Risk Factors Associated with Late Presentation to HIV Care in the "Treat All" Era in Sub-Saharan Africa: A Systematic Literature Review
Daniel Mwamba*
Department of Science, Lusaka Apex Medical University, Zambia, Lusaka
- *Corresponding Author:
- Daniel Mwamba
Department of Science, Lusaka Apex Medical University, Zambia, Lusaka
Tel: 260978625288
E-mail: mwambalonga@gmail.com
Received Date:February 08, 2022, QC No. IPCIID-22-12358; Revised date: March 23, 2022, Manuscript No. IPCIID-22-12358 (R); Published date: March 31, 2022, DOI: 10.36648/ipciid/6.3.001
Citation: Mwamba D (2022) Risk Factors Associated With Late Presentation To HIV Care In The "Treat All” Era In Sub-Saharan Africa: A Systematic Literature Review. Clin Immunol Infect Dis Vol:6 No:3
Abstract
Introduction
Late presentation to HIV care (with CD4 count <350 cells or WHO clinical stage 3 or 4) remains a norm in sub-Saharan Africa. This is despite the 2016 World Health Organization HIV guidelines recommending the initiation of Antiretroviral Therapy (ART) in all people living with HIV regardless of clinical and immunological status.
The aim of this systematic review was to describe the prevalence and demographics of adults aged >15 years who are late to present to HIV care in sub-Saharan Africa.
Methods
PubMed, Embase, ISI web of knowledge, Health System Evidence Global Index Medicus databases, web engines and conference websites were searched for relevant studies, grey literature and abstracts conducted between 2015 and 2020.
Results
9 studies were included in the review. Males represented 58% of the total 714, 929 participants aged >15. The prevalence of late presentation to care was 44%, (95% CI: 37–51). The odds of late presentation to care for males was 1.54 (95% CI: 1.05–2.36); aged >36 was 1.55 (95% CI: 0.98–2.69); not being married was 1.065 (95% CI: 0.99–1.15).
Conclusion
Late presentation to HIV care remains high among adults living with HIV in sub-Saharan Africa. Being male, not married, and being above 35 years of age were found to be associated with higher odds of late presentation to care. Strategies that allow early HIV detection and treatment and innovative approaches targeting population at risk are needed to achieve expected HIV program outcome of the treat all policy.
Keywords
Carinii pneumonia; Morbidity and mortality weekly report; Acquired immunodeficiency syndrome
Introduction
On the 5th of June 1981, the Morbidity and Mortality Weekly Report (MMWR); a U.S. Center for Disease Control (CDC) journal; published an article reporting cases of a rare chest infection. This was a case series of five young gay men from Los Angeles showing signs of Pneumocystis Carinii Pneumonia (PCP) and other rare conditions (including Kaposi’s sarcoma) consistent with a defective immune system [1]. This article was later considered as the first official report of the HIV epidemic.
In 1985, a study conducted by H C Lane et al. found that there was a significant association between the clinical presentation of patients with Acquired Immunodeficiency Syndrome (AIDS) and their immunological disorders as a result of a reduced number of CD4 T lymphocytes. This important finding has been crucial for the development of therapeutic HIV trials and guidelines for treatment.
The race to find a cure for HIV led to the discovery of Azidothymidine (AZT); an old failed anti-cancer drug; as an antiviral for HIV in 1987. In a double-blind, placebo-controlled trial (2) AZT administration for duration of 24 weeks was found to significantly increase the number of CD4 cells and decrease mortality and the frequency of opportunistic infections in patients with HIV. However, in the long run AZT monotherapy resulted in the emergence of HIV viral mutants and resulting drug resistance [3].
Several clinical trials and cohort studies were conducted in the subsequent years to identify treatment regimens that were most effective and feasible in clinical practice. In one clinical trial, found that a combination of triple Antiretroviral Therapy (ART) was associated with a decreased risk of death compared to a double combination; this was consistent with findings from that showed that patients receiving triple ART were at reduced risk of death [Relative Risk (RR), 0.15; 95% Confidence Limit (CL), 0.12, 0.17], followed by those on dual ART (RR, 0.24; 95% CL, 0.22, 0.26), and monotherapy (RR, 0.38; 95% CL, 0.36, 0.40) [4,5].
According to the MMWR published on 28th February 1997, the use of the triple ART combination in the USA resulted in 6% decline in the incidence of opportunistic infection in 1996 compared to 1995, and 23% less HIV-related deaths over the same period. Globally the use of this Highly Active Antiretroviral Therapy (HAART) has contributed to the drop in new HIV infections by 39% of and HIV-related deaths by 51% between 2000 and 2019, representing 15.3 million lives saved [6].
Although the United States reported the first cases of AIDS, by the mid-1990 s sub-Saharan Africa accounted for the majority of the estimated 20 million people living with HIV [7]. Data from the World Health Organization (WHO) shows that the virus has infected 76 million people and claimed 33 million lives since the beginning of the HIV epidemic in the early 1980 s. As of 2019, between 31.6 and 44.5 million people worldwide was living with HIV, 25.4 million of whom had access to antiretroviral therapy, with Sub-Saharan Africa accounting for more than two-thirds of the world's HIV burden.
While the therapeutic effect of ART and its preventive benefits of reducing HIV transmission were well established since mid-90 s, the appropriate time for ART initiation remained a topic of debate for years [8].
A review of three major HIV technical working group recommendations found that the United States Department of Health and Human Services (DHHS) Guidelines for Use of Antiretroviral Therapy in Adults and Adolescents, the European AIDS Clinical Society (EACS) guidelines and the WHO Antiretroviral Therapy Guidelines for Adults and Adolescents recommended ART initiation when CD4 counts fell below 200 cells/mL in their first guidelines [9]. These recommendations were backed by the evidence from “Optimal time of initiation of antiretroviral therapy for asymptomatic, HIV infected, treatment-naive adults”, a systematic review. The Review Included Randomized Controlled Trials (RCTs) and cohort studies, in which ART initiation was stratified according to CD4 cell count [10]. The author concluded that “there is evidence of moderate quality that initiating ART at CD4 levels higher than 200 or 250cells/microL reduces mortality rates in asymptomatic patients”
Additionally, concerns about ART-related adverse events, drug resistance and medication stock-outs were unanswered barriers to the widening of the treatment eligibility criteria particularly in high HIV burden settings like the sub-Saharan region [11].
ART guidelines evolved and changed as the body of evidence supporting earlier initiation of ART grew over time. The CIPRA HT-001 study, a single-centre randomized, open-label trial in Haiti, looked at early (CD4 count between 200 and 350 cells/mL) versus standard ART (CD4 count below 200 cells/mL) for HIV-Infected asymptomatic adults; the study endpoint was survival. The study recorded 29 deaths: 23 in the standard-treatment group and 6 in the early-treatment group (P=0.001) [12,13]. Similarly, the Strategies for Management of Antiretroviral Therapy (SMART) Study Group also evaluated the benefit of early ART initiation in a multicentre large randomized clinical trial conducted in both high-income and low/middle-income countries; in a subgroup analysis 477 patients who were ART-naive were randomized to start ART with CD4 of >350 cells/mL or delayed until CD4 dropped to <250 cells/mL. The study found that delaying ART significantly increased the risk of opportunistic disease and death.
Furthermore, reduced morbidity and mortality among patients who initiate ART early was also found in four other observational cohort studies from resource-limited and resource-rich countries. The compilation of the above evidence is cited in the WHO 2010 guidelines as justification to the change of the CD4 eligibility threshold from 200 to 350 cells/mL. As additional evidence and programmatic experience continued to favour earlier initiation of ART, the WHO further raised the CD4 threshold from 350 to 500 cells/mL in their 2013 guidelines [14-17].
In 2012, taking into consideration the availability of well tolerated Antiretrovirals (ARVs) and accumulating opinions from experts in the field, the United States and Canada abolished the CD4 based treatment eligibility criteria and recommended ART to everyone living with HIV (18). They were followed by South Korea and Brazil in 2013 and Thailand in 2014 [18].
Between 2009 and 2014, the Strategic Timing of Antiretroviral Therapy (START) study; conducted by the International Network for Strategic Initiatives in Global HIV Trials (INSIGHT); examined the occurrence of AIDS-defining conditions or death among people who were randomized to receive immediate ART at CD4 counts above 500 cells/mL versus deferring it until the CD4 count decreased to 350 cells/mL or until the development of the Acquired Immunodeficiency Syndrome (AIDS). The study found that early ART provided benefits over starting therapy after the CD4 count had declined; the occurrence of both AIDS related, and non-AIDS related cancer was lower in the early ART group. The study also found no difference in the occurrence of drug-related adverse event between the two groups. These findings were similar with those from the TEMPRANO trial conducted in Ivory Coast from 2008 to 2015; this was a multi-center randomized controlled trial. The study demonstrated that that initiating ART at a CD4 cell count above 500 cells/mL was associated with less severe HIV morbidity compared with treatment initiation at a CD4 cell count at or below 500 cells/mm.
Besides the evidence of benefit on an individual level, the HIV Prevention Trials Network (HPTN) 052 studies showed that heterosexual transmission of HIV could significantly be reduced if ART is started early, and viral suppression is achieved. The above findings revamped the debate around ART Treatment as Prevention (TasP) and the Universal Testing and Treatment (UTT) recommending ART initiation immediately after HIV diagnosis (test and treat) in all settings and particularly high burden regions like the sub-Saharan Africa with the aim of reducing the risk of HIV transmission [9].
Cohort and national programmatic data predicted that the number of people needing treatment could increase by up to 35% if ART is initiated regardless of CD4 count rather than at or below 500 cells/mL, however field experience claimed that this increase would be gradual as not all eligible patients will be put on ART at the same time, therefore this will not overburden the health system. Estimates from The HIV modeling Consortium projected up to 14% fewer HIV-related deaths during the next decade if the UTT was implemented globally. The Consortium modeling also indicated that the benefit of earlier ART would outweigh its cost by decreasing hospitalization, increasing productivity, and preventing people from acquiring HIV [19].
The WHO warned that early ART initiation would not have a large impact in the absence of increased HIV case finding strategies, prompt linkage to care and treatment and long-term retention on treatment. De Cock and El-Sadr suggested resource limited countries to strengthen health systems, address shortages of health workers budget constraints if individual health had to benefit from the UTT guideline while altering the overall epidemic picture.
A comparative study assessed the population level impact, cost and feasibility of UTT using data from four trials and experimental studies initiated in sub-Saharan Africa. Peterson M, et al (20) examined data from the ANRS 12,249 TasP (ANRS TasP) trial in South Africa, the HPTN 071 (PopART) trial in South Africa and Zambia, the SEARCH trial in Uganda and Kenya and the BCPP/YaTsie (BCPP) trial in Botswana, all four trials had cumulative HIV incidence as primary endpoint. The analysis found that the pooled HIV incidence declined by 0.07 per 100 person years (95% CI, 0.05-0.10) for each 1% absolute decrease in viremia These findings suggested that UTT could potentially reduce HIV incidence at population level, if effectively implemented, even if to a modest level [20].
In 2016 the World Health Organization (WHO) released the “treat all” policy also known as UTT, recommending Antiretroviral Therapy (ART) initiation in all people living with HIV (PLHIV) regardless of CD4 count and/or WHO clinical stage, with priority given to patients with advanced HIV disease. By the end of 2017, 80% of all Low and Middle Income Countries (LMIC) and almost all sub-Saharan countries had adopted Treat All, but only 50% of 139 LMICs WHO member states had fully implemented the Treat All in the majority of their ART sites. More than 90% of fast-track countries (countries with highest HIV global burden) had implemented the treat all policy in more than 50% of their treatment sites by mid-2018 [21].
This expanded programme is expected to alter the trend of people presenting to the health facilities with low CD4 count or with an AIDS-defining condition and alleviate health care costs related to treating advanced HIV diseases. Raising the CD4 threshold for ART initiation has resulted in a reduction of late presentation to HIV care between 2004 and 2015; from 75% to 34% in Haiti, 73% to 37% in Mozambique and 80% to 41% in Namibia [22]. However, despite large-scale HIV testing campaigns to hasten diagnosis and the abolition of the CD4 based treatment eligibility criteria, data from 85 countries that reported to UNAIDS, showed that up to a third of people presenting to care in 2016 had a CD4 cell count of less than 200. In 2017 up to 20% of PLHIV in eastern and southern Africa presented to care with advanced HIV disease [23].
A prerequisite to developing interventions to reduce late presentation to care is an understanding of who these late presenters are and their reasons for doing so. Several definitions of late presentation to care are cited in literature, however for the purpose of this review the WHO and the European consensus definition was used; it defines:
- Late presentation to HIV care: Persons presenting for care with a CD4 count below 350 cells/mL or presenting with an AIDS-defining event, regardless of the CD4 cell count.
- Presentation with advanced HIV disease: Persons presenting for care with a CD4 count below200 cells/mL or presenting with an AIDS-defining event, regardless of the CD4 cell count [24].
Therefore, patients with a CD4 count below 200 cells/mL and/or with an AIDS defining event will meet both criteria and will be both ‘late presenters’ and ‘presenters with advanced HIV disease’. The WHO clinical staging refers to the use of a tool that determines a patient’s eligibility for ART based on clinical signs and symptoms. The tool categorizes the health status of PLHIV into four stages (I, II, III, and IV), each of which gives an indication of the level of HIV disease progression [25].
Monitoring prevalence of individuals who present late for care and with advanced HIV disease among persons initiating Antiretroviral Therapy (ART) can help understand HIV program outcomes while informing HIV prevention strategies.
Although there is a lot of published literature on late presentation to HIV care prior to the UTT, there are fewer studies done after 2015. A review and compilation of the estimate of patients who present to HIV care late under the UTT and their demographics would be of value to policy makers and program managers and will allow targeted interventions.
Aim of the study
The aim of this study is to describe risk factors associated with late presentation to care among People Living with HIV (PLHIV) in the "treat all” era in sub-Saharan Africa
Study objectives
- To describe the proportion of PLHIV in sub-Saharan Africa who present late for HIV care in the treat all era.
- To describe the risk factors of PLHIV associated with presenting late to HIV care in the treat all era in sub-Saharan Africa
- To evaluate the quality of published studies on late presentation to HIV care in the treat all era in sub-Saharan Africa.
Literature Review
Methods
A systematic literature review of late presentation to HIV care in the treat all era in sub – Saharan Africa was conducted.
Study selection criteria
Studies that meet the following criteria were included: cohort, case series, trials and quasi-experimental studies conducted in sub-Saharan Africa between January 2015 and July 2020
All HIV-positive individuals aged ≥ 15 years in care who had a documented baseline CD4 count at HIV diagnosis and/or WHO clinical staging were included
To avoid the introduction of language bias into the review, studies conducted in any language but published and translated in English were included.
Studies conducted outside sub-Saharan Africa, qualitative studies, studies including individuals aged below 16 years and adult patients who were in care for the purpose of preventing mother-to-child transmission of HIV (PMTCT) were excluded. Studies that stipulate a threshold for starting ART ie are not “treat all” were not included.
Search strategy
PubMed, Embase, ISI web of knowledge, Health System Evidence and Global Index Medicus (GIM) databases were searched for publication. In addition, web engines (Google scholar, Bing) were searched for grey literature and conference websites (International AIDS conference, the International Conference on AIDS and STIs in Africa (ICASA) and the Conference on Retroviruses and Opportunistic Infections (CROI)) for relevant abstracts Table 1.
| Database | Search terms |
|---|---|
| Pubmed to 14th July 2020 | HIV+late presentation+Africa |
| HIV+late presentation+Low and middle-income countries | |
| HIV+advanced diseases+Africa | |
| HIV+advanced diseases+Low and middle-income countries | |
| ISI Web of Knowledge to 14th July 2020 | HIV+late presentation+Africa |
| HIV+late presentation+Low and middle-income countries | |
| HIV+advanced diseases+Africa | |
| HIV+advanced diseases+Low and middle-income countries | |
| Health System Evidence to 10th July 2020 | HIV+late presentation+Africa |
| HIV+late presentation+Low and middle-income countries | |
| HIV+advanced diseases+Africa | |
| HIV+advanced diseases+Low and middle-income countries | |
| Global Index Medicus (GIM) to 9th July 2020 | HIV+late presentation+Africa |
| HIV+late presentation+Low and middle-income countries | |
| HIV+advanced diseases+Africa | |
| HIV+advanced diseases+Low and middle-income countries | |
| IAS abstract archives 2015 to 2020 | Late presentation |
| Advanced HIV | |
| CROI abstract archives 2015 to 2020 | Late presentation |
| Advanced HIV | |
| ICASA abstract archives 2015 to 2020 | Late presentation |
| Advanced HIV | |
| Web search engine (Google scholar) | Scanned titles |
Table 1: Search strategy.
The search was limited to studies that were conducted after January 2015; although the WHO treat all guideline was launched in late 2016, a few trials initiating ART regardless of CD4 count were being implemented prior to 2016.
A combination of free text searching using synonyms and Medical Subject Heading (MeSH) for each search concept was used. Truncations were included to search for terms that might have several different endings, and Boolean operators (AND and OR) helped to connect sets of synonyms for the same search concept. The MESH terms comprised a combination of “HIV or AIDS”, “late presentation or advanced disease” and “Africa or low and middle-income countries”. The search was conducted until 14th July 2020. The Prisma flow chart was used to show the study search and screening cascade.
Critical appraisal of studies
For quality assessment of all studies the following parameters were considered: appropriateness of study design to the research objective, risk of bias, choice of outcome measure, quality of reporting and generalizability. An extensive search through multiple databases and using various search terms was done to minimize the risk of bias.
The Modified Newcastle–Ottawa scale was adapted and used to appraise studies found to meet the inclusion criteria, the following domains were included in the scale: (Table 2).
| Selection | Comparability | Outcome | ||||
|---|---|---|---|---|---|---|
| Representativeness of the sample | Sample size | Ascertainment of exposure | The subject in different outcome groups are comparable,andConfounding factors are controlled. | Assessmen t of outcome | Statistical test | |
| Carmonaet al | 1 | 0 | 1 | 2 | 1 | 1 |
| Lilian | 1 | 1 | 1 | 2 | 1 | 1 |
| Blankleyeta | 2 | 0 | 1 | 3 | 1 | 1 |
| Lifsonet al | 1 | 0 | 1 | 3 | 1 | 1 |
| Kerschbergeret al | 2 | 1 | 1 | 3 | 1 | 1 |
| FridahT | 2 | 0 | 0 | 3 | 1 | 1 |
| sogbanmuet a | 2 | 1 | 1 | 3 | 1 | 1 |
| Brennanet al | 1 | 0 | 1 | 0 | 1 | 1 |
| Brennanet al | 1 | 0 | 1 | 0 | 1 | 1 |
Table 2: Modified new castle ottava scale.
Selection domain (maximum score of 4 points)
Representativeness of the sample
- Truly representative of the average in the target population (PLHIV)=2 points
- Somewhat representative of the average in the target population=1 point
- Selected group of users=0 point
- No description of the sampling strategy=0 point
Sample size
- Justified and satisfactory=1 point
- Adequately powered to detect a difference=1 point
- Not justified=0 point
Ascertainment of the exposure (HIV status)
- Medical records=1 points
- Self-report=0 point
- No description=0 point
- Comparability domain (maximum score of 3 points)
Comparability of subjects in different outcome groups and control of confounding factors
- Study controls for age and sex or analysis separated by gender=2 points
- Study controls for any additional sociodemographic characteristic=1 point
- Subjects in different outcome groups no comparable=0 point
- Outcome domain (maximum score of 2 points)
Assessment of outcome (CD4 count/WHO clinical stage)
- Medical records=1 point
- Self-report=0 point
- No description=0 point
Statistical test
- The statistical test used to analyse the data is appropriate and well described=1 points
- The statistical test is inappropriate or not described=0 point
Studies were therefore classified as of good, fair and poor quality following the below scoring:
- Good quality: 3 or 4 points in selection domain AND 2 or 3 points in comparability domain AND 1 or 2 points in outcome domain
- Fair quality: 2 points in selection domain AND 1 or 2 points in comparability domain AND 1 or 2 points in outcome/exposure domain
- Poor quality: 0 or 1 point in selection domain OR 0 point in comparability domain OR 0 or 1 point in outcome/exposure domain
All studies were considered to be at low risk of detection bias, because appropriate statistical methods were used, and the outcomes measured were objective and extracted from subjects’ routine medical records.
Data extraction strategy
Both initial assessments of articles for inclusion in the review and data extraction were made by the first author with reference to the protocol. A standardized data extraction Excel sheet was used to extract the following variables from each source reviewed: source reference, study aim and design, date study conducted, country/geographical region of the study, population included (age and sex, area of residence, level of education and marital status) inclusion/exclusion criteria and sample size, definition of outcome, method of measurement. (Table 3 and Table 4)
| S.No | First author | Database | Study design | Country | Setting | Pub Year | Sample Siz | Outcome | Define late presentation | Proportion of late presenters | Weight | % | Std. err. | 95% Conf. interval |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 714,929 | ||||||||||||||
| 1 | Carmonaet al. | GlobalIndex Medicus | Cohort | LaboratorySouth Africa | Urban | 2018 | 6,54,868 | Distributionof ART naïve patients presenting with advanced HIV disease | EnteringHIV care with CD4<200 cells | 32.90% | 1 | 91.5 | 0.000581 | 32.8%33% |
| 2 | Lilianet al. | Embase | RetrospectiveCohort | Clinic-South/Africa | 2019 | 39,306 | Trendin ART initiation and baseline CD4 counts | 38% | 2 | 5.4 | 0.002448 | 37.5%38% | ||
| 3 | Blankleyet al | Pubmed | RetrospectiveCohort analysis | ClinicZimbabwe | Semiurban | 2019 | 16,007 | Riskfactor for presentation with Advanced HIV disease | Presentingfor HIV care with a CD4 count <200 cells/mL or presenting with WHO stage 3or 4 | 47.40% | 3 | 2.2 | 0.003947 | 46.6%48% |
| 4 | Lifsonet al | Embase | Survey | ClinicEthiopia | Rural | 2018 | 1559 | Predictorsof presentation to care with advanced HIV disease | Presentingfor HIV care with a CD4 count <200 cells/mL or presenting with WHO stage 3or 4 | 61% | 4 | 0.2 | 0.011501 | 58.7%63% |
| 5 | KerschbergerBet et al. | GoogleScholar | Prospectiveimplementation study | ClinicEswatini | Rural | 2019 | 1231 | Timefrom facility-based HIV care enrolment to ART initiation. | presentingwith CD4 cell counts <200 cells/mm3 and/or WHO clinical stage III/I. | 36.50% | 5 | 0.2 | 0.011588 | 34.2%39% |
| 6 | FridahT. | GoogleScholar | Across-sectional, descriptive quantitative study | ClinicSouth Africa | Urban | 2017 | 349 | Prevalenceof late ART initiation | Firstpresentation to care with CD4 <200 or WHO stage 4 | 46% | 6 | 0.04 | 0.026566 | 40.7%51% |
| 7 | Sogbanmuet al. | Embase | Crosssectional study | ClinicalSouth Africa | Urban | 2019 | 330 | Prevalenceof late presentation to HIV care | Presentingfor HIV care with a CD4 count <350 cells/mL or presenting with an AIDSdefining event | 60% | 7 | 0.04 | 0.026766 | 54.5%65% |
| 8 | Brennanet al | Pubmed | Non-blindedindividually randomized pragmatic trials | ClinicSouth Africa | Periurban | 2019 | 273 | Eligibilityfor Same day initiation | Firstpresentation to care with CD4 <200 | 36% | 8 | 0.04 | 0.027925 | 30.7%42% |
| 9 | Brennanet al | Pubmed | Non-blinded,individually randomized, pragmatic trials | Clinic-Kenya | Rural | 2019 | 221 | Eligibilityfor Same day initiation | Firstpresentation to care with CD4 <200 | 37% | 9 | 0.04 | 0.031179 | 31.0%44% |
Table 3: Search match review.
The Adjusted Odd Ratio (AOR) of variables reported in the primary studies was extracted because of its assumed to have controlled for possible confounders. Due to time constraint no attempt was made to reach primary authors for missing data (Table 4).
| Lifson et al. | Sogbanlll et al. | Carmona et al. | Blankley et al. | Fridah T. | lilian et al. | kerschberger et al | Brennan et al. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographiccharacteristics | LateN=942(%) | EarlyN=617 (%) | LateN=197(%) | EarlyN=133(%) | LateN=215162(%) | EarlyN=439706(%) | LateN=7587(%) | EarlyN=8420(%) | Laten=162(%) | Earlyn=187(%) | Laten=1163(%) | Earlyn=18041(%) | Late3333(%) | Early6302(%) | Laten=494(%) | Earlyn=737(%) | Laten=82(%) |
| Pvalue | <001 | 0 | <001 | <001 | <001 | <001 | |||||||||||
| Male | 424(66) | 216(34) | 82(78.8) | 22(21.2) | 104269(26.5) | 288501)(73.4) | 3463(57.9) | 2517(42.0 | 58(51.3) | 55(48.6) | 5255(49.8) | 5294(50.1) | 1347(47.7) | 1471(52.2) | 266(54.9) | 218(45) | |
| Female | 518(56) | 401(44) | 115(50.9) | 111(49.1) | 110893(42.3) | 151305(57.6) | 4124(41.1) | 5903(58.8) | 104(43.5) | 135(56.4) | 6375(33,3) | 12747(66.6) | 1986(29.1) | 4831(70.8) | 228(30.5) | 519(69.4) | |
| Pvalue | 0.002 | 0.026 | <001 | <001 | 0.56 | ||||||||||||
| median(range) | 35(29-41) | 31(26-37) | 41.4 | 37.0(16-79.1) | 33.7(15-78.1) | 39(16.1-78.2) | 34.7(15.0-79.3) | ||||||||||
| 16-25 | 188(52) | 171(48) | 121(55.5) | 97(44.5) | 16 | 75(18.2) | 336(81.7) | ||||||||||
| 26-35 | 614(63) | 361(37) | 251 | 492(41.3) | 699(58.6) | ||||||||||||
| >35 | 124(62) | 76(38) | 77(68.1) | 36(31.9) | 84 | 64(51.6) | 60(48.3) | ||||||||||
| Pvalue marital status | 0.31 | 0.604 | <0.001 | <001 | |||||||||||||
| single | 145(64) | 83(36) | 163(60.8) | 105(39.2) | 582(53.2) | 511(46.7) | 85(47.7) | 93(52.2) | 376(33.3) | 753(66.6) | |||||||
| married | 454(59) | 321(41) | 30(56.6) | 23(43.3) | 4806(44.5) | 5993(55.4) | 47(45.6) | 56(54.3) | 233(43.7) | 300(56.2) | |||||||
| widowed/divorced | 340(62) | 212(38) | 5(55.6) | 4(44.4) | 2193(53.4) | 1910(46.5) | 30(42.2) | 41(57.7) | |||||||||
| residence | |||||||||||||||||
| urban | 43(36.7) | 74(63.2) | |||||||||||||||
| periurban | 95(51.9) | 88(48.0) | |||||||||||||||
| rural | all | 24(46.1) | 28(53.8) | ||||||||||||||
| travelto clinic(%) | |||||||||||||||||
| yes | 85(46.7) | 97(53.2) | |||||||||||||||
| no | 77(456.2) | 93(54.7) | |||||||||||||||
| pvalue | 0.74 | 0.002 | 0.215 | <001 | |||||||||||||
| education(%) | |||||||||||||||||
| no education | 232(60) | 156(40) | 35(83.3) | 7(16.7) | 4(50) | 4(50) | 33(48.5) | 35(51.4) | |||||||||
| primary | 442(60) | 297(40) | 35(66.0) | 18(34.0) | 941(47.2) | 1050(52.7) | 27(56.2) | 21(43.7) | 140(41.6) | 196(58.3) | |||||||
| secondary | 267(62) | 164(38) | 100(56.5) | 77(43.5) | 2529(46.7) | 2883(53.2) | 109(46.1) | 127(53.8) | 331(34.5) | 627(65.4) | |||||||
| tertiary | 28(48.3) | 30(51.7) | 58(55.2) | 47(44.7) | 13(21.6) | 47(78.3) | 9(34.6) | 17(65.3) | |||||||||
| employment(%) | <001 | <.001 | |||||||||||||||
| employed | 782(59) | 550(41) | 135(40.4) | 1963(54.9) | 1611(45.0) | 93(41.5) | 131(58.4) | ||||||||||
| unemployed | 131(73) | 48(27) | 199(59.6) | 5319(46.9) | 6000(53.0) | 69(53.9) | 59(46.0) |
Table 4: Characteristics of included studies
Ethical considerations
Since there is no direct contact with patients and only published data were used, no ethical issues are were anticipated.
Data analysis
The choice of analysis method was heavily determined by the type of data included. The numerical data collected were captured into a Microsoft Excel spreadsheet and imported into STATA (statistics software) for analysis.
An initial descriptive synthesis was done using a table containing details about study type, interventions, numbers of participants, a summary of participant characteristics, outcomes (table 2 and 3); followed by a narrative synthesis. The random effects model was used to estimate the pooled prevalence of late presentation to HIV care and pooled AOR of identified variables. A forest plot was done using the 95% confidence interval and pooled prevalence of late presentation. I squared test was used to assess the statistical heterogeneity.
I squared statistic was interpreted according to the threshold recommended by the Cochrane Handbook of Systematic Reviews of Interventions [26].
- 0% to 40%: low heterogeneity.
- 30% to 60%: moderate heterogeneity.
- 50% to 90%: substantial heterogeneity.
- 75% to 100%: considerable heterogeneity.
The causes of statistical heterogeneity could not be investigated by carrying out a subgroup analysis by participants' age group because all included studies only recruited adults aged 15 and above and only two studies subsequently used similar age bands subgroups. All studies were conducted in Sub Saharan Africa; a regional analysis was not feasible as only two regions, southern and eastern, were represented. Study populations, methods used, and outcome measure were assessed and did not reveal clinical heterogeneity. An attempt to perform funnel plot analysis was made to assess for publication bias but this was not done as there were fewer than 10 studies in the review.
Results
Search results
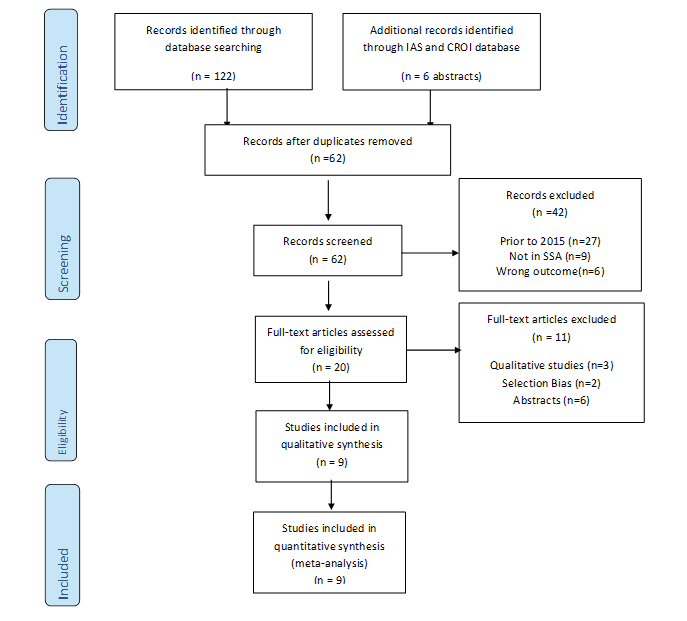
A total of 122 articles and 6 conference abstracts were searched from all databases, potentially relevant to our review. 67 articles were found from PubMed, 32 from Google scholar, 20 from Embase and 9 from GIM. After excluding 60 duplicates, 62 records were screened and 42 publications were excluded (27 were conducted before 2015, 9 studies were from outside sub-Saharan Africa and 6 had wrong outcome). 20 full text articles were assessed for eligibility, 11 were excluded (3 were qualitative studies, 2 had selections bias and 6 abstracts). After excluding studies that did not meet the geographic, population, content, or design criteria of our review, 9 full-length articles were eligible for the review (Figure 2).
Characteristic of studies included
A total of 9 studies accounting for 714,929 participants were included in the review; 3 studies were retrospective cohort studies, 2 were cross sectional studies, 2 randomized pragmatic trials, 1 was prospective implementation study and 1 survey. Five sub-Saharan countries were represented with 5 studies conducted in South Africa, 1 in Kenya, 1 in Eswatini, 1 in Zimbabwe, 1 in Ethiopia. 8 out of the 9 studies were conducted in hospital or clinic settings and one was laboratory based.
The studies settings varied from rural in Ethiopia, Kenya and Eswatini to peri urban in Zimbabwe and South Africa and urban in South Africa of the studies included in the review four defined late presentation as presenting to care or entering HIV care with CD4 count below 200 cell); four studies defined it as presenting for HIV care with a CD4 count <200 cells/mL or presenting with a WHO stage 3 or 4 condition and one study considered late presentation as presenting for HIV care with a CD4 count <350 cells/mL or presenting with an AIDS defining event [27-33].
To identify factors associated with late presentation to care, socio demographic variables like sex, age, level of education, employment and marital status were analysed [34,35].
Participants from all included studies were adults with lowest age band including individuals aged 15 to 18 years. For the purpose of this review the age was categorized as 15 to 25 years, 26 to 35 years and above 35 years.
The marital status was categorized into married and not married; widows and divorcees were considered as not married.
Education level was classified into no education, primary education, secondary education, and tertiary education. For the purpose of this review, we grouped no education and primary education in one category, while secondary and tertiary education constituted a second category.
Employment status was categorized into employed (this included any form of income generating activity as well as self-employment) and unemployed
Proportion of late presentation to care
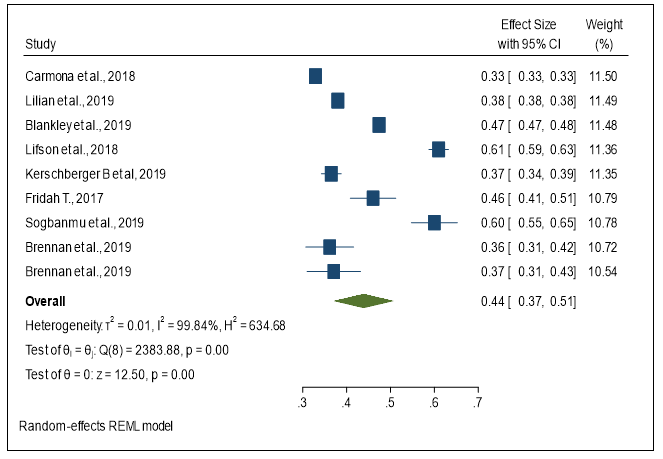
All 9 studies were considered when estimating the proportion of late presenter to care. Late presentation to care varied from 32% in South Africa to 61% in Ethiopia. A forest plot was used to calculate and display the pooled prevalence of late presentation, 44% (95%CI 37 – 51; I2=99.84% P=0.00) (Figure 2).
Risk factors associated with late presentation
Sex
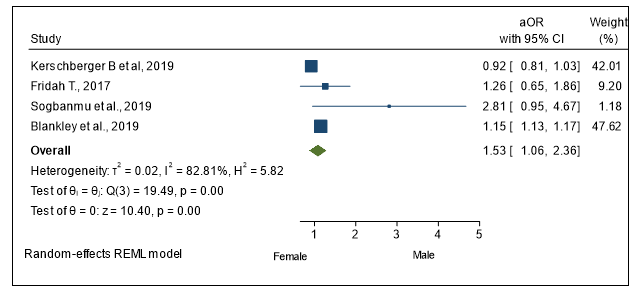
Across the 7 studies with documented sex, it was reported that male sex was associated with risk of late presentation. 40.8% of males presented late to care versus 27.3% of females. A pooled adjusted Odds ratio of late presentation to care for male versus female gender from 4 studies was 1.54 (95% CI: 1.05–2.36) (Figure 3).
Age
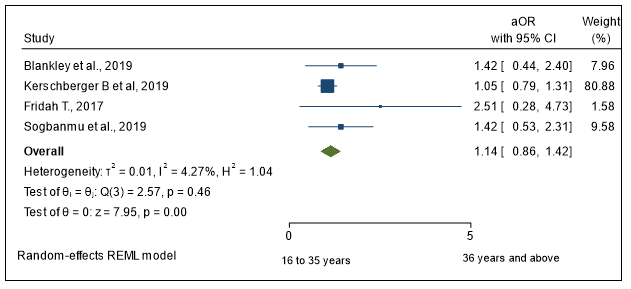
Three studies with age records showed that being of age 36 and above was associated with late presentation to care, 52.6% of those aged 36 years and above were late presenters versus 39,1% of those aged 16 to 35 years. However, a pooled adjusted Odd ratio of late presentation to care among those aged 36 and above compared to those aged 18 to 35 years from four studies was 1.55 (95 CI: 0.98–2.69) (Figure 4).
Marital status
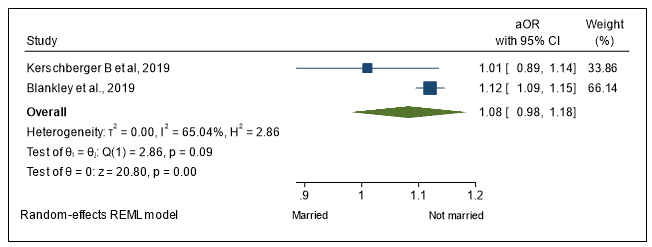
Data from 2 studies from Ethiopia and South Africa showed that 64% and 60.8% respectively of unmarried participants presented to care late, similarly 3 other studies from Zimbabwe, South Africa and Eswatini showed that 44.5%, 45.6% and 43.7% respectively of married subjects presented late to HIV care. A pooled adjusted Odd ratio of late presentation among those who were married compared to those not married from two studies was 1.065 (95% CI: 0.99–1.15) (Figure 5).
Employment status
Four studies showed that the proportion of late presenters among unemployed patients was 73%, 59.6%,46.9% and 53.9% while the proportion of late presentation among the those in employment was 59%, 40.4%, 54.9% and 41.5%. Because there was no documented adjusted odd ratio, the pooled AOR could not be extracted.
Residence
Two studies from Eswatini and Ethiopia were conducted in rural setting and all study participants resided in the same catchment area [27,29]. Data from one study with recorded residence details from urban setting in South Africa showed that 51% of those residing in peri urban set up were late presenters compared to 46% and 43% of those living in rural and urban areas, respectively. There was not sufficient statistical data to extract a pooled adjusted odd ratio for different type of setting
Level of education
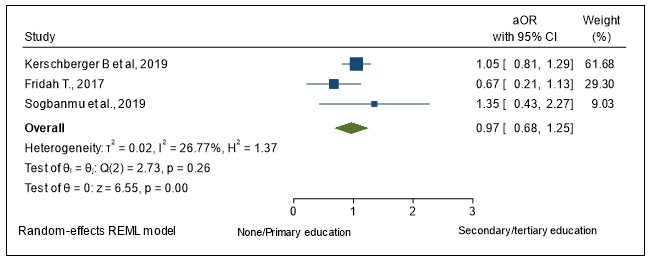
Five studies had level of educations records; one study revealed that up to 83.3% of people with no education and 66% of those with only primary education were late presenters to HIV care versus 56.5% and 48.3% of those with secondary and tertiary education respectively. No substantial difference was noted in the different education status from the remaining four studies. The pooled adjusted odd ratio from three studies looking at the odd of presenting to care late when one has no education or only has primary education compared to those with secondary and or tertiary education was 1.02 (95%CI 0.63 – 1.71) (Figure 7).
Discussion
A total of 9 studies from sub-Saharan Africa; a majority from South Africa; were included in this review. Male sex represented 58% of the total 714,144 participants. The study population included adults aged 16 years and older from urban and rural areas. In this review the pooled prevalence of late presentation to care in treat all era was 44% (95% CI) [37–51].
These findings are in line with what was observed in studies conducted before the treat all era in sub-Saharan Africa as well in countries that implemented treat all before 2015. The prevalence of late presentation in Kenya was 33% between 2013 and 2015, 65.5% in Ethiopia in the period between 2004 and 2014 [36,37]. Similarly, in rural India two thirds of patients-initiated ART late between 2007 and 2011; and in Italy a Cohort of PLHIV found that 60% of those initiating ART were late, in China late presentation was 45.1% in Guangxi during 2012–2016 and 72% during 2003–2012 across Asia [41].
Following the changes in CD4 count threshold for ART initiation, there has been a reduction of late presentation to HIV care between 2004 and 2015; from 75% to 34% in Haiti, 73% to 37% in Mozambique and 80% to 41% in Namibia but finding from this review suggests that late presentation to care remains a norm in many sub-Saharan countries [42].
This review revealed that male subjects had 1.5 times the odds presenting to care late compared to females. These findings are concurrent with those from and [43,44]. This could be partly due to the poor health seeking behaviour of men, but also attributable to the fact that women are more likely to be tested for HIV and offered ART while attending service like antenatal care, family planning or child health [45]. A Canadian qualitative study conducted by Fred and Yves to understand why men do not access the health care system for medical problems found that systemic barriers in the service delivery such as long waiting time before seeing the health care provider, limited clinic operating time; and having to disclose the reason for the visit to a female health care providers prevented men from seeking help when they are sick. Additionally, focused group discussions in this study revealed that traditional beliefs and social norms giving a sense of immunity and immortality to men was perceived as personal barrier to better men’s health seeking behaviour. Findings from an in-depth interview conducted to understand the extent to which gender influences men’s health-seeking behaviour showed that masculinity and cultural norms remain major influencers of men's health-seeking behaviour despite high educational attainments. Relationship with medical personnel was also mentioned as a barrier to seeking care; respondents admitted that their relationship with healthcare providers was distant or simply non-existent [46]. Some participants believed that being attended to by a female heath care provider was taboo because culturally certain matters are not supposed to be disclosed to a woman. Providing male friendly health services like Men’s ONLY clinic with male health care providers and other modes of differentiated HIV service delivery can be suggested as a way of improving health seeking behaviour among men. Community HIV testing was found to identify asymptomatic HIV-positive men at higher CD4 and facilitate earlier linkage to ART in a systematic review summarizing evidence on the effectiveness of strategies to increase men’s HIV-testing in sub-Saharan Africa [47]. Males’ involvement in antenatal care by escorting their female partners is an approach that should be encouraged.
Although the overall proportion of late presenters in this review were aged 36 and above the pooled adjusted Odd ratio from four studies analyzed did not statistically favor any age category. Fomundam, Lahuerta, Sun and Palmer found that older age (46-55 years) was associated with late presentation to care in sub-Saharan Africa as well as in higher income countries. Low level of HIV knowledge among subjects aged 50 and above has been mentioned as a contributing factor to late presentation to HIV care [48-50].
From this review it appears that not being married was associated with presenting to HIV care late, although the pooled adjusted odd ratio from two studies was not statistically important. Findings from one study done in four sub–Saharan African countries revealed that those who were not married had higher odds of presenting to HIV care late [49]. This could be due to the lack of psychosocial support and non-disclosure of HIV status when one has no life partner. A Californian survey, in the USA found that married subjects were more likely to be convinced by their partner to seek health care than unmarried (W A Norcross et al, 1996). Promoting HIV couple counseling and testing is likely to increase the number of males’ partner accessing HIV services.
It appears that being unemployed was associated with late presentation to care; similar findings were seen in the Italian cohort study [39]. It is assumed that unemployment might negatively impact on the socioeconomic status of HIV-infected individuals. Studies have shown that there is a strong association between low socioeconomic status and increased risk of HIV disease progression [51]. This study, therefore, refutes the finding from [46].
The association between area of residence, distance from home to clinic and late presentation to HIV care could not be established in this review. Only one study showed that residing in per urban area was associated with likelihood of presenting to care late when compared to rural and urban residents. Those who travel long distance to reach the clinic were not found to be at increased risk of late presentation to care when compared to those living within the clinic vicinity in this review. However, in a qualitative study from Zambia, distance from home to the clinic, transportation challenges and costs of travel were mentioned as contributing factors to disengagement from HIV care. Differentiated service delivery consisting of various community-based approaches to bring the service near the community has been highly recommended by WHO as remedial solution to long distance between the clinic and the community.
The level of education was not identified to be a predictor for late presentation to HIV care in this review. This finding is similar to that from Nigeria that observed that educational attainments and the employment status had no impact on health-seeking behaviour. However, this is contrary to findings from Ethiopia that found that people with no education were more likely to present late for ART initiation when compared to those who hold a certificate. Studies showed that persons with low health literacy also had limited knowledge about HIV disease; however, it has not been established that low HIV literacy leads to late presentation to care or advanced diseases.
Wawrzyniak et al. suggest measuring health literacy specific to HIV/AIDS, aside from level of education especially when HIV-related health behaviours and health outcomes are of interest.
Conclusion
Late presentation to HIV care remains a high among adults living with HIV in sub-Saharan Africa. Being male, aged 36 and above and not married was found to be significantly associated with high odds of late presentation to HIV care. Strategies that allow early HIV detection and innovative approaches targeting men are needed to achieve the health benefit and expected HIV program outcome of the treat all policy.
The time period between the treat all guideline adoption, its implementation in most sub-Saharan countries and this review was not sufficient to gather a larger number of publications. More recent data on new ART enrolees is needed to inform estimates of the current prevalence of late presentation to HIV care.
Study limitation
It is important to bear in mind the scarcity of studies on late presentation to HIV care conducted after the adoption of the WHO treat all guideline in sub-Saharan Africa when interpreting findings of this review. All included studies were conducted in sub-Saharan, therefore generalizability of finding from this review cannot be applied to other regions or settings.
Some included studies did not have patient-level characteristics, such as education, marital status and socioeconomic status this could not allow us to identify the association between those characteristics and late presentation to care.
All studies were screening, reviewed and assessed by one author, however this was done with strict reference to the protocol in the aim of remaining objective.
The studies included were of various design and different outcome measures; this could result in a degree of heterogeneity. The I squared test for heterogeneity was significantly high, leading to several review limitations, hence random effect model was used to address this.
References
- https://www.hiv.gov/hiv-basics/overview/history/hiv-and-aids-timeline
- Margaret A, Richman DD, Grieco MH, Gottlieb MS, Volberding PA, et al. (1987) The Efficacy of Azidothymidine (AZT) in the Treatment of Patients with AIDS and AIDS-Related Complex. N Engl J Med 317:185-191
- Richman DD, Fischl MA, Grieco MH, Volberding PA, Laskin OL, et al. (2010) The development of antiretroviral therapy and its impact on the HIV-1/AIDS pandemic. Antiviral Res 85:1-18
- Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, et al. (1997) A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med 337:725-733
- McNaghten AD, Hanson DL, Jones JL, Dworkin MS (1999) Effects of antiretroviral therapy and opportunistic illness primary chemoprophylaxis on survival after AIDS diagnosis. AIDS 13:1687-1695
- Kevin MDC, HW Jaffe, JW Curran (2012) The evolving epidemiology of HIV/AIDS. AIDS 26:1205-1213
- Cohen MS, Chen YQ, McCauley M, McCauley M, Hakim JG, et al. (2016) Antiretroviral Therapy for the Prevention of HIV-1 Transmission. N Engl J Med 375:830-839
- De Cock KM, El-Sadr WM (2013) When to start ART in Africa an urgent research priority. N Engl J Med 368:886-889
- Nandi Siegfried, OA Uthman (2010) Optimal time for initiation of antiretroviral therapy in asymptomatic, HIV-infected, treatment-naive adults. Cochrane Database Syst Rev 2010:008272
- World Health Organization (2010) Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach-2010 revision. Department of Health.
- Collins SE, Juste MJ, Koenig SP, Secours R, Ocheretina O, et al. (2015) CD4 deficit and tuberculosis risk persist with delayed antiretroviral therapy: 5-year data from CIPRA HT-001. Int J Tuberc Lung Dis 19:50-57
- Severe P, Jean Juste MA, Ambroise A (2010) Early versus Standard Antiretroviral Therapy for HIV-Infected Adults in Haiti. N Engl J Med 363:257-265
- Sterne JA, May M, Costagliola D, Phillips AN, Harris R, et al. (2009) Timing of initiationTiming of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet 373:1352-1363
- Badri M, Bekker LG, Orrell C, Pitt J, Cilliers F, et al. (2004) Initiating highly active antiretroviral in sub-Saharan Africa: an assessment of the revised World Health Organization scaling-up guidelines. AIDS 18:1159-1168
[Crossref][Googlescholar][Indexed]
- Wong KH, Lee SS, Low KHK, Wan WY (2003) Temporal Trend and Factors Associated with Late HIV Diagnosis in Hong Kong, a Low HIV Prevalence Locality. AIDS Patient Care STDS. 17:461-469
- Tymejczyk O, Brazier E, Yiannoutsos CT, Vinikoor M, Van Lettow M, et al. (2019) Changes in rapid HIV treatment initiation after national “treat all” policy adoption in 6 sub-Saharan African countries: regression discontinuity analysis. PLoS Med 16:1002822
- UNAIDS U (2014) The gap report: Adolescent girls and young women. Department of Health Sciences.
- Perriat D, Balzer L, Hayes R, Lockman S, Walsh F, et al. (2018) Comparative assessment of five trials of universal HIV testing and treatment in subSaharan Africa. J Int AIDS Soc 21:25048
- World Health Organization (2016) Progress report (2016) Prevent HIV, test and treat all: WHO support for country impact. Department of Health.
- Andrew FA, Sawadogo S, Baughman AL (2017) Trends in Prevalence of Advanced HIV Disease at Antiretroviral Therapy Enrollment-10 Countries, 2004–2015. MMWR Morb Mortal Wkly Rep 66:558-563
- Alfvén T, Erkkola T, Ghys PD, Padayachy J, Warner-Smith M, et al. (2017) Global AIDS reporting-2001 to 2015: lessons for monitoring the sustainable development goals. AIDS Behav 21:5-14
- Antinori A, Johnson M, Moreno S (2010) Report of a European Working Group on late presentation with HIV infection: recommendations and regional variation. Antivir Ther 15:31–35
- World Health Organization (?2007) WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. Department of health care.
- Enhancing the Quality and Transparency Of health Research (2009) CRD’s guidance for undertaking reviews in health care. Department of Systematic reviews in health care.
- Lifson AR, Workneh S, Hailemichael A (2019) Advanced HIV Disease among Males and Females Initiating HIV Care in Rural Ethiopia. J Int Assoc Provid AIDS Care 18:2325958219847199
- Brennan AT, Maskew M, Larson BA, Tsikhutsu I, Bii M, et al. (2019) Who is seeking antiretroviral treatment for HIV now? Characteristics of patients presenting in Kenya and South Africa in 2017-2018. J Int AIDS Soc 22:25358
- Kerschberger B, Jobanputra K, Schomaker M, Kabore SM, Teck R, et al. (2019) Feasibility of antiretroviral therapy initiation under the treat-all policy under routine conditions: a prospective cohort study from Eswatini. J Int AIDS Soc 22:25401
- Blankley S, Gashu T, Ahmad B, Belaye AK, Ringtho L, et al. (2019) Lessons learned: Retrospective assessment of outcomes and management of patients with advanced HIV disease in a semi-urban polyclinic in Epworth, Zimbabwe. PloS one 14:0214739
- Hemelaar J, Elangovan R, Yun J, Dickson-Tetteh L, Fleminger I, et al. (2019) Global and regional molecular epidemiology of HIV-1, 1990–2015: a systematic review, global survey, and trend analysis. Lancet Infect Dis 19:143-155
- Carmona S, Bor J, Nattey C (2018) Persistent High Burden of Advanced HIV Disease among Patients Seeking Care in South Africa's National HIV Program: Data from a Nationwide Laboratory Cohort. Clin Infect Dis 66:111-117
- Sogbanmu OO, Goon DT, Obi LC, Iweriebor BC (2019) Socio-demographic and clinical determinants of late presentation among patients newly diagnosed with HIV in the Eastern Cape, South Africa. Medi 98:14664
- Lilian RR, Rees K, Mabitsi M, McIntyre JA, Struthers HE, et al. (2019) Baseline CD4 and mortality trends in the South African human immunodeficiency virus programme: Analysis of routine. South Afr J HIV Med 20:963
- Van der Kop ML, Thabane L, Awiti PO, Muhula S, Ekström AM, et al. (2016) Advanced HIV disease at presentation to care in Nairobi, Kenya: Late diagnosis or delayed linkage to care? -a cross-sectional study. BMC Infect Dis 16:169
- Gesesew HA, Ward P, Woldemichael K, Mwanri L (2018) Late presentation for HIV care in Southwest Ethiopia in 2003-2015: Prevalence, trend, outcomes and risk factors. BMC Infect Dis 18:59
- Alvares Uria G, Midde M, Pakam R (2012) Factors Associated with Late Presentation of HIV and Estimation of Antiretroviral Treatment Need according to CD4 Lymphocyte Count in a Resource-Limited Setting: Data from an HIV Cohort Study in India. Interdiscip Perspect Infect Dis 2012:293795
- Monforte, M Law, W el Sadr, O Kirk, N Friis-Moller (2011) Trends in underlying causes of death in people with HIV from 1999 to 2011 (D: A:D): a multicohort collaboration. Lancet 384:241-248
- Hu XI, Liang B, Zhou C, Jiang J, Huang J, et al. (2019) HIV late presentation and advanced HIV disease among patients with newly diagnosed HIV/AIDS in Southwestern China: a large-scale cross-sectional study. AIDS Res Ther 16:6
- Jeong SJ, Italiano C, Chaiwarith R, Vanar S (2016) Late Presentation into Care of HIV Disease and Its Associated Factors in Asia: Results of TAHOD. AIDS Res Hum Retroviruses 32:255-261
- Andrew FA, Shiraishi RW, Oboho I, Ross C (2017) Trends in Prevalence of Advanced HIV Disease at Antiretroviral Therapy Enrollment-10 Countries, 2004–2015. MMWR Morb Mortal Wkly Rep 66: 558-563
- Nash D, Tymejczyk O, Gadisa T (2016) Factors associated with initiation of antiretroviral therapy in the advanced stages of HIV infection in six Ethiopian HIV clinics, 2012 to 2013. J Int AIDS Soc 19:20637
- Sun J, Liu L, Shen J, Chen P, Lu H, et al. (2017) Trends in baseline CD4 cell counts and risk factors for late antiretroviral therapy initiation among HIV-positive patients in Shanghai, a retrospective cross-sectional study. BMC Infect Dis 17:285
- Tudiver F, Talbot Y (1999) Why don't men seek help? Family physicians' perspectives on help-seeking behavior in men. J Fam Pract 48:47-52
- Olanrewaju FO, Ajayi LA, Loromeke E, Olanrewaju A, Allo T, et al. (2019) Masculinity and men’s health-seeking behaviour in Nigerian academia. Cogent Soc Sci 5:1682111
- Hensen B, Taoka S, Lewis JJ, Weiss HA, Hargreaves J, et al. (2014) Systematic review of strategies to increase men's HIV-testing in sub-Saharan Africa. AIDS 28:2133-2145
- Fomundam HN, Tesfay AR, Mushipe SA, Mosina MB, Boshielo CT, et al. (2017) Prevalence and predictors of late presentation for HIV care in South Africa. S Afr Med J 107:1058-1064
- Lahuerta M, Ue F, Hoffman S, Elul B, Kulkarni SG, et al. (2013) The problem of late ART initiation in Sub-Saharan Africa: a transient aspect of scale-up or a long-term phenomenon? J Health Care Poor Underserved 24:359-383
- Palmer AK, Cescon A, Chan K, Cooper C, Raboud JM, et al. (2014) Factors associated with late initiation of highly active antiretroviral therapy among young HIV-positive men and women aged 18 to 29 years in Canada. J Int Assoc Provid AIDS Care 13:56-62
- Ngum PA, Fon PN, Ngu RC, Verla VS, Luma HN, et al. (2017) Depression among HIV/AIDS patients on highly active antiretroviral therapy in the southwest regional hospitals of Cameroon: a cross-sectional study. Neurol Ther 6:103-114
- Zanolini A, Sikombe K, Sikazwe I, Eshun-Wilson I, Somwe P, et al. (2018) Understanding preferences for HIV care and treatment in Zambia: evidence from a discrete choice experiment among patients who have been lost to follow-up. PLoS med 15:1002636
- Beyene H (2015) National assessment: Ethiopia gender equality and the knowledge society. Addis Ababa, Ethiopia.
- Wawrzyniak AJ, Ownby RL, McCoy K, Waldrop-Valverde D (2013) Health literacy: impact on the health of HIV-infected individuals. Curr HIV/AIDS Rep 10:295-304
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences