ISSN : 0976-8505
Der Chemica Sinica
Separation and Activity against Drug-resistant Bacteria of Tetrandrine and Fangchinoline in Lipophilic Akaloids from Stephania tetrandra
Shao-bin Fu1*, Hong-qiang Yang1, Jia-jia Tu1, Qing-feng Meng2*, Shi-ji Xiao1, Mao-sheng Zhang1, Ze-Hui Chen3
1Department of Pharmacy, Zunyi Medical College, Zunyi, China
2Department of Public Health, Zunyi Medical College, Zunyi, China
Abstract
Tetrandrine and fangchinoline were separate in total alkaloids from Stephania tetrandra and the activities against drug-resistant bacteria in-vitro were investigated. Preparative medium pressure liquid chromatography and semi-preparative high performance liquid chromatography were applied to separate and purify tetrandrine and fangchinoline; Agar dilution method was used to test the anti-drug–resistant bacteria activity and determine the minimum inhibitory concentration (MIC) of MRSA 13366 and ESBLproducing Escherichia coli 13025. Tetrandrine and fangchinoline were identified by TLC and NMR spectra; The viable count absorbance regression equations were Y=9.5715x-0.3439(R2=0.9907) for MRSA 13366 and Y=8.6245x-0.1802(R2=0.9972) for ESBL-producing Escherichia coli 13025; MICs of tetrandrine were 80 μg/ml for MRSA 13366 and 160 μg/ml for ESBL-producin E. coli 13025; MICs of fangchinoline were 160 μg/ml for MRSA and 320 μg/ml for ESBL-producing Escherichia coli 13025. High performance liquid chromatographic method was considered convenient, fast and high-purity method to separate and purify tetrandrine and fangchinoline from total lipophilic alkaloids. Both tetrandrine and fangchinoline showed significantly inhibiting activity on MRSA and ESBL-producing Escherichia coli especially for tetrandrine.
Keywords
Alkaloids, Tetrandrine, Fangchinoline, Separation, Activity against drug-resistant bacteria, MIC
Introduction
With the widespread use of antibiotics in clinic, agriculture and animal husbandry, bacterial resistance has become a major threat to human health [1]. Members of Mohnarin (CARSS) in 2015 reached a total of 1427 hospitals, monitoring from October 2014 to September 2015. A total of 2400786 non duplicate bacterial strains, including 695066 strains of gram positive bacteria (28.9%), 1705720 strains of gram negative bacteria (71.1%) were reported during this period. The first rank of gram positive bacteria strains of were Staphylococcus aureus with number of 223, 7583 (about 2.2%) while the first rank of gram negative bacteria was Escherichia coli, with number of 510140 [2]. It was reported that the highest isolated rate of gram negative bacteria was also Escherichia coli, the positive bacteria was Staphylococcus aureus from pathogenic bacteria infected in hospital. Moreover, strains with the high resistance to antibiotics were Escherichia coli producing ESBLs and MRSA which separation rates were as high as 70.4% and 56.5% [3]. Hospital infections caused by drug-resistant strains had serious impacts on medical safety and patient safety that were the main causes of failure anti-infection treatment in clinical. Besides, the mortality rate led by drug resistant infections was twice higher than sensitive bacterial infection. Antibiotic resistance leads to higher medical costs, prolonged hospital stays, and increased mortality. In china the problem of bacterial resistance caused by misuse and overuse of antibiotics was very serious. The number of infections caused by resistant bacteria in the hospital was about 30% of the total number of hospital infection. In 2010, a "super bacteria"-Escherichia coli containing a metal called New Delhi-metal-β-lactamase (NDM-1) was found in India and the bacteria was resistant to almost all current antibiotics [4]. More worrying, the incidence of resistant strains and multi-drug resistant strains increased every year. The problem of bacterial resistance, a global public health problem in 21 Century has become the hot spots and becomes extremely urgent [5]. WHO has been appealing that the world needs to change the way it prescribes and uses antibiotics. Even if new medicines are developed, without behaviour change, antibiotic resistance will remain a major threat. It is worth noting that there was no reports on drug-resistant strains to Traditional Chinese Medicine (TCM) while the emergence of widespread resistance to chemical drugs. In addition, TCM, natural medicines with rich resources attracted more and more attentions because of little side effect, application security, low price, difficult to produce drug residues and environment friendly. In recent years, more and more reports on special effects of TCM with anti-bacterial, anti-tumor, anti-inflammatory, reversal of bacterial resistance and tumor resistance were reported [6-8].
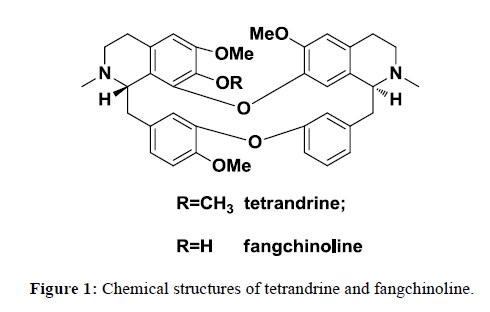
Stephania tetrandra containing alkaloids, polysaccharides and other ingredients has long history in TCM. Tetrandrine and fangchinoline, the main active components, showed anti-inflammatory, analgesic, anti-hypertensive, anti-tumor, anti-hypoxic pulmonary hypertension activities (Figure 1) [9-12]. In the previous study, it was found that the lipophilic alkaloids exhibited inhibitory activity to MRSA 13366 and ESBL-producing Escherichia coli 13025. Sun et al. [13] reported that tetrandrine and fangchinoline can reverse multidrug resistance by inhibiting P-glycoprotein activity in multidrug resistant human cancer cells. Herein, preparative medium pressure liquid chromatography and semi-preparative high performance liquid chromatography were applied to separate and purify tetrandrine and fangchinoline from lipophilic alkaloid. Anti-bacteria activity of tetrandrine and fangchinoline in clinical drug resistant bacteria activity was carried out and MICs were determined which lay the basis for the development of new antimicrobial substances.
Materials and Methods
Materials
Test strain: MRSA 13366 and ESBL-producing Escherichia coli 13025 were provided by affiliated hospital of Zunyi Medical College.
Lipophilic total alkaloids
The crude powders of Stephania tetrandra were soaked with 0.5% sulfuric acid and extracted by percolation. The percolated liquid was adjusted to pH 9~10 with fresh lime milk. Let stand for enough time, the supernatant was discarded then the mud yellow solid was obtained after filtration. The yellow solid and sodium borohydride were mixed evenly, and dehydrated on a water bath. The lipophilic total alkaloids were obtained by ethyl acetate for continuous extraction.
Methods
General experimental: Preparative medium pressure liquid chromatography, BUCHI, Switzerland; sem-preparative high performance liquid chromatography (LC3000), chuangxintongheng science and Technology Co. Ltd, Beijing, China; YMC liquid chromatography column (250 ODS-A* 10 mml.D S-5 m.12 nm), Beijing Hui Tak Technology Co Ltd, Beijing, China; autoclaves sterilizers (YXQ-LS-50S II) Shanghai Boxun Industrial Co. Ltd. Shanghai, China; Ultraviolet spectrophotometer (UV Power), Beijing Puxi instrument factory, China; Thermostatic incubator (TS-2102C), Shanghai tiancheng Technology Co. Ltd, China; Rotary evaporator (EYELAN-1100), Japan; NMR spectrometer (Agilent, 400 MHz).
Separation and purification of tetrandrine and fangchinoline
The lipophilic total alkaloids extracts were subjected to preparative medium pressure column chromatography on silica gel (49 mm* 460 mm, 300-400 mesh) eluted with petroleum-ethyl acetate-diethylamine (9:1:0.1~5:1:0.1) with rate 80 ml/min. Fr 1 containing tetrandrine and Fr 2 containing fangchinoline were collected through TLC detection. Fr 1 and Fr 2 were further purified by semi-preparative HPLC (YMC-pack chromatography column, 250*10 mmD, S-5 μm, 12 nm) eluted with methanol-water (85:15) at 4 ml/min and 230 nm. Purified tetrandrine and fangchinoline were gained and identified by TLC (petroleum-ethyl acetate-diethylamine=3:2:1) which colored by Bismuth potassium iodide spray reagent and NMR data.
Anti-bacterial activity of tetrandrine and fangchinoline to drug-resistant bacteria in-vitro
Serial concentrations of 400, 800, 1600, 3200 and 6400 μg/m (dissolved in DMSO) of tetrandrine and fangchinoline were prepared by two fold dilution method. 1 ml various concentrations liquid and 9 ml sterile MH agar medium were mixed into 40, 80, 160, 320 and 640 μg/ml concentrations. 2 μg/ml positive control drug imipenem, vancomycin and negative control penicillin sodium (dissolved in 50% ethanol) were prepared.
Spectrophotometric method was used to prepare 0.5 mic concentration of bacteria liquid. First, 4-5 colonies were inoculated in sterile physiological saline, and mixed with a vortex oscillator after the strain was recovered, and then two times dilution was used to obtain the bacteria of the 5 gradients. OD values were measured at 600 nm wavelength. Next, bacteria were diluted 1000 times and 10 μL was taken to coat on MH agar plates. Colonies were counted in the microscope after 24 h culture at 37°C. The results are shown in Table 1.
| Strains | Number | Absorbance | Living cells (× 108)/ml |
|---|---|---|---|
| MRSA | 1 | 0.080 ± 0.001 | 0.6 ± 0.05 |
| 2 | 0.153 ± 0.001 | 1.4 ± 0.05 | |
| 3 | 0.291 ± 0.002 | 2.1 ± 0.1 | |
| 4 | 0.534 ± 0.001 | 4.4 ± 0.4 | |
| 5 | 0.950 ± 0.002 | 9.0 ± 0.9 | |
| ESBL | 1 | 0.049 ± 0.002 | 0.3 ± 0.05 |
| 2 | 0.091 ± 0.001 | 0.7 ± 0.05 | |
| 3 | 0.166 ± 0.003 | 1.1 ± 0.05 | |
| 4 | 0.305 ± 0.002 | 2.4 ± 0.2 | |
| 5 | 0.595 ± 0.003 | 5.0 ± 0.5 |
Table 1: Results of living cells for MRSA and ESBL producing Escherichia coli.
0.1 ml drug-resistant bacteria of 0.5 mic concentration of were coated on the agar plate with drugs. Cultures were observed after 24 h at 37°C. Experiments were carried out in triplicate.
Results and Discussion
Separation and structural identification of tetrandrine and fangchinoline
Tetrandrine 1 and fangchinoline 2 were separated from the lipophilic total alkaloids by preparative medium pressure column chromatography and semi-preparative HPLC. Compared with standers, the colors and Rf values of the isolated Tetrandrine 1 (Rf ≈0.6, Petroleum: ethyl acetate: diethylamine=3:2:1) and fangchinoline 2 (Rf≈0.5, Petroleum: ethyl acetate: diethylamine=3:2:1) were consistent (Figure 2) in TLC.
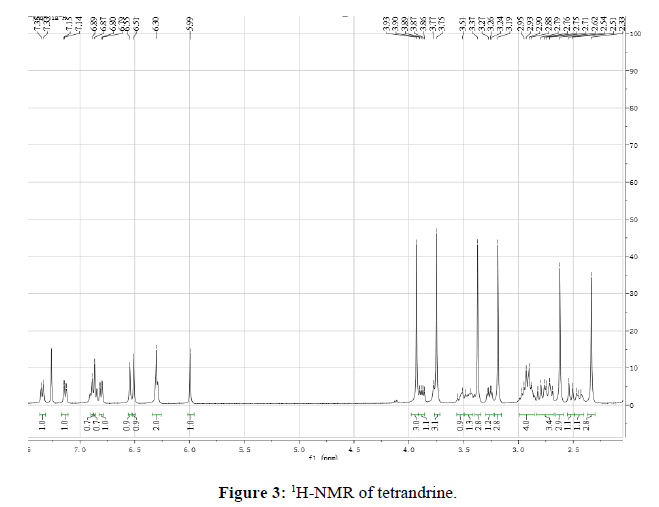
Tetrandrine 1: white power, mp 223°C. 1H-NMR(400 MHz, CDCl3) (Figure 3): 2.33(s, 3H), 2.41~2.47(m, 1H), 2.52(d, J=12.0 Hz, 1H), 2.62( s, 3H), 3.19(s, 3H), 3.25(dd, J=12.0, 8.0 Hz, 1H), 3.37(s, 3H), 3.43~3.48(m, 1H), 3.51~3.56(m, 1H), 3.75(s, 3H), 3.88(dd, J=12.0, 4.0Hz, 1H), 3.93(s, 3H), 5.99(s, 1H), 6.29(s, 1H), 6.30(m, 2H), 6.51(s, 1H), 6.55(s, 1H), 6.81(d, J=8.0 Hz, 1H), 6.86(dd, J=8.0, 4.0 Hz, 1H), 6.87(s, 1H), 6.89(s, 1H), 7.13(dd, J=8.0, 4.0 Hz, 1H), 7.34(d, J=8.0 Hz, 1H).
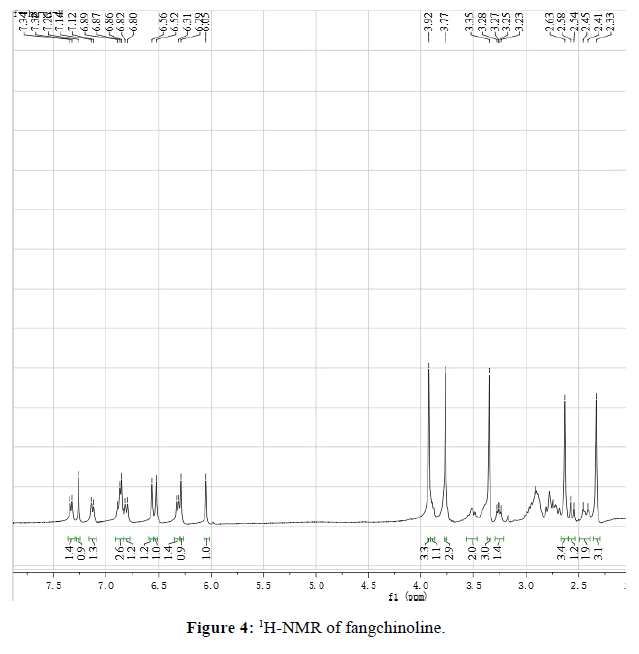
Fangchinoline 2: white power, mp 231°C. 1H-NMR(400 MHz, CDCl3) (Figure 4): 2.33(s, 3H), 2.41~2.45(m, 2H), 2.56(d, J=16.0 Hz, 1H), 2.63( s, 3H), 3.26(dd, J=12.0, 8.0 Hz, 1H), 3.35(s, 3H), 3.48~3.54(m, 2H), 3.77(s, 3H), 3.88~3.91(m, 1H), 3.92(s, 3H), 6.05(s, 1H), 6.29(s, 1H), 6.32(m, 1H), 6.52(s, 1H), 6.56(s, 1H), 6.81(d, J=8.0 Hz, 1H), 6.86(o, 1H), 6.87(o, 1H), 6.89(o, 1H), 7.13(dd, J=8.0, 4.0 Hz, 1H), 7.34(d, J=8.0 Hz, 1H).
The difference between tetrandrine and fangchinoline was the substitutes at C-7. There was methoxyl group at C-7 in tetrandrine while there was phenolic hydroxyl group in fangchinoline. From 1H-NMR spectrum, four methoxyl signals appeared bewteen 3.0-4.0,3.19(s, 3H, H-7), 3.37(s, 3H, H-6´), 3.75(s, 3H, H-6), 3.93(s, 3H, H-12) in tetrandrine while only four methoxyl signals 3.35(s, 3H, H-6’), 3.77(s, 3H, H-6), 3.92(s, 3H, H-12) in tetrandrine.
Results of antibacterial activity of tetrandrine and fangchinoline in-vitro
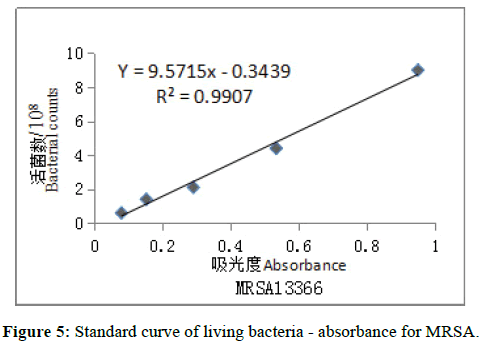
Plate counting living bacteria method: The relationship between the number of viable bacteria and absorbance was in Table 1, according five different concentrations of MRSA and Escherichia coli producing ESBL.
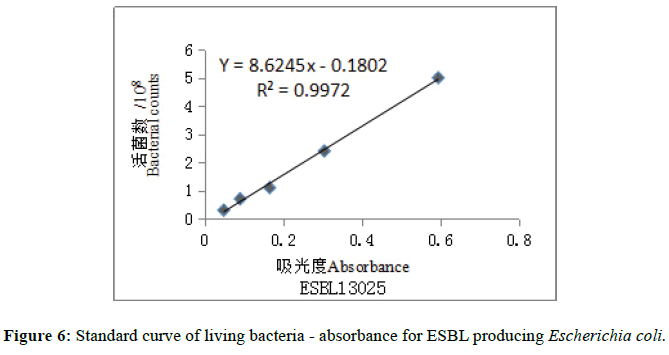
The standard curves were drawn in the number of viable bacteria and corresponding absorbance by Excel software (Figures 5 and 6). The regression equation of MRSA 13366: Y=9.5715x-0.3439 (R²=0.9907); ESBLproducing Escherichia coli 13025: Y =8.6245x-0.1802 (R2=-0.9972). The required concentration was calculated according to regression equation and then the corresponding absorbance of bacteria was prepared for antibacterial activity test in-vitro. The required concentrations were 1.5 × 108 cfu/ml, the corresponding absorbances were 0.193 for MRSA 13366 and 0.195 for ESBL-producing Escherichia coli 13025.
Antibacterial activity of tetrandrine and fangchinoline
MIC was determined by agar plate dilution method (Table 2). From Table 2, all the negative control of bacteria grew a lot, indicating that penicillin had no inhibitory effect on the two kinds of bacteria which indicating that they were drug-resistant strains. In addition, all the solvent control and blank control of bacteria grew fast, indicating that tetrandrine and fangchinoline were the active ingredient that had obvious inhibitory effect on MRSA and Escherichia coli producing ESBL. The results showed that tetrandrine exhibited stronger antibacterial activity to MRSA with MIC 80 μg/ml and Escherichia coli producing ESBL with MIC 160 μg/ml. The MIC of fangchinoline to MRSA was 160 μg/ml, and the MIC of ESBL-producing Escherichia coli was 320 μg/ml.
| Groups | Test strains | Test groups (μg/ml) | Control groups | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 40 | 80 | 160 | 320 | 640 | Negative | Positive | Solvent | Blank | ||
| Tetrandrine | MRSA | + | + | - | - | - | + | - | + | + |
| + | + | - | - | - | + | - | + | + | ||
| + | + | - | - | - | + | - | + | + | ||
| ESBL | + | + | + | - | - | + | - | + | + | |
| + | + | + | - | - | + | - | + | + | ||
| + | + | + | - | - | + | - | + | + | ||
| Fangchinoline | MRSA | + | + | + | - | - | + | - | + | + |
| + | + | + | - | - | + | - | + | + | ||
| + | + | + | - | - | + | - | + | + | ||
| ESBL | + | + | + | - | - | + | - | + | + | |
| + | + | + | + | - | + | - | + | + | ||
| + | + | + | + | - | + | - | + | + | ||
Notes: Imipenem and cilastatin sodium were positive drug control for ESBL producing Escherichia coli, vancomycin hydrochloride was positive drug control for MRSA, penicillin sodium was negative drug control for two kinds of bacteria, tablet plus solvent without drugs as solvent control, sterile water as blank control.
Table 2: MIC of inhibition activity of MRSA and ESBL producing Escherichia coli.
According to the previous literature, column chromatography, TLC and recrystallization methods were in common use for separation and purification of the effective components of natural medicine that usually time-consuming, low yield, low purity of the sample. In this study, preparative medium pressure column chromatography and semi-preparative high performance liquid chromatography were applied to separate tetrandrine and fangchinoline the first time with a highly automatic, convenient, fast, high purity process. The widespread use of antibiotics in clinical is serious that led to the number and types of drug-resistant bacteria increased every year and will pose the greatest health threats [14,15]. Therefore, developing new antibiotics was urgent. The experimental results showed that tetrandrine and fangchinoline exhibited obvious activity against drug-resistant bacteria, special tetrandrine for MRSA with MIC 80 μg/ml and for ESBL-producing Escherichia coli with MIC 160 μg/ml. The two alkaloids provided lead compounds for the development of new antibiotics as effective natural composition that unmodified structures.
Conclusion
The method of preparative medium pressure chromatography combined with high performance liquid chromatography was applied to separate and purify tetrandrine and fangchinoline showed the advantages of convenience, high speed and high purity. Structures of active compounds were identified by TLC and 1H-NMR spectrum. Tetrandrine and fangchinoline had obvious inhibitory effects on MRSA and ESBL-producing Escherichia coli and the activity of tetrandrine was stronger.
Acknowledgment
This research was financially supported by The National Natural Sciences Foundation of China (No.21462057), Guizhou Science and Technology Department (QKHLH-2014-7555).
References
- Liang H, Peng GJ, Zhang W, Liu J, et al. (2012) Comparative in vitro activity of tigecycline and minocycline agaist multidrug-resisitant clinical isolates. Chin J Infect Chemother 12: 390-392.
- National Bacterial Resistance Surveillance Report (2015) China Antimicrobial Resistance Surveillance System.
- Chen X, Yand Q, Zhang WL (2011) Mohnarin report of 2010: bacterial resistance in northwest China. Chin J Nosocomiol 21: 4933-4938.
- Kumarasamy KK, Toleman MA, Walsh TR (2010) Emergence of a new antibiotic resistance mechanism in India, Pakistan and the UK: a molecular, biological and epidemiological study. Lancet Infect Dis 10: 597.
- Patrick DM, Hutchinson J (2009) Antibiotic use and population ecology: how you can reduce your "resistance footprint". Cmaj 180: 416-421.
- Nobuo Y, Takanao U, Numhamant A, Yimit D, Hoxur P, et al (2015) Bi-​directional regulation by chinese herbal formulae to host and parasite for multi-​drug resistant Staphylococcus aureus in humans and rodents. Open J Immunol 5: 18-32.
- Prtar K Verica A, Natasa S (2016) Antimicrobial activity of Eucalyptus camaldulensis essential oils and their interactions with conventional antimicrobial agents against multi-​drug resistant Acinetobacter baumannii. J Ethnopharmacol 178: 125-136.
- Shaheen AY, Sheikh AA, Rabbani M (2015) Antibacterial activity of herbal extracts against multi-​drug resistant Escherichia coli recovered from retail chicken meat. Pak J Pharm Sci 28: 1295-1300.
- Liu W, Kou B, Ma ZK (2015) Tetrandrine suppresses proliferation, induces apoptosis, and inhibits migration and invasion in human prostate cancer cells. Asian J Androl 17: 850-853.
- Cui XY, Zhu W, Wang P (2015) Tetrandrine inhibits the intracellular calcium ion level and upregulates the expression of Brg1 and AHNAK in Hep-​2 cells. Clin Lab 61: 1569-1576.
- Guo BB, Xie P, Su JY (2016) Fangchinoline suppresses the growth and invasion of human glioblastoma cells by inhibiting the kinase activity of Akt and Akt-​mediated signaling cascades. Tumour Biol 37: 2709-2719.
- Li D, Lu Y, Sun P (2015) Inhibition on proteasome β1 subunit might contribute to the anti-​cancer effects of fangchinoline in human prostate cancer cells. PLoS ONE 10: e0141681.
- SunYF, Wink M (2014) Tetrandrine and fangchinoline, bisbenzylisoquinoline alkaloids from Stephania tetrandra can reverse multidrug resistance by inhibiting P-glycoprotein activity in multidrug resistant human cancer cells. Phytomedicine 21: 1110-1119.
- Willyard C (2017) The drug-resistant bacteria that pose the greatest health threats. Nature 543: 15.
- Kemp C (2017) Drug-resistant bacteria in children a growing problem. AAP News & Journals.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences