflict of Interest
The authors declare that they have no competing interests.
Credit Author Contribution Statement
PM: Conceptualization, project administration, data curation, methodology, writing-original draft; MO: Conceptualization, methodology, project administration, writing-original draft; MT: Conceptualization, data curation, writing-review and editing; SV: Conceptualization, writing-review and editing; DSV: Conceptualization, formal analysis, data curation, visualization, writing-review and editing; AZ: Conceptualization, formal analysis, data curation, visualization, writing-review and editing; MP: Conceptualization, writing-review and editing; LFN: Conceptualization, writing-review and editing; PB: Conceptualization, writing-review and editing; BE: Conceptualization, supervision, writing-review and editing; PH: Conceptualization, supervision, writing-review and editing; KH: Conceptualization, supervision, project administration, writing-original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This study was not supported by any funding.
Ethical Approval
No ethical approval was required, as all data were already published. No patients were involved in the design, conduction, or interpretation of our study.
Data Availability
The datasets can be found in the full-text articles included in the systematic review and meta-analysis.
Acknowledgments
We would like to express our gratitude to Maryam Saleh, a medical student at Semmelweis University, for her help in translating articles written in Persian.
References
- Ye F, Zhai M, Long J, Gong Y, Ren C, et al. (2022) The burden of liver cirrhosis in mortality: Results from the global burden of disease study. Front Public Health 10:909455
[Crossref] [Google Scholar] [PubMed]
- Pike JW, Meyer MB, Lee SM, Onal M, Benkusky NA (2017) The vitamin D receptor: Contemporary genomic approaches reveal new basic and translational insights. J Clin Invest 127:1146-1154
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Zhu J, deLuca HF (2012) Where is the vitamin D receptor? Arch Biochem Biophys 523:123-133
[Crossref] [Google Scholar] [PubMed]
- Llibre-Nieto G, Lira A, Vergara M, Sole C, Casas M, et al. (2021) Micronutrient deficiencies in patients with decompensated liver cirrhosis. Nutrients 13:1249
[Crossref] [Google Scholar] [PubMed]
- Lim LY, Chalasani N (2012) Vitamin d deficiency in patients with chronic liver disease and cirrhosis. Curr Gastroenterol Rep 14:67-73
[Crossref] [Google Scholar] [PubMed]
- Pop TL, Sirbe C, Benta G, Mititelu A, Grama A (2022) The role of vitamin D and vitamin D binding protein in chronic liver diseases. Int J Mol Sci 23:10705
[Crossref] [Google Scholar] [PubMed]
- Paternostro R, Wagner D, Reiberger T, Mandorfer M, Schwarzer R, et al. (2017) Low 25-OH-vitamin D levels reflect hepatic dysfunction and are associated with mortality in patients with liver cirrhosis. Wien Klin Wochenschr 129:8-15
[Crossref] [Google Scholar] [PubMed]
- Ravaioli F, Pivetti A, di Marco L, Chrysanthi C, Frassanito G, et al. (2022) Role of vitamin D in liver disease and complications of advanced chronic liver disease. Int J Mol Sci 23:9016
[Crossref] [Google Scholar] [PubMed]
- Sun J, Zhang YG (2022) Vitamin D receptor influences intestinal barriers in health and disease. Cells 11:1129
[Crossref] [Google Scholar] [PubMed]
- Triantos C, Kalafateli M, Aggeletopoulou I, Diamantopoulou G, Spantidea PI, et al. (2020) Vitamin D-related immunomodulation in patients with liver cirrhosis. Eur J Gastroenterol Hepatol 32:867-876
[Crossref] [Google Scholar] [PubMed]
- Udomsinprasert W, Jittikoon J (2019) Vitamin D and liver fibrosis: Molecular mechanisms and clinical studies. Biomed Pharmacother 109:1351-1360
[Crossref] [Google Scholar] [PubMed]
- Merli M, Berzigotti A, Zelber-Sagi S, Dasarathy S, Montagnese S, et al. (2019) EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol 70:172-193
[Crossref] [Google Scholar] [PubMed]
- Lai JC, Tandon P, Bernal W, Tapper EB, Ekong U, et al. (2021) Malnutrition, frailty, and sarcopenia in patients with cirrhosis: 2021 practice guidance by the American association for the study of liver diseases. Hepatology 74:1611-1644
[Crossref] [Google Scholar] [PubMed]
- Bischoff SC, Bernal W, Dasarathy S, Merli M, Plank LD, et al. (2020) ESPEN practical guideline: Clinical nutrition in liver disease. Clin Nutr 39:3533-3562
[Crossref] [Google Scholar] [PubMed]
- Bjelakovic M, Nikolova D, Bjelakovic G, Gluud C (2021) Vitamin D supplementation for chronic liver diseases in adults. Cochrane Database Syst Rev 8:CD011564
[Crossref] [Google Scholar] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, et al. (2021) The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Rev Esp Cardiol 74:790-799
[Crossref] [Google Scholar] [PubMed]
- Booth A, Clarke M, Dooley G, Ghersi D, Moher D et al. (2013) PROSPERO at one year: an evaluation of its utility. Syst Rev 2:4
[Crossref] [Google Scholar] [PubMed]
- Munn Z, Stern C, Aromataris E, Lockwood C, Jordan Z (2018) What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Med Res Methodol 18:1-9
[Crossref] [Google Scholar] [PubMed]
- Scherer RW, Saldanha IJ (2019) How should systematic reviewers handle conference abstracts? A view from the trenches. Syst Rev 8:264
[Crossref] [Google Scholar] [PubMed]
- Sterne JA, Savovic J, Page MJ, Elbers RG, Blencowe NS, et al. (2019) RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 366:14898
[Crossref] [Google Scholar] [PubMed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences