ISSN : 0976-8505
Der Chemica Sinica
Synthesis and Application of 3-Hydroxy-2-[3-(4-Methoxyphenyl)-1-Phenyl-4-Pyrazolyl]-4-Oxo-4h-1-Benzopyran for Extractive Spectrophotometric Determination of Vanadium (V)
Rajesh Agnihotri1*, Nivedita Agnihotri2, Vikas Kumar3 and Raj Kamal4
1University Institute of Engineering and Technology, Kurukshetra University, Kurukshetra, Haryana, India
2Department of Chemistry, Mahrishi Markandeshwar University, Mullana(Ambala), Haryana, India
3Department of Biotechnology, Mahrishi Markandeshwa University, Mullana(Ambala), Haryana, India
4Department of Chemistry, Kurukshetra University, Kurukshetra, India
Abstract
3-Hydroxy-2-[3-(4-methoxyphenyl)-1-phenyl-4-pyrazolyl]-4-oxo-4H-1-benzopyran (HPMEPB) has been found to form a green 1:3 (M:L) complex with vanadium(V) in weakly acidic medium at 0.02-0.12 M CH3COOH. The complex was extracted into carbon tetrachloride and showed absorption maximum at 408-417 nm, molar absorptivity, Sandell’s sensitivity and detection limit of the method were calculated to be 2.70 × 104 l mol-1 cm-1, 0.0018 μg V cm-2 and 7.913 × 10-5 gl-1, respectively at 415 nm. Linear regression equation was Y=0.527 X+0.004, with correlation coefficient, r=0.9951. Beer’s law was obeyed over the vanadium concentration range of 0-2.2 μg ml-1. Effect of large number of cations, anions and complexing agents was studied. Out of these, ascorbic acid, oxalate and hydrogen peroxide interfered seriously. The present method is simple, rapid, sensitive and has been satisfactorily applied to the determination of vanadium in various synthetic and technical samples.
Keywords
Vanadium determination, Spectrophotometry, Benzopyran derivative
Introduction
Vanadium affects favourably the diuretic kidney function, the cardiac muscle and cell growth. Antidiabetic function of vanadium is a subject of intense studies [1]. It has been established that drink water containing 25-75 ppb vanadium normalises the sugar level of rats suffering from diabetes [2]. Traces of vanadium in drinking water are suggested to affect favourably the human health, while higher level of vanadium content causes toxification. There are cases of vanadium poisoning, the symptoms of which are nervous depression, coughing, vomiting, diarrhoea, anaemia and increasing risk of lung cancer; such afflictions are sometimes fatal [3]. Vanadium has also been reported as the index element in urban environmental pollution, especially air pollution [4]. Vanadium in environmental samples has been determined by NAA [5], ICP-AES [6] and AAS [7]. The first two methods are disadvantage in terms of cost and instruments used in routine analysis. AAS is often lacking of sensitivity and is affected by matrix conditions of samples such as salinity. A number of catalytic methods having high sensitivity were reported [8-11]. Catalytic solvent extraction methods are highly sensitive but generally lacking of simplicity.
Several organic reagents have also been used for the spectrophotometric determination of vanadium. Of these, diphenylbenzidine [12] requires the preliminary separation of other elements associated with vanadium. Similar prior removal of Cu, Fe, Ti, Cr, Ce, MO, W, Pd, Zr, Bi and rare earths is needed for the determination of vanadium as its cupferron [13] complex in chloroform solution. 8-Hydroxyquinoline [14] has the required photometric sensitivity, but Fe, Al, Cr, MO, Ti, and W interferes. Later investigations have shown that derivatives of hydroxamic acid, such as salicylhydroxamic acid [15], nicotin [16] and isonicotino [17] hydroxamic acid and benzohydroxamic acid [18], are useful for the determination of vanadium either in aqueous or non-aqueous solutions, but these reagents suffer from the same defects as the earlier ones, i.e. they need the removal of numerous interfering elements including Fe, U, Ti, Al, Th, Sn, MO, Zr and W. Various hydroxylamine derivatives, such as N-benzoyl- N-phenyl- [19,20], N-cinnamoyl- N-phenyl- [21], N-2-thiophene carbonyl-N-p-tolyl- and N-2-thiophene carbonyl-N-phenyl- [22] hydroxylamines are valuable as calorimetric reagents for the determination of small amounts of vanadium and some of these are very sensitive, but still are of no use in presence of Zr, Ti, Mo, W etc.
Various chromone derivatives [23-26] have also been used for spectrophotometric determination of vanadium in weakly acidic media but most of these methods are not suitable for routine analysis of the metal ion due to their lack of selectivity and sensitivity [23]. In the present investigation, it has been observed that a new reagent 3-hydroxy-2- [3-(4-methoxyphenyl)-1-phenyl-4-pyrazolyl]-4-oxo-4H-1-benzopyran (HPMEPB), which possess similar chelating ability but differs in sensitivity and selectivity with the above mentioned reagents, forms a green colored complex with vanadium(V) in acetic acid medium which is extractable into carbon tetrachloride. This forms the basis of the present method that can be successfully applied for the determination of the metal ion in various synthetic and standard samples including high-speed steel and reverberatory flue dust.
Materials and Methods
Apparatus
UV-visible (Shimadzu-140-02) spectrophotometer with 10 mm matched quartz cells was used for absorbance measurements and spectral studies.
Reagents and solutions
A stock solution of vanadium (V) containing 1 mg ml-1 of metal ion was prepared by dissolving 0.2218 g of sodium metavanadate (A.R.) in deionized water to give 100 ml solution and standardized by ferrous sulphate method [27] volumetrically. Aliquots were suitably diluted to give 100 and 10 μg ml-1 solutions. Solutions of interfering ions were prepared by dissolving their A.R. salts in water or dilute acid to give ≤10 mg ml-1 concentrations. Acetic acid (2 mol l-1) was prepared by suitable dilution of glacial acetic acid (17.4 mol l-1).
3-Hydroxy-2-[3-(4-methoxyphenyl)-1-phenyl-4-pyrazolyl]-4-oxo-4H-1-benzopyran (HPMEPB) was synthesized by the literature method [28] and dissolved in acetone to give 0.1% (w/v) solution. Chemical composition of (HPMEPB) is C25H18N2O4 and structure shown in two as well as three dimensions by the use of computational technique is as:
The spectral analysis of the compound is given as:
IR (νmax, in KBr): 1611 cm-1 (CO stretch); 3298 cm-1 (OH stretch).
1H NMR (CDCl3, 300 MHz, δ): 3.93 (s, 3H, OCH3); 7.03 (d, 2H, J=8.7 Hz); 7.36-7.40 (m, 2H, ArH); 7.50-7.57 (m, 4H, ArH); 7.67 (d, 2H, J=8.7 Hz); 7.87 (d, 2H, J=7.8 Hz); 8.25 (dd, 1H, J=1.5, 8.1 Hz); 8.85 (s, 1H, C5-pyrazole proton).
Rf Value: 0.43
Yield: 52%, Melting Point: 202°C to 204°C
Carbon tetrachloride (RANBAXY) was used as such.
Synthetic Samples
Synthetic samples (some of them analogous to palau, crocar and permendur) were prepared by mixing solutions of vanadium(V) and other ions in suitable proportions (Table 1).
| Composition of sample (mg) | V(V), μg | |
|---|---|---|
| Added | Found* | |
| Co(0.5), Mn(0.5), Ni(0.1) | 5.0 | 5.2 ± 0.12 |
| Mn(0.1), Sr(1), Zn(0.5) | 10 | 10.3 ± 0.09 |
| Cu(0.01), Sn(0.01), Ag(0.001) | 7 | 7.1 ± 0.008 |
| Pt(0.01), Au(0.01), Mg(5) | 10 | 9.8 ± 0.018 |
| Se(0.01), Os(0.01), Pt(0.01) | 10 | 10.2 ± 0.04 |
| Ni(0.06), Pt(0.02), Pd(0.01)a | 10 | 10.1 ± 0.06 |
| Fe(0.7), CrVI(0.01), Co(0.018)a,b | 7.5 | 7.34 ± 0.06 |
| Co(0.3), Fe(0.2), Mn(0.02)a,b | 5 | 5.10 ± 0.05 |
| Reverberatory flue dust (100)c | 10 | 10.02 ± 0.09 |
| 5 | 4.98 ± 0.11 | |
| High speed steel super rapid extra 500c | 1 %d | 0.986 ± 0.036 |
| *Average of triplicate analyses (Mean ± % RSD). aComposition analogous to palau, crocar and permendur, respectively. bIn presence of 50 mg sodium thiocynate. cIn the presence of 0.5 mg sodium fluoride. dCertified value. |
||
Table 1: Analysis of vanadium(V) in synthetic and real samples by the proposed method
Reverberatory flue dust
Reverberatory flue dust sample (100 mg) from copper manufacture unit containing no vanadium was mixed with a solution of known vanadium content (1 mg) in a silica crucible and dried in an oven at 110°C to 120°C. It was then fused with sodium peroxide (0.8 g). The fused mass was dissolved in 25-30 ml hot water and neutralized with concentrated H2SO4. Iron was precipitated as its hydroxide and separated by filtration. The final volume was made to 100 ml and aliquots (1 and 0.5 ml) were analyzed for vanadium(V) by the proposed method in the presence of 0.5 mg sodium fluoride.
High speed steel super rapid extra 500
Steel sample (0.1 g) was dissolved by boiling with minimum amount of aqua regia [27]. Iron was precipitated as Fe(OH)3 by adding 1-2 g of ammonium chloride followed by aqueous ammonia. The precipitates were filtered off and washed with 1% ammonia solution. The filtrate along with washings was heated to remove excess of ammonia, cooled and adjusted to 100 ml final volume. Aliquot (1 ml) was taken for the determination of vanadium(V) by the proposed method in the presence of 0.5 mg sodium fluoride.
Procedure for extraction and determination
To a sample solution containing ≤ 22 μg V and/or other metal ions taken in a 100 mL separartory funnel, were added 0.5 ml of 2 M CH3COOH, 1.5 ml of 0.1% (w/v) HPMEPB in acetone and enough deionized water to raise the aqueous volume to 10 ml. It was then equilibrated with 10 ml of carbon tetrachloride, once for 10 sec and the layers were allowed to separate. The green coloured organic phase was passed through Whatmann filter paper No. 41 pretreated with carbon tetrachloride to remove water droplets, if any. The absorbance of the extract then was measured at 415 nm against a similarly treated reagent blank. The amount of vanadium was determined from the calibration curve obtained as per the proposed method. Modifications for samples containing Th(IV), Ti(IV), Mo(VI), Zr(IV), Sn(II) and W(VI). For each of 0.3 mg Th(IV), 0.1 mg Mo(VI), 0.04 mg Sn(II) and 0.08 mg W(VI), 5 mg of sodium fluoride and for each of 0.1 mg Zr(IV) and 0.3 mg of Ti(IV), 5 mg of sodium phosphate were added as masking agent prior to the addition of reagent in 10 mL aqueous volume.
Results and Discussion
Absorption spectra
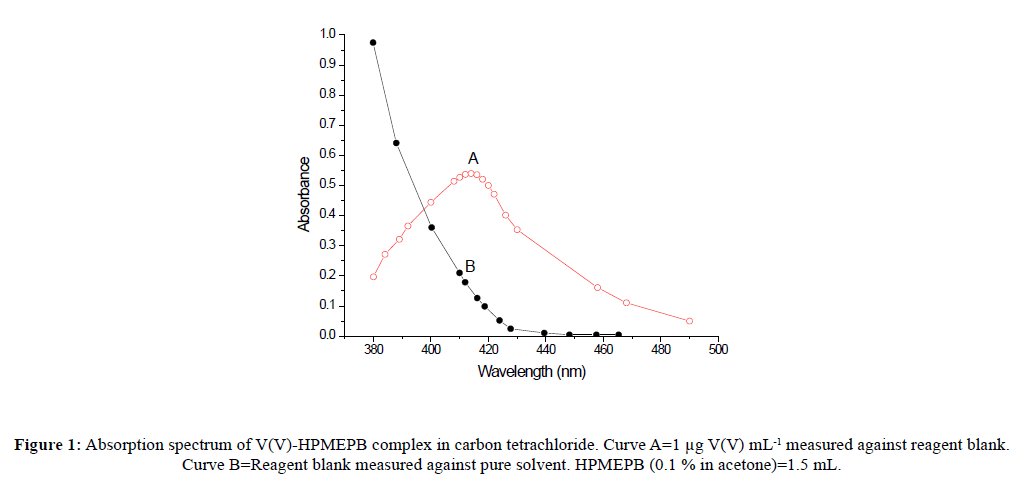
Vanadium(V) formed a green colored complex with HPMEPB in acetic acid medium, which was quantitatively extractable into carbon tetrachloride. It showed absorption maximum in the range 408-417 nm, where the reagent blank had also some absorbance (Figure 1).
Standardization of experimental conditions
The extraction showed a decreased trend in CH3COOH, H3PO4, HCl, H2SO4, HClO4 media. The decrease in extraction in the presence of strong acids may be due to the formation of oxonium salts of the reagent whereas in H3PO4 it may be due to the masking effect of excess phosphate ion concentration over the tolerance level. Since CH3COOH gave maximum absorbance therefore, it was selected for further studies (Table 2).
| Acida | CH3COOH | H3PO4 | HCl | H2SO4 | HClO4 |
|---|---|---|---|---|---|
| Absorbance | 0.530 | 0.330 | 0.240 | 0.180 | 0.050 |
a0.2 M acidity of the aqueous phase, other conditions same as given in the procedure.
Table 2: Effect of nature of acids on absorbance of V(V)-HPMEPB complex
Vanadium(V) complex had been extracted into a large number of water immiscible organic solvents. The extraction of the complex decreased in the order: benzene>toluene=carbon tetrachloride>dichloromethane1,2-dichloroethane >chloroform>isoamyl alcohol>isoamyl acetate>isobutyl methyl ketone>ethyl acetate>cyclohexane. Benzene, being carcinogenic and toluene in which the stability of the complex was only for 15 min, were not preferred as solvents for further studies. As carbon tetrachloride gave stable absorbance for more than 3 hrs with a rapid and clear phase separation, it was selected as the most suitable extractant. The colored complex of vanadium(V) with HPMEPB formed under optimum conditions was stable for more than 3h in carbon tetrachloride at 415 nm. A maximum and constant absorbance was observed in 0.02-0.12 mol l-1 acetic acid in presence of 1.3 ml-2.4 ml of 0.1% (w/v) acetonic solution of HPMEPB at room temperature when the aqueous phase was shaken with 10 cm-3 of carbon tetrachloride for 7-12 s (Table 3).
| CH3COOHa/M | 0.00 | 0.01 | 0.02-0.12 | 0.14 | 0.16 | 0.20 | 0.24 | ||
| Absorbance | 0.140 | 0.460 | 0.530 | 0.480 | 0.460 | 0.405 | 0.330 | ||
| HPMEPBb/mL | 0.1 | 0.3 | 0.5 | 0.9 | 1.2 | 1.3-2.4 | 2.5 | 2.7 | 3.0 |
| Absorbance | 0.010 | 0.130 | 0.330 | 0.410 | 0.460 | 0.530 | 0.490 | 0.460 | 0.380 |
| Equilibration timec/sec | 0 | 5 | 6 | 7-12 | 15 | 20 | 60 | 90 | |
| Absorbance | 0.015 | 0.440 | 0.490 | 0.530 | 0.480 | 0.450 | 0.400 | 0.370 |
aVanadium(V)=10 mg; CH3COOH=variable; 0.1 % HPMEPB=1.5 ml; equilibration time=10 sec; solvent=carbon tetrachloride; aqueous phase=organic phase=10 ml; number of extraction=one; λmax=415 nm.
bCH3COOH=0.1 M; other conditions as in (a) except for the variation in HPMEPB concentration.
c0.1 % HPMEPB in acetone=1.5 ml, other conditions same as in (b) except for the variation in equilibration time.
Table 3: Effect of various parameters on the absorbance of V(V)-HPMEPB complex
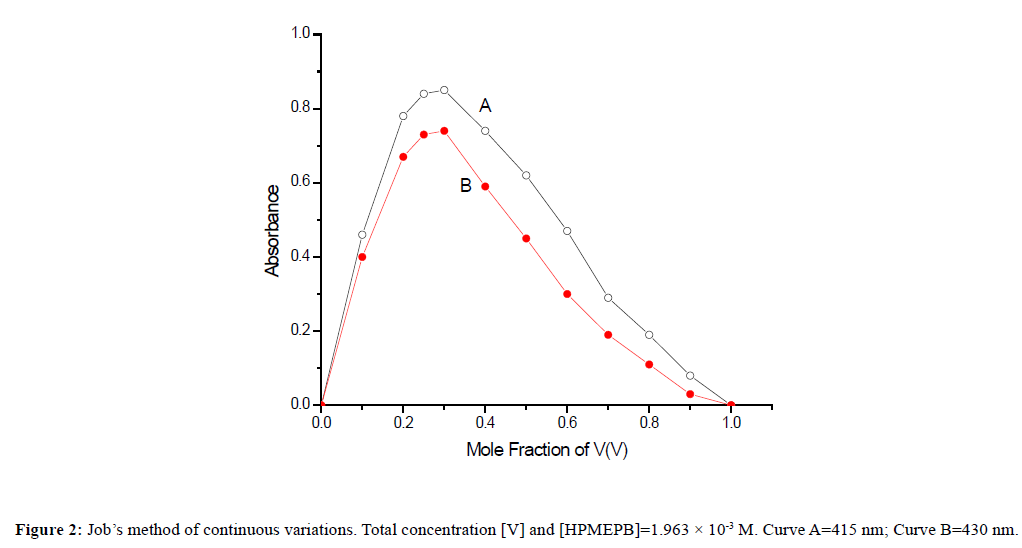
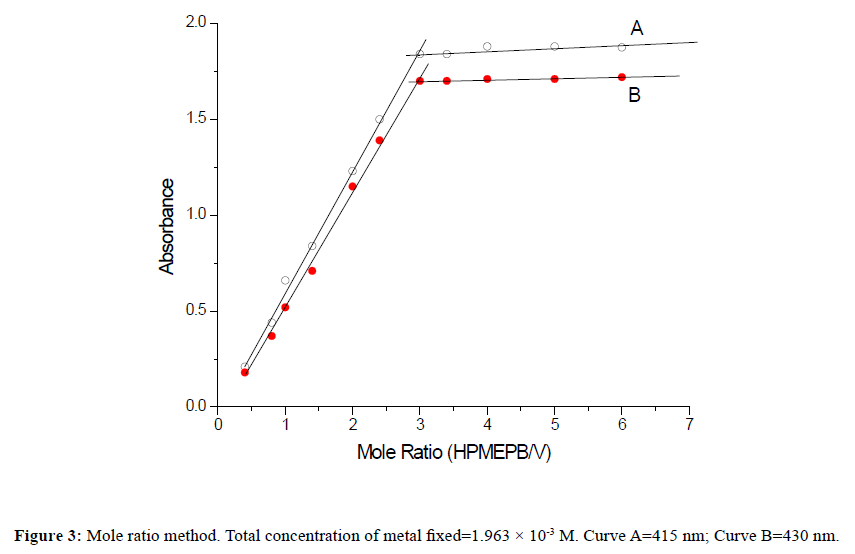
Composition of the complexThe ratio of vanadium(V) and HPMEPB in the extracted species was determined as 1:3 by Job's method of continuous variations [29] as modified by Vosburgh and Cooper [30,31] for a two phase system (Figure 2), and also by the mole ratio method [32] (Figure 3). On the basis of above studies, the probable structure of V(V)-HPMEPB complex in the organic extract may be assigned as shown below.
Analytical features
The metal complex obeyed Beer's law in the range 0-2.2 μg VV ml-1 and after that concentration it deviated from linearity. The Ringbom plot [31] between log ppm of vanadium concentration and percentage transmittance showed that the optimum concentration of the metal ion, which could be measured most accurately, lied in the range 0.29-1.91 ppm V(V). The molar absorptivity and Sandell's sensitivity were: 2.72 × 104 l-1 mol-1 cm-1 and 0.0018 μg V(V) cm-2, respectively at 415 nm. The linear regression equation was, Y=0.527X+0.004 (Y=absorbance, X=microgram V(V) ml-1) and the correlation coefficient, r=0.9951. The detection limit of the method is 7.92 × 10-5 gl-1. The colour was stable in CCl4 for more than 3 hours and no change in absorbance was observed during this period. The reproducibility of the method has been tested by performing ten sets of experiments keeping the same (1 μg V ml-1) amount of the metal ion each time with the relative standard deviation of ± 0.52% absorbance units.
Effect of diverse ions
In order to assess the analytical potential of the proposed method, effects of some diverse ions, which often accompany vanadium, were examined by carrying out the determination of 1 μg ml-1 of vanadium(V) in the presence of a number of other ions. Chloride, bromide, iodide, phosphate, thiocynate, nitrate, sulphate and thiourea (100 mg each each); sulfosalicylic acid (30 mg); fluoride and tartrate (5 mg each); citrate and hydrazine sulphate (1 mg each); EDTA (0.1 mg) and glycerol (1.5 ml) caused<1% error. However, ascorbic acid, oxalate and hydrogen peroxide interfered seriously. Among the cations Co(II), Mn(II), Mg(II), Ba(II), Ca(II), Al(III), Pb(II), Cd(II), Se(IV), Ag(I) and Sr(II) (10 mg each); Ni(II) and Zn(II) (9 mg each); Hg(II) and Ce(IV) (8 mg each); As(III) (7 mg); Be(II) (5 mg); Cr(III) and Sb(III) (4 mg each); Au(III), Pb(II) and Os(VIII) (1 mg each); Rh(III), Cr(VI), Cu(II) and Fe(II) (0.8 mg each); Pt(IV), U(VI) and Fe(III) (0.5 mg each); Bi(III) and Re(VII) (0.1 mg each); Ta(V) (0.08 mg) and Ir(III) (0.06 mg) has no effect on the absorbance of the complex. Th(IV), Ti(IV) (0.3 mg each) Mo(VI) (0.1 mg) W(VI) (0.08 mg), Sn(II) (0.04 mg) and Zr(IV) (0.1 mg) did not interfere in the presence of respective masking agents as given in procedure.
Applications
The proposed method is making use of a very simple, cheap and easy to handle UV-VIS spectrophotometric determination technique. The applicability of the developed method straightway to a wide variety of synthetic and technical samples especially reverberatory flue dust and steel is shown in Table 1 by their satisfactory analysis. The method has a better rapidity (takes only 5 min for a single determination and even less in series operation), sensitivity and freedom from disturbances from several important and frequently interfering elements as compared to most of the reported methods (Table 4). Compared with these, the method has a better precision and accuracy.
| S.No | Aqueous conditions | Solvent Λmax (nm) |
Molar absorptivity (l mol-1 cm-1) Sandell’s sensitivity (mg V cm-2) |
Interfering metal ions | Reference |
|---|---|---|---|---|---|
| 1. | V(V), acidic medium, benzohydroxamic acid. | 1-Hexanol 450 |
4.24 × 103 0.012 |
Fe(III), Bi(III), Al(III), Sn(II), Ti(IV), Zr(IV), Mo(VI), W(VI) | [33] |
| 2. | V(V), pH 3.5 - 4.5, shaking time 10 min picolinealdehydebenzothiozolylhydrazone, | Benzene 528 |
1.54 × 104 0.0033 |
Co(II), Pb(II), Fe(II, III) | [34] |
| 3. | V(V), 0.07-0.14 M CH3COOH, 3-hydroxy-2-(4-methylphenyl)-6-methylbenzopyran, shaking time 30 sec | Dichloromethane 400 |
2.54 × 104 0.00198 |
Mo(VI), Sn(II) | [35] |
| 4. | V(V), 0.04-0.06 M HCl, 6-chloro-3-hydroxy-7-methyl-2-(2-thienyl)benzopyran, at 32°C, shaking time 20 sec | Carbon tetrachloride 420 |
8.26 × 104 0.0006 |
Sn(II) | [36] |
| 5. | V(V), 0.15-0.20 M CH3COOH, 3-hydroxy-2-(4-methoxyphenyl)-6-methylbenzopyran, at 60-70 °C, shaking time 60 sec | Chloroform 410 |
3.3 ×104 | Zr(IV), Th(IV) | [37] |
| 6. | V(V), 0.04-0.30 M CH3COOH, 3-hydroxy-2-(2-thienyl)benzopyran, | Benzene 420 |
3.27 × 104 0.0016 |
Mo(VI), Zr(IV) | [38] |
| 7. | V(V), pH 5.2, acetophenone-2’,4’- dihydroxythiosemicarbazone, | Cyclohexanone 378 |
3.41× 103 0.01495 |
- | [39] |
| 8. | V(V), variamine blue | 570 | 1.65 × 104 0.003 |
- | [40] |
| 9. | V(V), pH 4±0.2, potassium iodide, HCl, thionin, sodium acetate | 600 | 2.294 × 104 0.0052 |
Ce(IV) | [41] |
| 10. | V(V), pH 4.5 - 5.5, H3PO4, 4-(2-pyridylazo)-resorcinol, tetrazolium violet, shaking time 2 min. | 1,2-Dichloroethane 555 |
3.05 × 104 | [42] | |
| 11. | V(V), 0.02-0.12 M CH3COOH, 1.3-2.4 ml of 0.1 % HPMEPB in acetone. | Carbon tetrachloride 415 |
2.72 × 104 0.0018 |
39 metal ions do not interfere | Proposed method |
Table 4: Comparison of the proposed method with some of the existing methods
Conclusion
The proposed method for trace determination of vanadium is very simple, rapid, sensitive and has much wider tolerance limits of foreign ions (39) thereby increasing its scope of applications. Micro amounts of the metal ion could be determined with greater accuracy with wider Beer’s law range. The validity of the proposed method has further been tested by analysing several synthetic samples of varying compositions, steel samples and reverberatory flue dust. The obtained results were in excellent agreement with the amount of metal ion added or certified. The proposed method was very simple, rapid and compared favourably with the existing methods of vanadium determination (Table 4).
Acknowledgements
Authors are thankful to the authorities, Kurukshetra University, Kurukshetra and Maharishi Markandeshwar University, Mullana for providing laboratory facilities.
References
- Gavazov K, Simeonova Zh, Alexandrov A (2000) Extraction spectrophotometric study of the system vanadium(V–C(2-thiazolylazo)-resorcinol(TAR)-triphenyltetrazolium chloride(TTC)-water-chloroform. Talanta 52: 539-544.
- Patrick P, Verma S, Grynpas MD, McNeill JH (1998) Vanadium and diabetes. Mol Cell Biochem 188: 73-80.
- Venugopal B, Luckey TD (1978) Metal toxicity in mammals. Chemical toxicity of metals and metalloids, Vol 2, Plenum Press: New York, pp.220.
- Langard S, Norseth T, Friberg L, Norberg GR, Vouk VB (1986) Handbook on the toxicology of metal, 2nd edn, Elsevier, Amsterdam.
- Greenberg RR, Kingston HM (1983) Trace element analysis of natural water samples by neutron activation analysis with chelating resin. Anal Chem 55: 1160-1165.
- Wang CF, Miau TT, Perng JY, Yeh SJ, Chiang PC, et al. (1989) Multielement analysis of airborne particulate matter by inductively coupled plasma atomic emission spectrometry. Analyst 114: 1067-1070.
- Yamashige T, Yamamoto M, Sunahara H (1989) Comparison of the decomposition methods for the analysis of atomic particulates by atomic absorption spectrometry. Analyst 114: 1071-1077.
- Mohamed AS, Fawy KH (2000) Catalytic determination of vanadium based on bromated oxidative coupling reaction of metal with phloroglucinol. Microchim Acta134: 229.
- Guangjin W, Yongfang W, Xuxiang S (1977) Lihua Jianyan, Huaxue Fence 33: 126.
- Balaji BK, Kumar SG, Murugesan P, Mishra G (1998) A modified catalytic-photolytic method for the determination of vanadium in chloride rich hydrogeochemical samples. Talanta 46:1299-1304.
- Ensafi AA, Amini MK, Mazloum M (1999) Spectrophotometric reaction rate method for the determination of trace amount of vanadium (V) by its catalytic effect on the oxidation of nile blue with bromate. Anal Lett32: 1927-1937.
- Eeckhout J, Weynants A (1956) Spectrophotometric determination of small amounts of vanadium with diphenylbenzidine. Analytica Chimica Acta 15: 145-153.
- Bertrand D (1942) Bulletin de la Société Chimique de France. Rue Saint-dominique, Paris.
- Talvitie NA (1953) Colorimetric determination of vanadium with 8-quinolinol. Anal Chem 25: 604-607.
- Bhaduri AS, Roy PZ (1957) Investigations of salicylyl-hydroxamic acid. Anal Chim 154: 103.
- Dutta RL (1958) J Indian Chem Soc 35: 243.
- Dutta RL (1959) J Indian Chem Soc 36: 285.
- Wise WM, Brandt WW (1955) Spectrophotometric determination of vanadium(V) with benzohydroxamic acid, Anal Chem 27: 1392-1395.
- Ryan DE (1960) The colorimetric determination of vanadium with benzoylphenylhydroxylamine. Analyst 85: 569-574.
- Priyadarshini U, Tandon SG (1960) J Chem Soc 29: 931.
- Priyadarshini U, Tandon SG (1961) Analyst 86: 544.
- Tandon SG, Bhattacharyya SC (1961) Use of N-2-thiphenecarbonyl-N-p-tolylhydroxylamine and N-2-thiophenecarbonyl-N-phenylhydroxylamine as reagents for vanadium. Anal Chem33: 1267.
- Kohara H, Ishibashi N, Ito Y (1967) Spectrophotometric determination of Vanadium (V) by solvent extraction with 3-hydroxyflavone. Bunseki Kagaku 16: 315.
- Chauhan RS, Kakkar LR (1991) Extraction Spectrophotometric determination of vanadium(III) with 3-hydroxyflavone. Annali di Chimica (Rome) 81:179.
- Kohara H, Ishibashi N, Hanamura Y, Ueno K (1966) Spectrophotometric determination of vanadium (V) by solvent extraction with morin. Bunseki Kagaku 15: 938-943.
- Shnaiderman SY, Prokof’eva GN (1970) Zhurnal Analiticheskoi Khimii 25: 2368.
- Charlot G, Bezier D (1957) Quantitative Inorganic Analysis. Methuen and Co Ltd, London.
- Oyamada T (1934) A new general method for the synthesis of the derivatives of flavonol. J Chem Soc Japan 55: 1256.
- Ringbom A (1938) Accuracy of colorimetric determinations I and II. Z Anal Chem 115: 332-343.
- Job P (1928) Formation and stability of inorganic complexes in solution. Ann Chim 9: 113-203.
- Vosburgh WC, Cooper GR (1941) Complex ions I. The identification of complex ions in solution by spectrophotometric measurements. J Am Chem Soc 63: 437-442.
- Yoe JH, Jones AL (1944) Colorimetric determination of iron with disodium-1,2-dihydroxybenzene-3,5-disulphonate. Ind Eng Chem Anal Ed 16: 111-115.
- Marczenko Z (1986) Spectrophotometric determination of elements. John Wiley, New York.
- Nishioka H, Suchiro S, Kumagai T, Uesugi K (1991) Kenkyu Hokoku-Himeji Kogyo Diagaku Kogakubu. Chem Abstr 44: 14.
- Agnihotri N, Dass R, Mehta JR (1999) Extractive spectrophotometric determination of vanadium(V) using 3-hydroxy-2-(4-methoxyphenyl)-6-methyl-4H-chromen-4-one. J Indian Chem Soc 76: 421-423.
- Agnihotri N, Dass R, Mehta JR (1999) A highly sensitive and selective spectrophotometric determination of vanadium(V) using 6-chloro-3-hydroxy-7-methyl-2-(2-thienyl)-4H-chromen-4-one. Anal Sci 15: 1261-1264.
- Agnihotri N, Dass R, Mehta JR (1997) Extraction spectrophotometric determination of vanadium using 3-hydroxy-2 - (4 - methoxyphenyl)- 6-methyl-4H-chromen-4-one. Chem Anal Warsaw 42: 397.
- Agnihotri N, Dass R, Mehta JR (1998) 3-Hydroxy-2-(2-thienyl)-4H-chromen-4-one as an analytical reagent for spectrophotometic determination of vanadium(V). J Indian Chem Soc 75: 514.
- Rana P, Lokhande R, Pitale S, Janwodkar S, Yadav D (2014) Spectrophotometric determination of vanadium with acetophenone-2’,4’-dihydroxythiosemicarbazone. Int J Chem Tech Res 6: 2295-2299.
- Kiran Kumar TN, Revanasiddappa HD (2005) Spectrophotometric determination of vanadium using variamine blue and its application to synthetic , environmental and biological samples. J Iran Chem Soc 2: 161-167.
- Cherian T, Narayana B (2005) A simple spectrophotometric determination of trace amounts of vanadium using thionin. Bull Chem Soc Ethiopia 19: 155-161.
- Gavazov K, Lekova V, Patronov G, Turkyilmaz M (2006) Extractive spectrophotometric determination of vanadium(IV,V) in catalysts using 4-(2-pyridylazo)-resorcinol and tetrazolium violet. Chem Anal Warsaw 51: 221-227.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences