ISSN : 2393-8854
Global Journal of Research and Review
The Influence of Juvenile Fish (Oreochromis niloticus) on Population Density of Pond Breeding Mosquitoes in the Degraded Wetlands of Yala Swamp, Western Kenya
1Department of Biological Sciences, Makerere University-College of Natural Sciences, P.O Box 7062, Kampala, Uganda
2School of Environment and Natural Resource Management, Makerere University-College of Agriculture and Environmental Sciences, P.O Box 7062, Kampala, Uganda
Abstract
The study whose findings are presented in this paper aimed to assess the potential of Nile tilapia (Oreochromis niloticus) juveniles to cause a significant reduction in larval population of mosquitoes breeding in the ponds. Mosquitoes always raise a concern for public health where they occur, as in Yala swamp. Edible fish can be an incentive for promoting both socioeconomic and ecologically appropriate mosquito management practice, in a setting where resource poor communities can be proactively involved. Field experiments were conducted from April to August 2006 in three habitats (transient pools, control and experimental ponds) located within a disturbed wetland of Yala swamp. Fingerlings of Nile tilapia were introduced into one of the ponds and mosquito sampling commenced in both control and experimental ponds a week after the introduction of fish. We analyzed data for mosquito larval population mean differences in three habitats between two periods (long rains and dry spell). The results show that Anopheles gambiae ssp was the most abundant in transient pools followed by control Pond, while fish stocked Pond had significantly lower abundance. Unlike transient pools, the association (r = 0.466) between increased larval density and dry season in ponds was highly significant (P = 0.02). These results suggest that effective use of fish for mosquito control in ponds should be limited to a period immediately following heavy rains.
Keywords
Nile tilapia, Mosquito control, Degraded wetland, Yala swamp.
INTRODUCTION
Mosquitoes that are commonly known for the transmission of various types of vector-borne diseases are true flying insects belonging to the order Diptera; families of Cuilicidae and Toxorhynchitinae; and they are natural part of ecosystems, both intact and disturbed such as wetlands. Under Cuilicidae, the three genera most recognized are Aedes, Anopheles and Culex. There are over 3,000 different species of mosquitoes in the world, and each species distribution and abundance, according to Bernués-Bañeres and Jiménez- Peydró and Rueda, varies with spatial and temporal differences worldwide [1,2]. Of all the mosquito species in the world, only those that belong to genus Anopheles, and particularly female anopheles mosquitoes are capable of transmitting parasites (Plasmodium) causing malaria. Anopheles species common to Afro-tropical regions include An. funestus, An. arabiensis and An. gambiae sl. In East Africa the common malaria vectors are Anopheles gambiae sl, while their siblings, Anopheles arabiensis occur in West Africa [3]. In Kenya Plasmodium falciparum is the parasite causing malaria, and it is transmitted most often by Anopheles gambiae, with Anopheles funestus as another major vector [4,5]. Indeed malaria related studies merit research attention, as the disease remains a leading cause of human morbidity and infant mortality in Kenya.
As insects, a mosquito’s life cycle involves four stages in development (from egg through larval and pupal to adult). The first three stages of mosquito development are aquatic, making immature population of mosquitoes less mobile and more often localized in identifiable aquatic habitats. This also implies that larvae can only survive on subsurface micro-organisms and nutrients. These limitations of mosquito larvae to aquatic environment and survival on subsurface nutrients make a variety of fish [6] potentially suitable for their control. Consequently, various species of fish have been introduced in ponds, ditches, ornamental ponds and stagnant pools of water near areas of human habitation, which dramatically reduced both the vector and non-vector populations of mosquito rather fast [7-9]. A part from the use of extracts from plants [10], essential oils [11] and Gambusia affinis [12] in mosquito bio-control, data on the use of edible indigenous fish is sparse and inconclusive.
Although several species of locally available fish have the potential to prey on mosquito larvae6,13 and eventually bring down the overall mosquito population in an area, previous mosquito bio-control projects over emphasized the used Gambusia affinis. Interestingly, while studies portray G. affinis, a native of North America, as efficient predator of mosquito larvae, its negative ecological impacts are clearly documented [12]. As an alternative, using indigenous edible fish like Nile tilapia (Oreochromis niloticus) may offer an attractive mosquito larvae control measure. Being a major source of animal protein, as well as financial income to most rural communities in malaria endemic areas around Lake Victoria, it is likely that using edible fish could attract communities’ active involvement in mosquito control initiative from the forefront.
The elimination of mosquito larvae from aquatic habitats by predation, or avoidance of predator infested waters by ovipositing female mosquitoes [14] has rarely been studied in East Africa, with most mosquito control studies reporting use of indoor and outdoor control measures for adult mosquitoes and rarely on larvae [15,16]. This study tested the hypothesis that juveniles of edible Oreochromis niloticus have the potential to cause significant reduction in larval population of pond breeding mosquitoes. The first mosquito larval control trial with Nile tilapia in Kenya was carried out in western Kenya where mature fish was stocked in abandoned ponds [13]. The study by Howard and others highlighted very important role of edible fish in mosquito larval control [13], but did not consider how fish size and age could influence fish predation on immature mosquitoes. Understanding mosquito larval predation potential of juvenile fish, of pond breeding mosquitoes between seasons can facilitate planning a well targeted larval control using edible Nile tilapia.
MATERIALS AND METHODS
Study area
The study was carried out in the disturbed wetland area of Kadenge within Yala swamp (approximately 2km from Lake Kanyaboli) in Siaya district. The wetlands of Yala swamp are located on the North- Eastern shore of Lake Victoria [17]. The swamp, which is the third largest in Kenya after the Lorian swamp and Tana River delta, is probably the most valuable riparian and floodplain wetland in the delta of River Yala [17,18]. The wetland covers a geographical area of 17,500 ha (175 km²) and has three components of fresh water lakes namely, Kanyaboli (15 Km²), Sare (5.0 km²) and Namboyo (2.0 km²). The dominant anthropogenic activities in the study area are fresh water irrigated agriculture and aquaculture. The crops Dominion Farms Limited cultivate in the drained part of the wetland are sun flower, vegetable varieties, rice and maize. Other natural resource based commercial activities include aquaculture practiced by the same Dominion Farms Limited, while members of the local communities do fishing, graze cattle and harvest papyrus from the wetland for their livelihood. Figure 1 illustrates the river catchments of Yala Swamp wetlands in western Kenya.
Experimental design
The experimental ponds were uniformly constructed in the degraded part of Yala swamp wetland and filled with fresh water. Fingerlings of Nile tilapia (Oreochromis niloticus) were obtained from the existing commercial fish ponds of the Dominion Farms Limited, Yala swamp, and transferred in two large plastic containers by road to the study site. Fifty fingerlings (initial weight 10.85±0.03 g) were stocked in the experimental pond measuring 9m³. Water temperature, pH, and dissolved oxygen were similar in both ponds before the introduction of fish into the experimental pond. Once in the pond, the fish were left to feed naturally on their preferred diet.
Mosquito larvae sampling
Anopheles and culicine mosquito larvae sampling exercise began in different larval habitats three days after introducing juvenile Oreochromis niloticus (Nile tilapia) into the experimental pond. Sampling was done using standard dippers (350 ml) twice every week between 8.00 hr and 10.00 hrs, whenever weather permitted. Apart from the ponds which retained water for longer periods, different types of small water bodies (transient pools) were identified each day at the beginning of the survey. Up to 3 dips were made in each type of transient water body encountered at each survey within the location of ponds. Ponds with relatively larger water surface received 10 dips for each observation during the survey. The presence or absence of mosquito larvae was recorded. Only mosquito larvae of third and forth instars were counted and recorded.
A week to the end of long rains fish were harvested and given to the community, and ponds emptied thereafter by the aid of generator operated water pump. The ponds stayed dry for a week, after which they were refilled with fresh water. Accordingly, one week into the beginning of dry spell, both ponds were refilled with fresh water, after which another batch of fingerlings was introduced into the experimental pond and mosquito larvae sampling began three days later. Larvae from each larval collection habitat were transferred to Ratuoro health centre in separate water filled containers for indoor breeding. The adult mosquitoes emerging from the breeder were counted, recorded and identified morphologically by the use of a light microscope [20]. The two ponds and their immediate environment were cleared of herbaceous vegetation on a regular basis and water level maintained identically in two ponds during the study.
Data analysis
SPSS for windows version 17.0 was used to carry out statistical analysis. We examined descriptive measures in terms of larval population means and standard error of mean. Association of immature mosquitoes’ distribution and abundance with the pond type (control and experimental ponds) was examined using Pearson correlation (r). Mosquito larval species abundance (expressed as mean number of immature in different breeding habitat types was examined for significant mean differences between habitats using a Post-Hoc test in one way analysis of variance (ANOVA). We estimated mosquito population mean differences between two seasons (wet and dry), for both species and habitat type, using paired sample t-test. All numbers of sampling units per habitat, including those with no larvae, were included in larval population density analysis. We calculated larval density for each mosquito habitat by dividing total larvae sampled by number of dips. Finally, we generated a graph illustrating the effect of Nile tilapia on mosquito larval population by indicating changes in larval population at the beginning (soon after stocking pond with fish) through monthly trend to the end of each season for two ponds.
RESULTS
The results showed that two species of anopheles mosquitoes, often associated with transmission of malaria in East Africa, are abundantly present in Yala swamp. Of these two species of anopheles mosquitoes, the most abundant was Anopheles gambiae complex, which was found to be breeding successfully throughout the year. The larval habitat type had important influence on the distribution and relative abundance of different species of mosquitoes. For instance, the most preferred breeding habitats for mosquitoes of Yala swamp were the transient pools of water.
On the contrary, ovipositing Anopheles gambiae and culicine mosquitoes had less preference for large pond waters, and in particular fish pond was least preferred. It was also observed that, at mosquito species level, breeding activity of Anopheles funestus was high in the fish pond (151) compared to the control pond which had only 104 larvae. Similarly, a higher proportion of Anopheles gambiae sampled in fish pond compared to Anopheles funestus larvae could be explained by the overall abundance of Anopheles gambiae in the study area during the sampling period (Table 1).
Table 1: Proportion of mosquito species and their distribution in three habitats
| Habitat | An. gambiae | % proportion | An funestus | % proportion | Culines | % proportion |
|---|---|---|---|---|---|---|
| Control pond | 244 | 54.3 | 104 | 23.2 | 101 | 22.5 |
| Fish pond | 164 | 47.0 | 151 | 43.3 | 34 | 9.7 |
| Transient pools | 573 | 52.5 | 380 | 34.9 | 137 | 12.6 |
*Column counts show number of mosquito species identified during the field experiments
On mosquito larval density the results showed that mosquito breeding activity was intense in the transient water pools, which were comparatively smaller in size than the ponds. A single observation made in the transient water pools during the study period was likely to yield an average of 27 mosquito larvae, compared to 11 and 9 for control pond and experimental pond respectively. These results further confirm that transient water pools had significantly higher abundance of mosquito larvae compared to control pond and experimental pond (Table 2).
Table 2: Mean mosquito larval population in each of the three habitats
| Habitat type | Observations | Number of dips | Total larvae | Larval density/dip | Mean (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Control pond | 40 | 400 | 449 | 1.12 | 11.2(8.6 - 13.8) | 0.08 |
| Fish pond | 40 | 400 | 349 | 0.87 | 8.7(7.0 - 10.4) | 0.12 |
| Transient pools | 40 | 400 | 1090 | 2.73 | 27.3(22.6 - 32.0) | 0.56 |
*Mosquito larval population abundance by habitat type was significant at P < 0.05
The results of statistical analysis indicate that mean mosquito larval population was significantly different between habitats. The highest mean larval population variability among sites occurred between transient water pools and fish pond, indicating that fish pond had the lowest larval population density during the study. Indeed control pond had slightly higher but significant (p < 0.05) mean mosquito density than fish pond. This is evidenced by a positive mean difference of 2.5 between control and fish seeded pond. Table 3 provides a summary of paired samples t-test for significant difference between two means, considering population abundance of mosquito larvae in the three habitats surveyed during field experiment.
Table 3: Summary of paired samples t-test for larval population abundance by habitat type
| Mean difference of mosquito larvae | |||||
|---|---|---|---|---|---|
| Variables | Habitat (I) | Habitat (J) | Mean Diff (I-J) | STD Err | P -value |
| Paired habitats | Transient pools | Fish pond | 18.5 | 0.86 | 0.001 |
| Control pond | 16.0 | 0.84 | 0.001 | ||
| Control pond | Fish pond | 2.5 | 0.43 | 0.001 | |
Mean difference for paired samples t-test was significant at P < 0.05 (2-tailed)
For the surveyed habitats, the results showed that mosquito species population varied significantly (p < 0.05) within habitats but between seasons. However, seasonal differences in mean population abundance of mosquito species among the three sampled larval habitats were significant for anopheline species. According to data obtained from transient pools of water, for larvae of culicine origin, mean larval population difference between seasons was not significant (P > 0.05). The dry season had lower mean abundance of culicines and Anopheles gambiae than wet season. On the other hand, Anopheles funestus population, unlike the An. gambiae and culicines, increased in abundance significantly (P < 0.05) in the three habitats during the same dry season. This suggests that An. funestus prefer relatively permanent sun-lit water bodies compared to other mosquito species (Table 4).
Table 4: Mosquito species relative abundance in their breeding habitats
| Habitat | Mosquito species | Wet season | Dry season | P- value |
|---|---|---|---|---|
| Mean ± SE | Mean ± SE | |||
| An. gambiae | 5.90 ± 0.37 | 6.30 ± 0.45 | 0.50 | |
| Control pond | An. funestus | 1.25 ± 0.22 | 3.95± 0.32 | 0.00 |
| Culicines | 3.15 ± 0.27 | 1.90 ± 0.27 | 0.00 | |
| An. gambiae | 4.80 ± 0.47 | 3.40 ± 0.27 | 0.01 | |
| Fish pond | An. funestus | 2.95 ± 0.29 | 4.60 ± 0.32 | 0.00 |
| Culicines | 0.45 ± 0.19 | 1.25 ± 0.22 | 0.01 | |
| An. gambiae | 16.35 ± 0.86 | 12.30 ± 0.39 | 0.00 | |
| Transient pool | An. funestus | 8.35 ± 0.62 | 10.60 ± 0.34 | 0.03 |
| Culicines | 3.60 ± 0.43 | 3.30 ± 0.55 | 0.62 |
*Mosquito species relative abundance by habitat type between seasons was significant at P < 0.05
It was further observed that total mosquito populations increased significantly (P< 0.05) in both control and fish pond during the dry season, while mosquito populations declined in the transient water pools during the same period. As shown in Table 5, population mean differences of mosquito larval between seasons in both fish pond and control pond were statistically significant (p < 0.05). On the contrary, population mean difference of mosquito larvae in transient water pools was not statistically significant (P>0.05) between wet season and dry season, with higher mean frequency occurring in wet season.
Table 5: Seasonal variability of mosquito population within larval habitat
| Larval habitats | Paired mean difference of mosquito larvae | ||||
|---|---|---|---|---|---|
| Wet season (I) | Dry season (J) | t | df | p - value | |
| Paired seasons (I - J) | |||||
| Control pond | 10.3(9.87 - 10.73) | 12.15(11.63 - 12.67) | -2.23 | 19 | 0.038 |
| Fish pond | 8.20(7.71 - 8.69) | 9.25(8.84 - 9.66) | -2.12 | 19 | 0.047 |
| Transient water pools | 28.3(27.34 - 29.26) | 26.20(25.77 - 26.63) | 1.28 | 19 | 0.216 |
*Mean difference for paired samples t-test was significant at P < 0.05 (2-tailed)
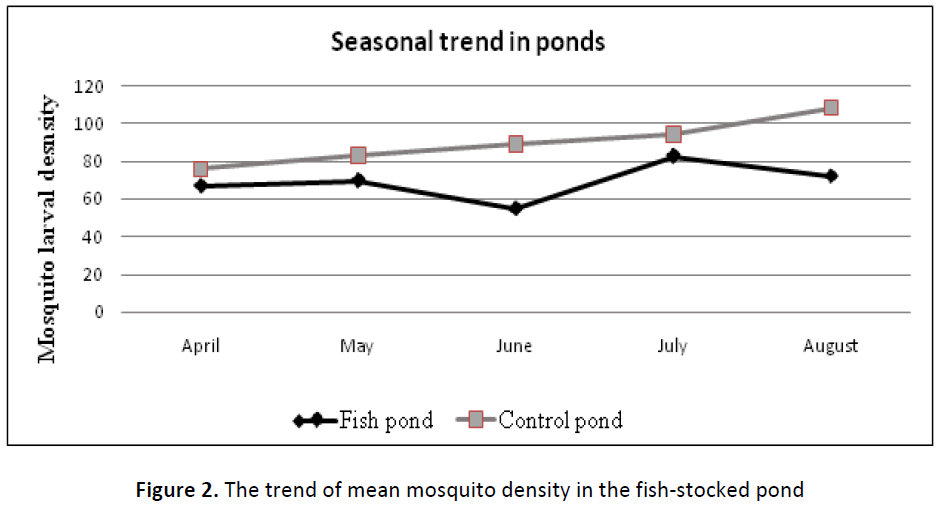
A low population mean of mosquito larvae in fish pond suggests that edible fish (Oreochromis niloticus) had influence on larval survivorship. Although mosquito larvae are not natural food for Nile tilapia (Oreochromis niloticus), low larval density in fish stocked pond indicates that fish significantly suppressed populations of mosquito larvae in the experimental pond (Figure 2). The suppression of larval population could have occurred either through direct predation on mosquito larvae or deterring ovipositing female anopheles mosquitoes from breeding in fish (predator) infested water.
Further observation showed that in the fish seeded pond, mosquito larvae occurred in larger numbers during the first part of the wet season (April) but declined towards June at the end of rainy season when juvenile fish had presumably adapted to a new pond. Similarly, compared to the month of August of phase two mosquito larvae sampling, mosquito larval population was significantly higher (p < 0.05) in the month of July when new fish had just been introduced into the pond for dry season experiments. Unlike the fish pond, the population of mosquito larvae increased progressively in control pond from April when numerous small water pools were widely distributed outside the ponds to August when a few relatively larger pools of water were available in the study area. Factors such as fish adaptation after being introduced into the pond and subsequent increased food requirement for growth of fish are important parameters in fish ecology that might explain mosquito larval population distribution patter shown in Figure 2 below.
DISCUSSION
This study first and foremost showed that ovipositing Anopheles mosquitoes, in the degraded area of Yala swamp wetland, favored smaller water collections more than larger pools for their breeding activities during the study period A description by Gillies and De Meillon and Service [20,21], on preferred breeding habitats for Anopheles funestus was not consistent with our findings. However, the results we presented agree with the findings of a study conducted in Dar es Salaam revealing that Anopheles species were likely to be found in low densities in large drains [22]. Another similar study also observed that besides inhibiting mosquito oviposition preference, large habitats tend to remain in place long enough for predators to establish [23]. While the latter view partly explains fairly low population density of Anopheles larvae in the ponds, it might not account for the absence of larvae in the natural swamp where even smaller pools of water registered no mosquito larvae.
Our study showed a significant reduction in population of mosquitoes breeding in fish seeded pond, with lower mean mosquito density than control pond. A similar field experiment with mature Nile tilapia (Oreochromis niloticus), carried out in western Kenya [13], found out that pond stocked with fish had significantly lower larval density than control ponds. However, there are a few cases where studies reported failure in the use of fish for mosquito control [24,25], suggesting that the practice can be unpredictable in some contexts. Although the presence of O. niloticus fingerlings in the pond was negatively correlated with mosquito larval density, this study did not investigate whether low larval density in fish seeded pond was due to predation or avoidance of the fish infested pond by gravid female mosquitoes. However, some studies have shown that immature fish of tilapia origin are usually omnivores, unlike adult tilapia which are thought to be herbivores [26]. Therefore, authors were of the opinion that mosquito larvae were being eaten by fish.
Season influence on mosquito breeding in ponds was also observed during the study. In dry season ovipositing mosquitoes showed significantly higher preference for larger pools of water (Fig. ponds vs transient pools). This could be due to the fact that small pools resulting from sprinkle irrigation did not often stay long enough in dry season to support the development of mosquitoes in aquatic environment. It was suspected that short life of transient water pools, resulting from agriculture irrigation activities in the study area, was a deterrent to mosquito breeding in smaller water pools in dry weather. For example, we observed during larvae sampling survey that most of the small transient pools dried before a repeat sampling. Subsequently, emerging new pools were exploited in succession for larval survey within the study site. More importantly, the findings confirm that edible indigenous fish can cause significant reduction of mosquito population, thereby making fish farming a good fit initiative for controlling anopheles larvae in a stagnant water pool.
Similarly, stocking relatively larger water pools with edible fish (Oreochromis niloticus) is likely to work for mosquito population reduction strategy when it is targeted at the dry season breeding mosquitoes. This is so, because dry season mosquito breeding habitats are usually semipermanent water sources. In most cases such water sources are often localized, have relatively larger surface and can sustain significantly high mosquito population. The larval habitat conditions for dry season could ensure the survival of malaria vectors in ecologically stressful conditions until weather changes for better. This facilitates subsequent population explosion of mosquitoes when weather conditions change from dry to wet, and rainfall creates new habitats. Thus, stocking edible fish in water sources that are likely to support mosquito population during the dry season could be a good management strategy for mosquito bio-control.
As shown in Table 5, there were significant mosquito population mean differences between wet and dry seasons in both control as fish ponds. However, dry season had higher mean population abundance of mosquito larvae in both control and fish pond. The higher abundance of mosquito larval density in ponds during dry season does not imply less predation by fish. Rather, it implies that more mosquitoes were breeding in permanent water sources due to limited habitats in dry weather conditions. This could be expected, because transient water pools were drying up as soon as they resulted from the irrigation activities in the disturbed part of the wetland. Indeed fish feeding on mosquito larvae was expected to be more intense in dry season when macrophytes which usually constitute the larger proportion of fish diet were low in supply. Dry weather conditions can affect the ability of aquatic macrophytes for rapid vegetative growth, which may in turn lead to low supply in ponds. A study conducted elsewhere ranked plant remains (macrophytes) higher among food items preferred by Nile tilapia (Oreochromis niloticus) [27], and also listed insect parts as well as larvae among feeding materials. Indeed previous studies on feeding habits of Oreochromis niloticus show that a wide range of food items constitute a diet for this species of Cichilidae [27,28].
The potential of Nile tilapia (Oreochromis niloticus) as predators of mosquito larvae was examined with a view to finding alternative to the use of Gambusia affinis which is efficient predator of mosquito larvae, but a threat to native species of fish due to their competitive feeding behaviour. Nile tilapia was also preferred for its economic value besides its ecological friendly nature. Globally the use of fish has been considered an appropriate component of malaria control strategy, especially where mosquito breeding sites are known and limited in number. The use of larvivorous fish has been in practice for many decades. Prior to 1970s the most used predator fish that eat mosquito larvae is Gambusia affinis (Mosquito fish), a fresh water species native to southeastern United States of America. Field use of Gambusia affinis has now been discouraged based on a number of studies showing that it was in fact responsible for disappearance of some native fish where it was introduced [29]. In India [7], China [9] and Ethiopia near Ethiopian-Somalia border [30] indigenous fish have been used to suppress breeding of malaria vectors in man-made water holding structures such as wells, cisterns and barrels. Furthermore, a study conducted in Asia revealed that management of fish for mosquito larvae control has been effective where pisciculture can provide additional financial benefits, as well as benefits associated with nutritional value [31].
While locally available edible fish, Nile tilapia, may not totally eradicate mosquito larvae in the ponds, or any other naturally occurring water bodies, they are much more environment friendly, and their use could be encouraged where feeding on mosquito larvae results in significant larval population reduction. Beside, In spite of several studies showing that biological agents that suppress larval population under laboratory experiments often do not yield much desired results in field conditions, the results of this study showed that fingerlings of edible Oreochromis niloticus have high mosquito larval population reduction potential under field condition. The natural tendency of Oreochromis niloticus to use a wide range of food items makes it suitable for both domestic farming as a source of rural households’ income, as well as mosquito bio-control agent in specific contexts.
In light of our findings and those of the previous studies, rearing both mature and immature fish together in relatively large water bodies, especially when such water pools are suspected to be providing breeding grounds for mosquitoes, could increase the effectiveness of edible fish for mosquito larvae population suppression. For instance, mature tilapia are generally herbivores and would eat plant parts that act as hiding places for larvae, thereby exposing the larvae for fingerlings of fish to pursue mosquito larvae and feed on them. What is more, evidently fast and easy growth of Nile tilapia [32] makes it appropriate for mosquito control initiatives that can mediate poverty reduction among communities targeted by mosquito control initiatives that rely on integrated environmental approach.
CONCLUSION
The drainage of Yala swamp, alongside irrigation agriculture and aquacultural activities in the swamp have created favorable conditions for the breeding and proliferation of mosquito vectors of malaria causing plasmodium. The results of this study revealed that Anopheles gambiae and Anopheles funestus preferred man-made habitats for breeding. They included fish ponds, transient water pools created by cultivation of crops in the wetland and agricultural trenches. Anopheles gambiae were the most abundant in Yala swamp followed by Anopheles funestus, while culicines occurred in very low densities in all habitats. A significantly high abundance of Anopheles gambiae could be attributed to ecologically suitable conditions created by anthropogenic activities, coupled with relatively short time they take to generate in aquatic habitats. Thus, it was appropriate to expect their larger population in transient water pools of Yala swamp compared to those of Anopheles funestus that require pools which are permanent and sunlit for a breeding success. The claim about ecological requirement for Anopheles funestus is evidenced by high abundance of Anopheles funestus observed during the dry season in long standing pools. Although the study findings provide leverage to consider fish for economic gains in environmental management for malaria control, especially in a setting like Yala swamp environment, the use of post-fingerling Oreochromis niloticus formerly known as Tilapia nilotica (Perciformes: Cichlidae) has some limitations. Any future study of biological mosquito control which relies on the use of Nile tilapia (Oreochromis niloticus) should consider concurrent use of both fingerlings and mature fish in the experiments. Similarly, this study showed that the most efficient malaria vector in the eastern African region, Anopheles gambiae complex, was the most dominant mosquito species in breeding habitats of Yala swamp. Therefore, further research should involve clinical survey of the local community, through blood samples screening for malaria parasites, in order to understand vectorial potential of Anopheles funestus and Anopheles gambiae sp. in the villages surrounding Yala swamp.
Ethical consideration
The clearance to conduct this research with fish as mosquito predators was obtained prior to the commencement of the study from Kenya’s Ministry of Education, Science and Technology. A clearance for research numbered MOEST 13/001/36C 37/2 was registered in the name of Nina Pius Mbuya. Other than field experiments with fish no human subjects were used for the purpose of experiment. Nevertheless, the consent of field assistants involved in the management of ponds during experiments was obtained in writing.
ACKNOWLEDGEMENTS
This study would not have accomplished its objectives without the collaboration and involvement of others at different stages of implementation. Thanks to the management team of Ratuoro health center where temporary insectaries were established for breeding larvae to adult mosquito and to Dominion Farms Limited for their cooperation. Mr. Geoffrey Nyachwara of ICIPE provided much needed support during mosquito species identification, while Mr. Gabriel Mattwale of Uganda vector control division took the author through intensive mosquito sampling and handling procedures prior to the study. I am indebted indeed to Dr. Wolfgang Richard Mukabana of School of Biological Sciences University of Nairobi for his guidance in study design and insightful comments that enriched the study. The authors thank Dr. Joseph Kisaakye of Department of Biology, Makerere University whose instructions facilitated the conceptualization of the problem. Finally the author returns his deepest appreciation and gratitude to the Society of Jesus for financing the entire study.
REFERENCES
- Bernués-Bañeres A. and Jiménez-Peydró, R., 2013: Diversity of mosquitoes (Diptera Culicidae) in protected natural parks from Valencian Autonomous Region (Eastern Spain). Biodiversity Journal, 4 (2), 335-342.
- Rueda L. M. 2008: Global diversity of mosquitoes (Insecta, Diptera, Culicidae) in fresh water.
- Taylor C. E., Y. T. Toure, M. Coluzzi, and M. Petrarca, 1993: Effective population size and persistence of Anopheles arabiensis during the dry season in west Africa. Medicine Veterinary Entomology, 7, 351- 357.
- Githeko A K, Service M W, Nbongo C M and Atieli FK. 1996: Resting behaviour, ecology and genetics of malaria vectors in large scale agricultural areas of Western Kenya. Parassitogia, 38, 481-489.
- Lehmann T., W. A. Hawley, H. Grebert, and F. H. Collins, 1998: The effective population size of Anopheles gambiae in Kenya: implications for population structure. Biological evolution, 15: 264-276.
- World Health Organization, 1981: Aquatic macrophytes as Habitat of vectors and Hosts of tropical Diseases, and Biological Control Using Fish (WHO technical report: https://www.fao.org//DOCREP/006/X7580E/ X7580E14.htm).
- Victor T J, Chandrasekaran B and Reuben R, 1994: Composite fish culture for mosquito control in rice fields in southern India. Southeast Asian J. Trop. Med Public Health 25, 522–527.
- Fletcher M, Teklehaimanot A and Yemane G, 1992: Control of mosquito larvae in the port city of Assab by an indigenous larvivorous fish, Aphanius dispar. Acta Trop, 52, 155–166.
- Wu N, G. Liao, D. Li, Y. Luo, and G. Zhong, 1991: The advantages of mosquito biocontrol by stocking edible fish in rice paddies. Southeast Asian J. Trop. Med. Pub. Health 22, 436–442.
- Tripathi A K, Upadhyay S, Bhuiyan M and Bhattacharya P R, 2009: A review on prospects of essential oils as bio pesticide in insect-pest management. Journal of Pharmacognosy and Phytotherapy; 1(5), 52- 63.
- Palanisami S, Natarajan E and Rajamma R, 2014: Development of eco-friendly herbal mosquito repellent. Journal of innovative biology, 1(3), 132-136.
- Segey O, Mangel M and Blaustein L, 2009: Deleterious effects by mosquito fish (Gambusia affinis) on the endangered fire salamander (Salamandra infraimmaculata. Animal conservation 12, 29-37.
- Howard A F V, Zhou G and Omline F X, 2007: Malaria mosquito control using edible fish in western Kenya: preliminary findings of a controlled study. BMC Public Health, 7, 199, 1-6.
- Marten G G, Suarez M F and Astaeza R, 1996: An ecological survey of Anopheles albimanus larval habitats in Colombia. J Vector Ecol., 21, 122–131.
- Scholte E. J., K. Ng'habi, J. Kihonda, W. Takken, K. Paaijmans, S. Abdulla, G. F. Killeen, and G. J. Knol, 2005: An Entomopathogenic Fungus for Control of Adult African Malaria Mosquitoes. Science, 308 (5728), 1641-1642.
- Okumu F. O., R. D. Sumaye, N. S. Matowo, S. P. Mwangungulu, E. W. Kaindoa, I. R. Moshi, E. P. Madumla, and D. W. Lwetoijera, 2013: Outdoor mosquito control using odour-baited devices: development and evaluation of a potential new strategy to complement indoor malaria prevention methods. Malaria world journal, 4(8), 1-9.
- Abila R. 2002: Utilization and Economic Valuation of the Yala Swamp Wetland, Kenya. In: Gawler, M. (Ed.). Best practices in participatory management. Proceedings of a workshop held at the 2nd International Conference on Wetlands and Development, Dakar, Senegal. Wetlands International, Wage ningen: IUCN –WWF Publications No 65, pp. 96–104.
- Nina P M, Kateyo E and Lunyolo F, 2014: Assessment of Anopheles Larval Source Reduction Using Cow Dung: Environmental Perspective on Pro-poor Tool for Malaria Vector Control. International Journal of Innovation and Applied Studies, 5(1), 30-42.
- Crafter S A, Njuguna S G, Howard G W (eds), 1992: Wetlands of Kenya. Proceedings of the KWWG Seminar on Wetlands of Kenya. National Museums of Kenya. Nairobi, Kenya, 3–5 July, 1991. Pp. viii and 183.
- Gillies M T. and De Meillon B. 1968: The Anopheline of Africa South of the Sahara (Ethiopian zoogeographical region). Johannesburg: The South African Institute for Medical Research, pp. 3-58.
- Service M W. 1993: Mosquito ecology: Field sampling methods, 2nd Ed. London and New-York, Elsevier Applied Science, 988pp.
- Yamagata Y. 1996: Final report of Japanese International Cooperation Agency (JICA)- urban malaria control project. Dar es Salaam, Japanese International Cooperation Agency, 46.
- Sunahara T, Shizaka K and Mogi M, 2002: Habitat size a factor determining the opportunity for encounters between mosquito larvae and aquatic predators. J Vector Ecol 27, 8-20.
- Bence J.R. 1988. Indirect effects and biological control of mosquitos by mosquitofish. Journal of Applied Ecolology, 25,505–521.
- Blaustein L. 1992: Larvivorous fishes fail to control mosquitoes in experimental rice plots. Hydrobiologia, 232, 219–232.
- Smith C. 1989: An investigation into the problem of conspecific predation among the fry of the Nile tilapia, Oreochromis niloticus (Linnaeus, 1775) in an intensive culture system. MSc thesis, Plymouth Polytechnic, 134 pp. In: Smith, C and Reay, P. 1991. Cannibalism in teleost fish. Reviews in Fish Biology and Fisheries, 1, 41-64.
- Oslo J A, Ayodele I A and Fagbuaro O, 2006: Fagbuaro Food and Feeding Habits of Oreochromis niloticus (L.) and Sarotherodon galilaeus (L.) in a Tropical Reservoir. World Journal of Zoology 1 (2), 118-121.
- Komolafe O O. and Arawomo, G A., 1998: Reproduction of Oreochromis niloticus (Linneaus) in Opa reservoir, Ile-Ife, Nigeria. Biosci. Res. Commun., 10, 167-174.
- Rupp HR. 1996: Adverse assessments of Gambusia affinis: an alternate view for mosquito control practitioners. J. Am. Mos. Control Assoc., 12, 155–166.
- Teklehaimanot A, Kassahun A and Fletcher M, 1993: Using fish against malaria: a local initiative, World Health Forum 14,176–177.
- Gupta D K, Sharma R C, Sharma V P 1989: Bioenvironmental control of malaria linked with edible fish production in Gujarat. Indian J. Malariology 26, 55–59.
- Cleber C, Figueredo C, Giani A. 2005: Ecological interactions between Nile tilapia (Oreochromis niloticus, L.) and the phytoplanktonic community of the Furnas Reservoir (Brazil). Freshwater Biology, 50, 1391–1403.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences