Successful Angioplasty of a Hepatic Vena Caval Branch in a Patient with Budd- Chiari Syndrome Due to Occlusion of the Three Major Hepatic Veins
Masanori Yamashita1*, Natsuko Hayashi1, Yoshito Takeuchi2, Osamu Tanaka3 and Kei Yamada1
1Department of Radiology, Kyoto Prefectural University of Medicine, Japan
2Department of Radiology, Fukuchiyama City Hospital, Japan
33Department of Radiology, Kayashima Ikuno Hospital, Japan
- *Corresponding Author:
- Masanori Yamashita
Department of Radiology
Kyoto Prefectural University of Medicine, Japan
Tel: +817525151111
E-mail: m-yama@koto.kpu-m.ac.jp
Received date: October 25, 2017; Accepted date: November 01, 2017; Published date: November 08, 2017
Citation: Yamashita M, Hayashi N, Takeuchi Y, Tanaka O, Yamada K. Successful Angioplasty of a Hepatic Vena Caval Branch in a Patient with Budd-Chiari Syndrome Due to Occlusion of the Three Major Hepatic Veins. J Clin Radiol Case Rep 2017, Vol.1 No.1:03.
Copyright: © 2017 Yamashita M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
In this case of a Japanese woman with Budd-Chiari syndrome, percutaneous venoplasty of a single narrow accessory hepatic vein successfully restored normal hepatic circulation. The three major hepatic veins were occluded, whereas the inferior vena cava was patent. In this setting, percutaneous venoplasty of the single remaining hepatic vena caval branch was considered clinically effective.
Keywords
Budd-Chiari syndrome; Accessory hepatic vein; Percutaneous venoplasty
Introduction
Budd-Chiari Syndrome (BCS) is an uncommon condition caused by the blockade of hepatic venous outflow in the hepatic veins (HV) or inferior vena cava (IVC) [1]. The classical symptom triad seen in BCS includes abdominal pain, ascites, and hepatomegaly, but patients can present with other symptoms, such as liver dysfunction, jaundice, portal hypertensive gastroenteropathy, gastroesophageal varices, splenomegaly, thrombocytopenia, venous thrombosis, and leg edema/ulcers [2,3]. These conditions are usually managed using diuretics, anticoagulants, sodium restriction, and other palliative measures, although such treatments often fail or are only transiently effective [2].
Invasive procedures can be performed in patients who do not respond to medical therapy. For example, portocaval shunts can be created surgically or by an interventionist to decompress the portal venous system and alleviate symptoms of portal hypertension [3,4]. The shunts that can be created by interventionists include a trans jugular intrahepatic portosystemic shunt (TIPS) and a direct intrahepatic portosystemic shunt (DIPS), with the latter being preferred when the three major HV are obstructed [4].
In addition, percutaneous transluminal venoplasty (PTV) can relieve hepatic congestion by resolving the blockage of hepatic venous outflow [1]. PTV, TIPS, and DIPS are all less invasive than surgery. PTV restores hepatic venous outflow and thus achieves greater physiological reconstruction of the intrahepatic circulation compared with creation of a portocaval shunt. Therefore, PTV is preferred if any of the HV are accessible, while portocaval shunting should be performed otherwise [5].
Liver transplantation is performed to treat patients with fulminant hepatic failure or those who are refractory to other invasive treatments [2]. PTV of a single HV is the treatment of choice for BCS when a patient has isolated HV occlusion [3] and PTV is most successful when performed on the least obstructed of the three major HV [5]. When all major HV are completely occluded, recanalization of hepatic vena caval branches is required for treatment efficacy [3]. This brief report presents a patient with BCS who was successfully treated by PTV of an accessory hepatic vein (AcHV). This case was challenging because all three major HV were obstructed and inaccessible.
Case Presentation
A health check-up detected liver dysfunction in a 49-yearold woman without significant symptoms. An ultrasound detected abnormalities of the HV. On admission, an endoscopic examination revealed bleeding gastroesophageal varices with the “red color” sign. Physical examination findings were unremarkable. Laboratory data showed a total bilirubin of 1.39 mg/dL, serum albumin of 3.8 g/dL, platelet count of 112 × 103/ μL, and prothrombin activity of 74%. Her Child-Pugh score was 6A.
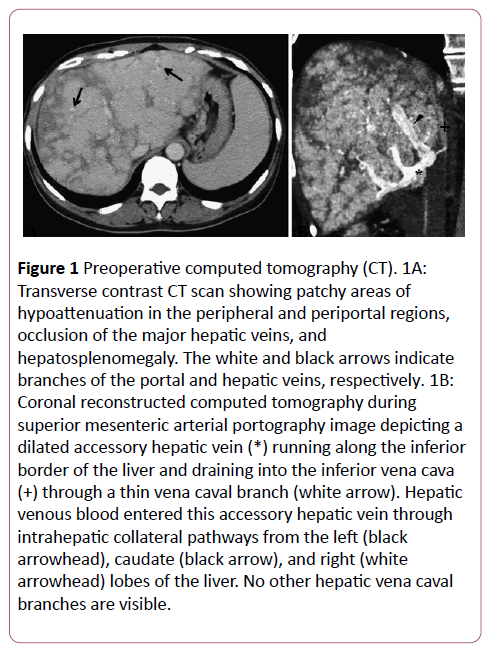
Abdominal contrast-enhanced computed tomography (CT) demonstrated patchy areas of hypoattenuation in the peripheral and periportal regions, occlusion of the major HV, hepatosplenomegaly, and scanty ascites (Figure 1A). The portal vein and IVC were both patent. CT during superior mesenteric arterial portography (CTAP) demonstrated a dilated AcHV that drained into the IVC via a small vena caval branch (Figure 1B). Hepatic venous flow through intrahepatic venovenous collaterals into this AcHV was noted. Since hepatic venous obstruction causes portal hypertension and liver cirrhosis, we planned emergent PTV to recanalize one of the HV.
Figure 1: Preoperative computed tomography (CT). 1A:Transverse contrast CT scan showing patchy areas of hypoattenuation in the peripheral and periportal regions,occlusion of the major hepatic veins, and hepatosplenomegaly. The white and black arrows indicate branches of the portal and hepatic veins, respectively. 1B:Coronal reconstructed computed tomography during superior mesenteric arterial portography image depicting a dilated accessory hepatic vein (*) running along the inferior border of the liver and draining into the inferior vena cava (+) through a thin vena caval branch (white arrow). Hepatic venous blood entered this accessory hepatic vein through intrahepatic collateral pathways from the left (black arrowhead), caudate (black arrow), and right (white arrowhead) lobes of the liver. No other hepatic vena caval branches are visible.
Procedure
The PTV was performed in the angiography suite under local anesthesia. An attempt to insert a catheter into the HV via the transfemoral transcaval approach was unsuccessful because the veins were almost completely occluded, and the associated branches were small with takeoff at an acute angle, making it impossible to insert catheters or guidewires.
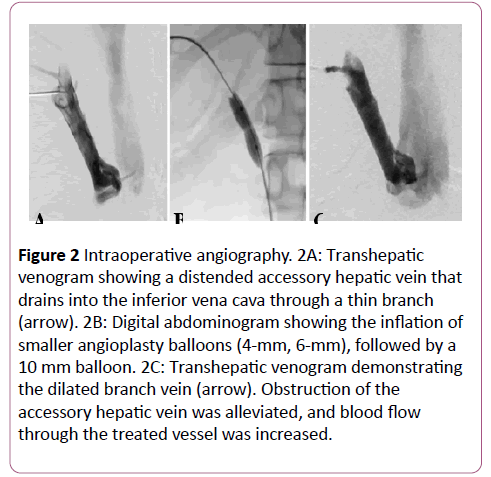
Instead, transhepatic puncture of the dilated AcHV was performed under ultrasonic guidance using a 20-G biliary needle (PTC; Hakko Co., Tokyo, Japan). A 4-Fr angiography sheath (XEMEX introducer set; Zeon Medical, Tokyo, Japan) was inserted over a 0.025-inch steel wire. Transhepatic venography showed stenosis at the venous exit (Figure 2A). The lesion was crossed with a 4.2-Fr multipurpose catheter (Hanako Excellent EN; Hanako Medical Inc., Saitama, Japan), followed by a 0.035-inch hydrophilic guidewire (Radifocus; Terumo, Tokyo, Japan).
Figure 2: Intraoperative angiography. 2A: Transhepatic venogram showing a distended accessory hepatic vein that drains into the inferior vena cava through a thin branch (arrow). 2B: Digital abdominogram showing the inflation of smaller angioplasty balloons (4-mm, 6-mm), followed by a 10 mm balloon. 2C: Transhepatic venogram demonstrating the dilated branch vein (arrow). Obstruction of the accessory hepatic vein was alleviated, and blood flow through the treated vessel was increased.
The transhepatic guidewire was then pulled out from the femoral venous sheath to stabilize the route through the lesion. Stepwise angioplasty was performed using balloons that were 4 mm, 6 mm, and 10 mm in diameter (Mustang; Boston Scientific, Marlborough, MA, USA) until the stenosis was dilated (Figure 2B). The inflation pressure was set at 10 atm for the 4-mm balloon and at 20 atm for the other balloons with an inflation time of 30 seconds each. After the angioplasty, vena caval return through the AcHV showed marked improvement on transhepatic venography (Figure 2C).
A metallic stent was not implanted because venography demonstrated an adequate response to the balloon dilatation. The percutaneous transhepatic track was embolized with a coil and gelatin particles. Procedural time was 130 minutes from the initiation of anesthesia to the sheath removal. No intraoperative events occurred apart from transient abdominal pain during the balloon inflation.
Postoperative Course
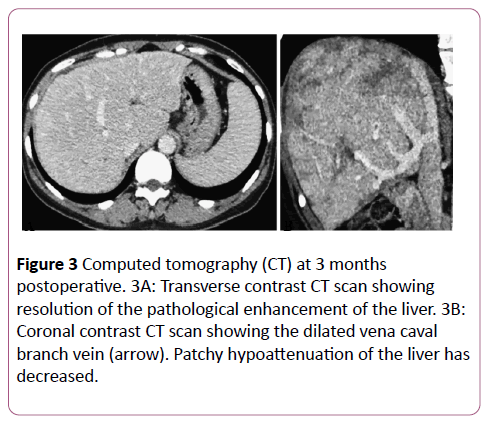
There were no procedural complications as defined by the Society of Interventional Radiology standards of practice committee classification during the postoperative period. Anticoagulant therapy was provided with intravenous heparin (10,000 units/day) for the first 24 hours, followed by oral warfarin (2-3 mg/day). The postoperative course was uneventful. A CT scan obtained at the 3-month follow-up showed that the treated lesion was still patent.
There was also a reduction in abnormal enhancement and a decrease in the size of the patchy areas of hypoattenuation (Figure 3). Esophagogastroduodenoscopy showed fewer esophageal varices and fading of the color of the remaining varices. After 8 months, the serum total bilirubin level was reduced (1.39 to 1.05 mg/dL) and the albumin level was increased (3.8 to 4.5 g/dL). None of the other parameters showed deterioration, and no new symptoms of BCS developed during the 8-month follow-up period.
Discussion
When the hepatic venous anatomy is normal, there are three major HV, with the middle and left HV forming a common trunk that drains into the IVC in 60% of people. The most common variant is the presence of an AcHV that drains separately into the IVC [6].
In typical BCS, contrast-enhanced CT shows occlusion of the IVC, the HV, or both. Multiple collateral pathways can develop in the systemic, portal, or hepatic venous systems, and intrahepatic collaterals are often found in BCS patients. In addition, CT demonstrates patchy decreased peripheral enhancement due to portal and sinusoidal stasis with stronger enhancement of the central liver parenchyma [7].
Okuda et al. performed a venocavography study in 157 Japanese patients and classified venous obstruction in BCS into the following four types: type 1, slight IVC obstruction with or without HV obstruction (frequency: 54.2%) type 2, marked IVC obstruction (frequency: 39.1%) type 3, isolated HV occlusion (frequency: 5.7%) and type 4, unclassified venous obstruction (frequency: 1.3%) [8].
In healthy individuals, AcHV are usually too small to drain much of the hepatic venous outflow. However, these vessels can provide an alternate route for venous drainage when the major HV are occluded, such as in type 3 obstruction, because blood drains into the AcHV through the intrahepatic venovenous collaterals [3].
CTAP is useful for evaluating intrahepatic hemodynamics, especially in BCS [9]. A single AcHV was found by CTAP but not contrast-enhanced CT in the present case. Thus, a detailed imaging assessment based on CTAP can be useful for identifying the best vein for angioplasty.
Recanalization or dilation of one of the three major HV after determining which intrahepatic collaterals will help drain the liver can sometimes resolve BCS-related symptoms [3,5]. Thus, an accessible HV should be targeted for angioplasty, even if it is not a major vessel.
Balloon venoplasty is associated with a greater risk than arterioplasty because arteries have a strong muscular layer within their walls (tunica media), whereas veins do not [10]. The diameter of the target HV/AcHV should be at least 5 mm to enable significant drainage of the liver. Venoplasty of an AcHV is usually done with balloons 6-11 mm in diameter [1].
In the present patient, balloons of increasing size were inflated stepwise in the stenotic segment of the AcHV because a single large balloon would have been too invasive. Ding et al. reported that the cumulative 1-2, 2-5, and 5-8-year primary patency rates after balloon venoplasty for BCS with hepatic venous obstruction were 97.5%, 92.9%, 90%, and 86.5%, respectively [11]. Zhang et al. found that the hepatic venous stent patency rate was 90.9% after 15-78 months of follow-up [3].
Thus, the outcomes of balloon angioplasty and stenting were similar. The main risk after stent deployment is migration. Therefore, stenting should be performed for lesions that respond inadequately to balloon dilatation, immediate recoil, or recurrent stenosis or occlusion [2].
When all three major HV are occluded, a portacaval shunt can be created by TIPS or DIPS [4]. In addition, PTV has the advantage of restoring anatomical hepatic venous blood outflow, which cannot be achieved with TIPS or other portacaval shunting methods [5]. Thus, PTV should be the treatment of choice when all three major HV are obstructed and a single AcHV or hepatic vena caval branch is identified.
The chief limitation of this case report is that the postoperative follow-up period of 8 months is too short, so further follow-up is needed to assess the outcome.
In conclusion, percutaneous venoplasty was successfully performed for a single stenotic AcHV to restore normal intrahepatic circulation in a patient with BCS due to an isolated HV obstruction. Percutaneous venoplasty can be effective for BCS if another hepatic vena caval branch remains patent when the three major HV have been occluded.
Conflict of Interest
The authors declare no conflicts of interest.
References
- Fu YF, Xu H, Zhang K, Zhang QQ, Wei N (2015) Accessory hepatic vein recanalization for treatment of Budd-Chiari syndrome due to long-segment obstruction of the hepatic vein: initial clinical experience. Diagnostic and Interventional Radiology 21: 148.
- Copelan A, Remer EM, Sands M, Nghiem H, Kapoor B (2015) Diagnosis and management of Budd Chiari syndrome: an update. Cardiovascular and Interventional Radiology 38: 1-12.
- Zhang CQ, Fu LN, Xu L, Zhang GQ, Jia T, et al. (2003) Long-term effect of stent placement in 115 patients with Budd-Chiari syndrome. World Journal of Gastroenterology 9: 2587-2591.
- Peynircioglu B, Shorbagi AI, Balli O, Cil B, Balkanci F, et al. (2010) Is there an alternative to TIPS? Ultrasound-guided direct intrahepatic portosystemic shunt placement in Budd-Chiari syndrome. Saudi Journal of Gastroenterology: Official Journal of the Saudi Gastroenterology Association 16: 315-318.
- Beckett D, Olliff S (2008) Interventional radiology in the management of Budd Chiari syndrome. Cardiovascular and Interventional Radiology 31: 839-847.
- Sahani D, Mehta A, Blake M, Prasad S, Harris G, et al. (2004) Preoperative hepatic vascular evaluation with CT and MR angiography: implications for surgery. Radiographics 24: 1367-1380.
- Brancatelli G, Vilgrain V, Federle MP, Hakime A, Lagalla R, et al. (2007) Budd-Chiari syndrome: spectrum of imaging findings. American Journal of Roentgenology 188: 168-176.
- Okuda H, Yamagata H, Obata H, Iwata H, Sasaki R, et al. (1995) Epidemiological and clinical features of Budd-Chiari syndrome in Japan. Journal of Hepatology 22: 1-9.
- Ueda K, Matsui O, Kadoya M, Yoshikawa J, Gabata T, et al. (1998) CTAP in Budd-Chiari syndrome: evaluation of intrahepatic portal flow. Abdominal Imaging 23: 304-308.
- Ueda K, Matsui O, Kadoya, M, Yoshikawa J, Gabata T, et al. (1998) CTAP in Budd-Chiari syndrome: evaluation of intrahepatic portal flow. Abdominal Imaging 23: 304-308.
- Ding PX, Zhang SJ, Li Z, Fu MT, Hua ZH, et al. (2016) Longâ€ÂÂterm safety and outcome of percutaneous transhepatic venous balloon angioplasty for Budd–Chiari syndrome. Journal of Gastroenterology and Hepatology 31: 222-228.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences