ISSN : 2393-8862
American Journal of Pharmacology and Pharmacotherapeutics
The Function of Gp130-Mediated Signaling Pathways in the Heart and How They May Affect Prospective Treatment Areas
Hari Prasad Sonwani*, Alka Sen, Seema Kishor Kumar and Ritu Sahu
Department of Pharmacy, Apollo College of Pharmacy, Anjora, Durg, India
- *Corresponding Author:
- Hari Prasad Sonwani
Department of Pharmacy, Apollo College of Pharmacy, Anjora, Durg, India
E-mail:
Received date: December 13, 2023, Manuscript No. IPAPP-23-18204; Editor assigned date: December 15, 2023, PreQC No. IPAPP-23-18204 (PQ); Reviewed date: December 29, 2023, QC No. IPAPP-23-18204; Revised date: January 13, 2024, Manuscript No. IPAPP-23-18204 (R); Published date: January 20, 2025, DOI: 10.36648/2393-8862.12.1.201
Citation: Sonwani HP, Sen A, Kumar SK, Sahu R (2025) The Function of Gp130-Mediated Signaling Pathways in the Heart and How They May Affect Prospective Treatment Areas. Am J Pharmacol Pharmacother Vol:12 No:1
Abstract
Cytokines of the IL-6 type attach themselves to complexes of plasma membrane receptors that contain the common signal transducing receptor chain, or Gp130, which is present in most organs, including the heart. It has been shown that the two main signaling cascades that the Gp130 receptor activates, the SHP2/ERK and STAT pathways, are crucial for the growth, hypertrophy, protection, and remodeling of the heart in response to normal and pathological stimuli. Experimental evidence suggests that Gp130 signaling promotes the proliferation and survival of cardiomyocytes in both in vitro and in vivo settings. On the other hand, it has been documented that in individuals with heart failure or following a myocardial infarction, increased serum levels of Gp130 proteins and IL-6 cytokines are robust predictors of morbidity and death. Furthermore, it has been demonstrated that failing human hearts have changes to the localgp130 receptor system. In The basic concepts of Gp130 signaling are outlined in this article. A balanced biological outcome needs the simultaneous activation of the STAT and ERK pathways, which is tightly regulated by positive and negative intracellular signaling modulators. Additionally, we draw attention to the critical roles that the Gp130 receptor and its main downstream effectors play in the heart's development and regeneration as well as in how it responds to different physiological and pathological stress scenarios. Lastly, we discuss the difficulties and variety among tissues in targeted pharmacological interference with Gp130 receptor system components.
Keywords
Heart; Gp130 receptor; Signalling diversification; Hypertrophy
Introduction
The glycoprotein-130 (gp130) is a major signal transducer of cytokines related to Interleukin-6 (IL-6) and is extensively expressed in mammals, including the developing and adult heart [1,2]. During embryogenesis, in response to inflammation, pressure overload, and ischemic injury, IL-6 type cytokines activate Gp130 in the heart, triggering three main signaling pathways:
•The Janus Kinase (JAK)/Signal Transducer and Activator of Transcription (STAT) pathway.
•The Ras/Mitogen-Activated Protein Kinase (MAPK and extracellular signal-regulated kinase (ERK)) signaling pathway; and
•The Phosphatidylinositol-3-Kinase-Dependent (PI3K)/Akt pathway.
According to reports in the past several years, individuals with congestive heart failure have higher levels of circulating IL-6 and the soluble IL-6 receptor (sIL-6R) and serum concentration of IL-6 increases during myocardial infarction (Figure 1) [3].
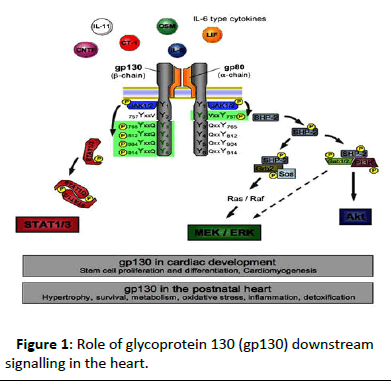
Figure 1: Role of glycoprotein 130 (gp130) downstream signalling in the heart.
Literature Review
Scheme of Gp130 receptor activation showing Interleukin-6 (IL-6) type cytokines binding to their transmembrane receptor α- subunits, which induces homodimerization of the gp130 receptor β-subunits or heterodimerization of gp130 with either one of the structurally and functionally similar Leukaemia Inhibitory Factor (LIF) receptor and Oncostatin M (OSM) receptor β-subunits. Following dimerization of the Gp130 receptor complex, Janus Kinase-1/2 (JAK1/2) constitutively connected to the intracytoplasmatic membrane proximal regions of the receptor subunits are catalytically activated and themselves transphosphorylate tyrosine residues in the gp130 receptor intracellular domain [4,5]. Subsequently, two major intracellular signalling cascades are triggered, the Signal Transducer and Activator of Transcription (STAT)-1/3 pathway and the SH2 domain-containing cytoplasmic protein tyrosine phosphatase (SHP2)/MEK/Extracellular signal-regulated kinase (ERK) pathway. Via the formation of complexes with Growth factor receptor binding protein-2 (Grb2)-Associated Binding protein-1/2 (Gab1/2) and Phosphatidylinositol-3-Kinase (PI3K), SHP2 activation furthermore initiates the Akt pathway and additionally enhances MEK/ERK signalling. Activation of gp130-mediated signalling plays essential roles in stem cells and cardiomyogenesis. In the adult heart, it is involved in numerous physiological and pathophysiological processes. MEK, Mitogen-Activated Protein Kinase (MAPK)/ERK kinase [6-9].
Additionally, in hypertrophy and dilative cardiomyopathy. Additionally, systemic IL-6 levels are a powerful independent predictor of the clinical outcomes that follow heart failure and myocardial infarction in patients, and they correspond with the severity of left ventricular dysfunction [10]. Similarly, the degree of heart failure is correlated with a considerable increase in plasma levels of the cytokine IL-6 type, Leukaemia Inhibitory Factor (LIF), and soluble gp130 receptor. More recently, research conducted in our lab on patients with end-stage heart failure revealed changes to the IL-6/gp130/JAK/STAT signaling cascade at the levels of ligands, receptors, and downstream signaling molecules. For instance, there is a decrease in the expression of IL-6 in the heart while there is an increase in the expression of LIF. The manifestation of Cardiotrophin-1 (CT-1), an additional constituent of There is no change in the IL-6 cytokine family. Failure hearts show increased gp130 activation (tyrosine phosphorylation), while gp130 expression is unchanged at the receptor level. Surprisingly, the activation state (tyrosinephosphorylation) of JAK2 and tyrosine kinase-2, the next downstream signaling molecules of the gp130 receptor, is reduced in failing hearts, but their expression is not affected. Most remarkably, failing hearts have significantly lower levels of both the effector signalling protein, STAT3, and its activation. STAT3 is part of the IL-6/Gp130/JAK/STAT cascade. The expression of the Suppressor of Cytokine Signaling (SOCS) proteins, which act as negative regulators of Gp130/JAK/STAT signaling, in failing human hearts was mostly unknown until recently. STAT transcription factors upregulate the expression of SOCS3, and in patients who are nearing the end of their lives, this expression appears to be diminished Podewski, et al., discuss hearts. Generally speaking, observations made from patient studies are primarily descriptive and do not pinpoint the specific functional roles that cause a certain component or signaling pathway. Regarding this, it is yet unclear how the expression of IL-6 type cytokines and the correspondingdownstream signaling processes affect human heart pathophysiology biologically [11-13]. It has been proposed that a systemically elevated IL-6 type cytokine signaling may play a detrimental function in patients by contributing to the development of heart failure and its thrombotic complications. On the other hand, an increasing amount of experimental research indicates that cytokine signaling related to IL-6 plays a role in compensatory hypertrophy, cardio protection, and neovascularization. Specifically, the examination of transgenic mice with knockouts for certain gp130 receptor system components clarify the precise functions of the Gp130 receptor system for different kinds of cardiovascular cells as well as the heart itself in the present review, we emphasize the critical function of the gp130 receptor and its principal downstream signaling mediators in the heart's development and postnatal physiology. Additionally, we provide an overview of the fundamental principles of Gp130 signaling and attempt to contextualize clinical observations and experimental results in order to clarify any possible differences between the observed and expected beneficial and detrimental roles of gp130 signaling in the pathophysiological conditions of the cardiovascular system [14,15].
The Gp130 receptor system plays a crucial role in early heart development and might therefore be important for cardiac regeneration: One of the first organs to form and begin to operate throughout development is the heart. According to Yoshida, et al., embryos with a homozygous null mutation for gp130 pass away between 12.5 days post coitum and term. They have hypoplastic ventricular myocardium without a septal or trabecular defect 16.5 days post coitum and later. Cardiomyocytes lacking in gp130 appear to have normal subcellular ultra-structures, but their cardiac phenotype suggests a function in the ventricular myocardium's compact layer growth [16]. The gp130 receptor system may be crucial for cardiac development because it regulates cardio myo-genesis, cardiomyocyte survival, and growth. This is supported by the high expression levels of the Gp130 ligands LIF and CT-1 during the course of cardio genesis, which also promote the proliferation and survival of embryonic cardiomyocytes. The characteristics of the postnatal heart as a terminally differentiated heart have changed in recent years [17]. organ that is incapable of myocardial regeneration and endogenous repair due to negligible rates of cardiomyocyte proliferation and quantitatively insignificant replenishment by endogenous progenitor cells. This led to the goal of replacing damaged cardiac tissue with ex vivo expanded and differentiated stem or progenitor cells. The current body of research centers on appropriate cell types and transplantation techniques, the identification of the niche microenvironment for cardiac stem cells, and-most importantly-the pathways and inducers of stem cell proliferation and directed differentiation into the major myocardial cell types, including cardiomyocytes [18]. In this regard, it is important to note that the G0/G1 cell cycle phase shift and the increase of hepatocyte proliferation following hepatectomy have been linked to the overexpression of IL-6 and the activation of STAT3. Mice lacking in the transcription factor NF-IL-6 or IL-6 have a disrupted proliferative response during liver regeneration, which partially leads to liver failure linked to STAT3 deficiency. activation and reduced expression of Myc, cyclin D1 mRNA, and adaptor-related protein complex-1 [19]. It is 2025 Vol.12 No.1:201 SHP2/MEK/Extracellular signal-Regulated Kinase (ERK) pathway. ICAM, intercellular adhesion molecule-1. still unknown, nevertheless, whether understanding the regenerative features of Gp130-mediated signaling in the liver can, at least partially, aid in understanding how other adult organs, like the heart, are modulated when it comes to cell cycle progression and cellular division. Using differential Gp130 signaling as a switch to control the fate of stem cells It is known that in the presence of Lipid Growth Factor (LEF) or on a feeder layer of mouse embryonic fibroblasts, murine Embryonic Stem (ES) cells can be kept in an undifferentiated condition. The gp130 system in murine ES cells is essential for self-renewal and requires LIF-mediated gp130 activation or alternatively induced gp130 dimerization. Of particular significance is the identification of STAT3 downstream signaling as the critical pathway. That retains ES cells in an undifferentiated condition and inhibits their development. Similarly, it has been demonstrated that the proliferation of hematopoietic precursor cells in the fetal mouse aorto-gonado-mesonephros area is mostly caused by Gp130 and the activation of its downstream mediator, STAT3. Nonetheless, it is highly probable that additional functions are played by other paracrine signaling pathways (Gp130-independent) in maintaining "stemness" and determining the fate of stem cells. Human ES cells, like their murine counterparts, contain a functional Gp130 receptor system (Figure 2) [20].
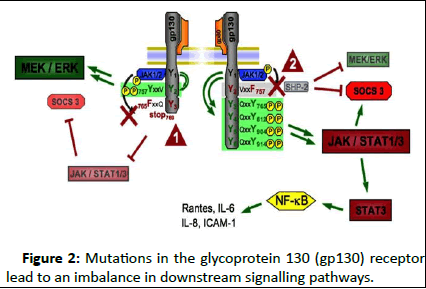
Figure 2: Mutations in the glycoprotein 130 (gp130) receptor lead to an imbalance in downstream signalling pathways.
Detailed schematic view of the Gp130 receptor structure shows tyrosine 757 (Y757) necessary for SH2 domain-containing cytoplasmic protein tyrosine phosphatase (SHP2) phosphorylation and Suppressor of Cytokine Signalling (SOCS)-3 binding. A point mutation (Y757 →F757, tyrosine → phenylalanine) abolishes binding of SHP2 and SOCS3 (arrowhead 1) and leads to uncontrolled Janus Kinase (JAK)/Signal Transducer and Activator of Transcription (STAT) activation with exaggerated STAT3 activation. As a consequence, proinflammatory pathways, that is Nuclear Factor-κB (NF-κB) activation, are promoted. The left side displays a truncated gp130 intracellular domain incapable of binding and activating the STAT1/3 pathway (arrowhead 2) from its membrane-distal phosphotyrosine residues. This mutant form is characterized by the substitution of Y765 by F765 and is truncated at the C-terminal side of amino acid 768. Deficient STAT1/3 signalling in this model is accompanied by enhanced signalling via the SHP2/MEK/Extracellular signal-Regulated Kinase (ERK) pathway. ICAM, intercellular adhesion molecule-1.
Discussion
MEK, Mitogen-Activated Protein Kinase (MAPK)/ERK kinase responds to IL-6-type cytokine stimulation by exhibiting robust activation of its primary signaling pathways, JAK/STAT, MEK (MAPK/ERK kinase)/ERK, and PI3K/AKT. Nevertheless, in human ES cells, activation of the JAK/STAT3 pathway and gp130 dependent signaling results in the loss of pluripotency markers, suggesting that the Human ES cells' ability to remain in an undifferentiated state is independent of STAT3 signaling and may rely on non-gp130 receptor system-related pathways. While the STAT3-mediated conservation of stemness in murine ES cells is stabilized by suppression of the gp130 receptor-triggered MAPK pathway, activation of the ERK1/2 pathway results in the loss of pluripotency and the start of differentiation. For example, adding bone morphogenetic protein-2 and LIF simultaneously can induce the expression of cardiomyocyte markers in murine ES cells as early as 2-4 days in culture, including connexin-43, Mef2c, Tbx5, Nkx2.5, GATA-4, and Myosin Heavy Chain (MHC). STAT3 and ERK1/2 are important factors in this determination of cell fate. Based on these experimental findings, it's probable that the harmony and mutual understanding between MAPK and STAT3 The stimulation of cardiomyocyte development in ES cells is explained by signaling. Gp130 signaling may also play a significant role in adult stem cell sources. Spermatogonial stem cells are thought to be promising candidates to restore the damaged heart because recent research has demonstrated their ability to develop into cardiomyocytes. Throughout the adult male animal's life, spermatogonial stem cells divide constantly in the test is to maintain spermatogenesis. Because there are so few spermatogonial stem cells in vivo, it is required to grow these cells in vitro in order to potentially use them for therapeutic purposes. According to a recent study, adding LIF to newborn testis cell cultures improved the development of germ cell colonies. It's interesting to note that OSM did not have the same effect as ciliary neurotrophic factor. LIFper did not change the stemness of spermatogonial stem cells, as evidenced by the lack of difference in phenotypic or functional characteristics of spermatogonial stem cells that have been maintained in the absence of LIF. According to these findings, LIF/gp130 signaling may be helpful in the development and propagation of spermatogonial stem cell cultures for potential therapeutic applications. It's interesting to note that new research also suggests a function for differentialgp130 receptor downstream signaling in tissue-committed adult cell plasticity. In particular, it was discovered that during tubulo-interstitial kidney fibrosis, OSM induces the epithelial-to-mesenchymal transition of proximal tubular cells into myofibroblasts in a gp130-dependent manner. This process involves the phosphorylation of STAT1 and STAT3, as well as the activation of ERK1, ERK2, and ERK5. Other cytokines may also stimulate the development of myofibroblast differentiation from resident, tissue-specific cells. These are created during inflammatory reactions and that activate STAT3 or the MAPK pathway. Furthermore, the trans differentiation mechanism of tissue committed cells may be crucial in the remodeling of processes that occur in different organs and t issues, including the heart, in response to pathological effects like ischemia. Therefore, understanding how the gp130 receptor system affects embryonic development, cell proliferation, or differentiation decisions made in embryonic or adult stem cells may aid in clarifying the processes that promote stem cell expansion and cell destiny determination. Characterizing the function of gp130-dependent signaling in the microenvironment of organ-specific stem cell niches will also be of great interest. In particular, the makeup of gp130-dependent paracrine factors that control adult stem cell biology and tissue/organ homeostasis in order to maximize regeneration processes.
Conclusion
The Gp130 receptor system may be involved in controlling cardiomyogenesis, cardiomyocyte survival, and growth, all of which are critical processes in heart development. It has been demonstrated in experimental models that Gp130 receptor signaling is critical for both the formation of the heart and cardioprotection against physiological and pathological stress. Furthermore, knowledge of and targeted modification of Gp130 dependent signal transduction pathways is necessary for cell type-specific pre-commitment, which may ultimately enable stem and progenitor cell-based treatments for cardiac regeneration and repair. Notably, balanced circuits of STAT and ERK signaling pathways appear to be necessary for the advantageous effects of gp130 signaling, where negative feedback mechanisms-that is, SOCS proteins-play a significant role. Failure in this signaling coordination causes cardiac cell death, maladaptive hypertrophy, and cardiac dysfunction. It may also account for differences in the protective and beneficial activities of the Gp130 receptor system that have been described in experimental models and the correlation adverse clinical outcomes in patients suffering from heart failure or following a myocardial infarction due to components of the activated Gp130 receptor system. Patients with end-stage heart failure showed diminished SOCS3 expression and decreased STAT3 activation. These findings could indicate a disruption in the physiological activity of the Gp130 signaling pathway. As a result, balanced Gp130 signaling seems to be cardioprotective, but imbalanced Gp130 signaling is more likely to cause heart failure and maladaptation.
References
- Ancey C, Menet E, Corbi P, Fredj S, Garcia M, et al. (2003) Human cardiomyocyte hypertrophy induced in vitro by gp130 stimulation. Cardiovasc Res 59:78-85
[Crossref] [Google Scholar] [PubMed]
- Berishaj M, Gao SP, Ahmed S, Leslie K, Al-Ahmadie H, et al. (2007) Stat3 is tyrosine-phosphorylated through the interleukin-6/glycoprotein 130/Janus kinase pathway in breast cancer. Breast Cancer Res 9:1-8
[Crossref] [Google Scholar] [PubMed]
- Berry MF, Pirolli TJ, Jayasankar V, Morine KJ, Moise MA, et al. (2004) Targeted overexpression of leukemia inhibitory factor to preserve myocardium in a rat model of postinfarction heart failure. J Thorac Cardiovasc Surg 128:866-785
[Crossref] [Google Scholar] [PubMed]
- Birks EJ, Latif N, Owen V, Bowles C, Felkin LE, et al. (2001) Quantitative myocardial cytokine expression and activation of the apoptotic pathway in patients who require left ventricular assist devices. Circulation 104:233
[Crossref] [Google Scholar] [PubMed]
- Burdon T, Smith A, Savatier P (2002) Signalling, cell cycle and pluripotency in embryonic stem cells. Trends in Cell Biol 12:432-438
[Crossref] [Google Scholar] [PubMed]
- Buzas K, Megyeri K, Hogye M, Csanady M, Bogats G, et al. (2004) Comparative study of the roles of cytokines and apoptosis in dilated and hypertrophic cardiomyopathies. Eur Cytokine Netw 15:53-59
[Google Scholar] [PubMed]
- Chin BS, Blann AD, Gibbs CR, Chung NA, Conway DG, et al. (2003) Prognostic value of interleukin-6, plasma viscosity, fibrinogen, von Willebrand factor, tissue factor and vascular endothelial growth factor levels in congestive heart failure. Eur J Clin Invest 33:941-948
[Crossref] [Google Scholar] [PubMed]
- Coles B, Fielding CA, Rose-John S, Scheller J, Jones SA, et al. (2007) Classic interleukin-6 receptor signaling and interleukin-6 trans-signaling differentially control angiotensin II-dependent hypertension, cardiac signal transducer and activator of transcription-3 activation, and vascular hypertrophy in vivo. Am J Pathol 171:315-325
[Crossref] [Google Scholar] [PubMed]
- Daheron L, Opitz SL, Zaehres H, Lensch WM, Andrews PW, et al. (2004) LIF/STAT3 signaling fails to maintain self-renewal of human embryonic stem cells. Stem Cell 22:770-778
[Crossref] [Google Scholar] [PubMed]
- Dani C, Chambers I, Johnstone S, Robertson M, Ebrahimi B, et al. (1998) Paracrine induction of stem cell renewal by LIF-deficient cells: A new ES cell regulatory pathway. Dev Biol 203:149-162
[Crossref] [Google Scholar] [PubMed]
- Eghbali M, Deva R, Alioua A, Minosyan TY, Ruan H, et al. (2005) Molecular and functional signature of heart hypertrophy during pregnancy. Circu Res 96:1208-1216
[Crossref] [Google Scholar] [PubMed]
- Eiken HG, Damas JK, Yndestad A, Bjerkeli V, Aass H, et al. (2001) Myocardial gene expression of leukaemia inhibitory factor, interleukin-6 and glycoprotein 130 in end-stage human heart failure. Eur J Clin Invest 31:389-397
[Crossref] [Google Scholar] [PubMed]
- El-Adawi H, Deng L, Tramontano A, Smith S, Mascareno E, et al. (2003) The functional role of the JAK–STAT pathway in post-infarction remodeling. Cardiovasc Res 57:129-138
[Crossref] [Google Scholar] [PubMed]
- Ernst M, Jenkins BJ (2004) Acquiring signalling specificity from the cytokine receptor gp130. Trends Genet 20:23-32
[Crossref] [Google Scholar] [PubMed]
- Fischer P, Hilfiker-Kleiner D (2007) Survival pathways in hypertrophy and heart failure: The gp130-STAT3 axis. Basic Res Cardiol 102:393-411
[Crossref] [Google Scholar] [PubMed]
- Florholmen G, Aas V, Rustan AC, Lunde PK, Straumann N, et al. (2004) Leukemia inhibitory factor reduces contractile function and induces alterations in energy metabolism in isolated cardiomyocytes. J Mol Cell Cardiol 37:1183-1193
[Crossref] [Google Scholar] [PubMed]
- Florholmen G, Andersson KB, Yndestad A, Austbo B, Henriksen UL, et al. (2004) Leukaemia inhibitory factor alters expression of genes involved in rat cardiomyocyte energy metabolism. Acta Physiol Scand 180:133-142
[Crossref] [Google Scholar] [PubMed]
- Freed DH, Cunnington RH, Dangerfield AL, Sutton JS, Dixon IM (2005) Emerging evidence for the role of cardiotrophin-1 in cardiac repair in the infarcted heart. Cardiovasc Res 65:782-792
[Crossref] [Google Scholar] [PubMed]
- Fuchs M, Hilfiker A, Kaminski K, Hilfiker-Kleiner D, Guener Z, et al. (2003) Role of interleukin-6 for left ventricular remodeling and survival after experimental myocardial infarction. FASEB J 17:1-20
[Crossref] [Google Scholar] [PubMed]
- Sansone P, Storci G, Giovannini C, Pandolfi S, Pianetti S, et al. (2007) p66Shc/Notch-3 interplay controls self-renewal and hypoxia survival in human stem/progenitor cells of the mammary gland expanded in vitro as mammospheres. Stem cell 25:807-815
[Crossref] [Google Scholar] [PubMed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences