ISSN : 0976 - 8688
Der Pharmacia Sinica
The Adherence to Treatment in Chronic Myeloid Leukemia with Imatinib: The Role of Prescribed Daily Dose
Fiorenzo S*
Hospital Pharmacist, Hospital Pharmacy, General Hospital of Pescara, Pescara, Italy
Abstract
Purpose: Adherence to treatment plays a key role in patients with chronic myeloid leukaemia (CML), among whom adherence rates range from 57% to 95% depending on the method of analysis. The aim of this study was to calculate the one- and two-year adherence of CML patients to imatinib treatment in real-world clinical practice using two different methods of analysis. Methods:The two methods of analysis compared were the ratio between the received daily dose (RDD) and the prescribed daily dose (PDD), and the medication possession ratio (MPR). Results:The medication adherence, in the first year, was 0.90 ± 0.15 when calculated using RDD/PDD, and 0.83 ± 0.20 when calculated using the MPR; the corresponding values in the second year were respectively 0.90 ± 0.10 and 0.82 ± 0.16. The differences in the values calculated using the two methods was therefore considerable, being 7% in the first year and 8% in the second Conclusion:In the case of diseases for which the prescribed dose may vary from one patient to another or during the course of treating individual patients, it is not suitable to use methods based on the DDD as a surrogate for the PDD.
Keywords
Leukemia, Imatinib, Received Daily Dose (RDD)
Introduction
Adherence to treatment is an increasingly debated subject and an essential means of increasing the likelihood of achieving the desired clinical outcome. It is particularly important in ensuring the optimal management of chronic treatments [1] because non-adherence reduces their therapeutic efficacy, increases morbidity and mortality, and leads to higher National Health Service costs [2].
Published data indicate that non-adherence rates range from 33 to 70%, above all in the case of patients with chronic diseases in whom the duration of treatment is an unfavourable factor [3-5]. Adherence to treatment plays a key role in patients with Chronic Myeloid Leukaemia (CML) [6-8], among whom adherence rates range from 57 to 95% [9-13] depending on the method of analysis. Measuring adherence accurately is important for researchers and clinicians, and may directly affect the patients themselves because inaccuracies may erroneously lead to a judgement of treatment failure or inadequacy, and consequently unnecessary or even harmful treatment intensification. Furthermore, clinical trial findings should also take adherence data into account in order to ensure a more complete picture of treatment efficacy [14].
The importance of treatment adherence means that every effort should be made to establish the most suitable and disease-specific method of measuring it [15]. There are currently a number of methods awaiting validation [16,17] and, starting from the assumption that there is no reference gold standard [18], the aim of this study was to calculate the one- and two-year adherence of CML patients to imatinib treatment in real-world clinical practice using two different methods of analysis.
Materials and Methods
This retrospective observational study was carried out at the pharmacy of Pescara Hospital in collaboration with the Hematology Department, and considered all of the CML patients attending the hospital who had received firstline treatment with imatinib for one or two years ± 60 days (a tolerance due to the fact that the patients preferred to collect the drug from the hospital pharmacy at the time of their hospital appointments). The data were taken from the pharmadd.it database, which contains all the information concerning the patients’ sociodemographic characteristics, disease, gender, age, the name and dose of the drug used, the amounts of drug dispensed, and the dates of dispensation. As the hospital pharmacy was the only place dispensing the study drug, it was not difficult to monitor the patients’ therapeutic behaviour and calculate their adherence to treatment.
The two methods of analysis compared were the ratio between the Received Daily Dose (RDD) and the Prescribed Daily Dose (PDD) [19], and the medication possession ratio (MPR) [20], which is calculated on the basis of the number of doses dispensed over a given period of time [21-23].
Prescribed Daily Dose (PDD)
The PDD is an expression of prescriptive intent that can be obtained from ad hoc studies of databases of drug prescriptions or patient interviews [24], and may vary depending on a patient’s diagnosis, race, clinical condition, and the tolerability of the drug. In this sense, it can be considered a dynamic parameter of drug use, and is particularly important in cases in which it differs from the Defined Daily Dose (DDD) or, as in the case of imatinib, when the PDD varies so much that there is no established DDD. In this study, the PDD was extrapolated from the specialist’s prescription, and updated every time the prescription was renewed. Using the PDD, when calculating adherence minimises the errors arising from the use of a DDD that does not coincide with the PDD [25].
Received Daily Dose (RDD)
The RDD is the ratio between the dispensed dose (and therefore probably the dose actually taken by the patient) and the number of days between successive dispensations. It was calculated for each patient and the weighted average of the time intervals between each dispensation, thus obtaining the weighted average RDD given by the following formula:
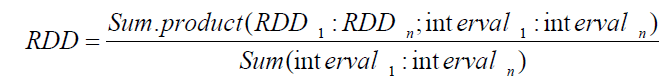
The PDD was calculated in the same way, and the ratio between the RDD and PDD was used to describe treatment adherence.
Results
Only the patients who had received imatinib for one or two years (365 or 730 ± 60 days) were considered. Sixty-two patients had been treated for one year (a median of 363 [305-479] days), and 50 for two years (a median of 365 [307- 413] days in the second year). The median age in both years was 65 (range 21-92), and there were more men than women (33 vs. 29 in the first year, and 28 vs. 22 in the second) (Table 1). The weighted prescribed daily dose (WPDD) in the first and second year was respectively 0.37 ± 0.07 g/day and 0.36 ± 0.06 g/day.
| First year | Second year | |
|---|---|---|
| No. of patients | 62 | 50 |
| Males | 33 | 28 |
| Females | 29 | 22 |
| Median age (years) | 65 (21-92) | 65 (21-92) |
| No. of days treated | 363 (305-479) | 365 (307-413) |
| WRDD (g/day) | 0.34 ± 0.09 | 0.33 ± 0.07 |
| WPDD (g/day) | 0.37 ± 0.07 | 0.36 ± 0.06 |
| Weighted adherence | 0.90 ± 0.15 | 0.90 ± 0.10 |
| MPR | 0.83 ± 0.20 | 0.82 ± 0.16 |
Table 1: Patient characteristics and adherence calculated as RDD/PDD and MPR
As shown in Table 2, the full imatinib dose of 0.4 g/day was taken by only 63% of the patients in the first year and 64% in the second, and respectively 16.13% and 12% changed dose during the course of treatment; all of the others took doses of 0.3, 0.2 or 0.1 g/day. These differences in PDD inevitably conditioned adherence which, in the first year, was 0.90 ± 0.15 when calculated using RDD/PDD, and 0.83 ± 0.20 when calculated using the MPR; the corresponding values in the second year were respectively 0.90 ± 0.10 and 0.82 ± 0.16 (Table 1). The differences in the values calculated using the two methods was therefore considerable, being 7% in the first year and 8% in the second.
| First year (No. of patients; %) |
Second year (No. of patients; %) |
|
|---|---|---|
| PDD=0.4 g | 39; 62.9 | 32; 64 |
| PDD=0.3 g | 10; 16.13 | 9; 18 |
| PDD=0.2 g | 1; 1.61 | 2; 4 |
| PDD=0.1 g | 1; 1.61 | 1; 2 |
| 0.11<PDD<0.39 | 10; 16.13 | 6; 12 |
| PDD=0.6 g | 1; 1.61 | - |
Table 2: PDD divided by dispensing intervals
Dsicussion
An analysis of the results revealed a substantial difference depending on the method of calculation: the use of the MPR underestimated adherence (0.83 and 0.82 vs. the 0.90 given by RDD/PDD) because it does not consider possible variations in doses between one patient and another. The WPDD of respectively 0.37 and 0.36 g/day in the two years bears witness to the fact that clinicians do not always use the full imatinib doses of 0.4 g/day, but doses ranging from 0.1 to 0.6 g/day. This has nothing to with adherence, but is exclusively due to individual drug tolerability and the question of better disease management [26]. As the MPR is based on the DDD, it is not surprising that it leads to discordance in the assessment of treatment adherence.
Conclusion
As none of the published methods of analysing treatment adherence is free of limitations, in order to make the assessment more reliable, it is always necessary to specify which method is being used and what data are being considered. In the case of diseases for which the prescribed dose may vary from one patient to another or during the course of treating individual patients, it is not suitable to use methods based on the DDD as a surrogate for the PDD.
References
- Sabaté, E.,World Health Organization, 2003. 35(3): p. 207.
- Brown, MT., Bussell, JK.,Mayo Clinic Proceedings, 2011.86(4): p. 304-314.
- Lavsa, SM., Holzworth, A.,Ansani, NT.,Journal of the American Pharmacists Association,2011.51(1): p. 90-94.
- Svarstad, BL., et al.,Patient Education & Counseling,1999. 37(2): p. 113-124.
- Osterberg, L., Blaschke, T.,New England Journal of Medicine2005. 353(1): p.487-497.
- Marin, D., et al.,Journal of Clinical Oncology2010. 28(14): p. 2381-2388.
- Ibrahim, AR., et al.,Blood2011. 117(14): p. 3733-3736.
- Jabbour, E., et al.,Clinical Lymphoma Myeloma and Leukemia,2012.12(4): p. 223-229.
- Dos, SR., et al.,Revista Brasileira de Hematologia e Hemoterapia2013. 35(3): p. 174-179.
- Darkow, T., et al.,Pharmacoeconomics2007. 25(1): p. 481-496.
- Wu, EQ., et al.,Current Medical Researchand Opinion2010. 26(1): p. 61–69.
- Tsang, J., Rudychev, I., Pescatore, SL.,Journal of Clinical Oncology2006. 24(suppl): p. 6119.
- Bollu, VK., et al.,Blood.2009. 114(22): p. 2209.
- Hurley, F., Cramer, J.,Spilker, B.,Patient Compliance in Medical Practice and Clinical Trials1991. 3(5): p. 243-250.
- Vitolins, MZ., et al.,Controlled Clinical Trials2000.21(5): p. 188S-194S.
- Vermeire, E.,et al.,Journal of Clinical Pharmacy and Therapeutics2001.26(5): p. 331-342.
- Steiner, JF.,Earnest, MA.,Annals of Internal Medicine2000. 132(11): p. 926-930.
- Farmer, KC.,Clinical Thereputics,1999. 21(6): p. 1074-1090.
- Santoleri, F., et al.,PLoS One2013. 8(2): p. e56813.
- Andrade, SE.,Pharmacoepidemiology and Drug Safety2006. 15(8): p. 565-574.
- Steiner, JF., et al.,Medical Care1998. 26(8): p. 814-823.
- Steiner, JF., Prochazka, AV.,Journal of Clinical Epidemiology1997. 50(1): p. 105-116.
- Cramer, JA.,Value Health2008. 11(1): p. 44-47.
- https://www.whocc.no/filearchive/publications/2017_guidelines_web.pdf
- Sinnott, SJ., et al.,Journal of Clinical Epidemiology2016. 69(1): p. 107-119.
- Lavallade, H., et al.,Journal of Clinical Oncology2008. 26(1): p. 3358-3363.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences
