ISSN : 2393-8862
American Journal of Pharmacology and Pharmacotherapeutics
Review on Hazardous Corticosteroids: Focus on Adverse Effects
Datta Ughade*
Department of Pharmacology, Jamia Hamdard University, New Delhi, India
- *Corresponding Author:
- Datta Ughade
Department of Pharmacology,
Jamia Hamdard University,
New Delhi,
India
Tel: 9112469093
E-mail: dattaughade1191@gmail.com
Received date: February 08, 2023, Manuscript No. IPAPP-23-15862; Editor assigned date: February 13, 2023, PreQC No. IPAPP-23-15862 (PQ); Reviewed date: February 28, 2023, QC No. IPAPP-23-15862; Revised date: April 05, 2023, Manuscript No. IPAPP-23-15862 (R); Published date: April 13, 2023, DOI: 10.36648/2393-8862.10.3.160
Citation: Ughade D (2023) Review on Hazardous Corticosteroids: Focus on Adverse Effects. Am J Pharmacol Pharmacother Vol:10 No:3
Abstract
During 1950's potential for corticosteroids therapy expanded in the management of chronic conditions. After this initial success in treating Rheumatoid arthritis, cortisone was used successfully to treat a range of other diseases as well. Various adverse effects are known to occur with their use. This review aimed to identify the most common and serious adverse effects. A search of review and research articles on the adverse effects of the corticosteroids class of steroid, as well as individual drugs from various publications made to study and analysis of various corticosteroid and their adverse effects. Data findings from the various research and review articles helped to understand the severity of the corticosteroid such as cortisone, prednisone, prednisolone, dexamethasone, methylprednisolone, fludrocortisone etc. The analysis of the more than 10 research articles and various online research publication website resulted adverse effects of the prednisone, prednisolone and dexamethasone which were responsible for the HPA axis suppression, hyperthyroidism and infection in which they produce susceptibility to the infections like varicella zoaster, aspergillus etc. There are many common adverse effects of the corticosteroid such as nausea, heartburn, headache, dizziness, fluid retention, irritability etc. Children weight gain, growth retardation and cushingoid features were most observed adverse effects due to the treatment of the prednisolone and budesonide.
Keywords
Steroid; Corticosteroids; Mineralocorticoids; Glucocorticoids; Adverse effects; Prednisone; Methylprednisolone
Introduction
Corticosteroids, often known steroids are an anti-inflammatory medicines prescribed for a wide range of conditions. They are man-made version of hormones normally produced by the adrenal glands (to small plants that sit on top of the kidney). The steroids form a group of structurally related compounds which are widely distributed in animals and plants [1]. The structures of the steroids are based on the 1,2-Cyclopentenophenthrene skeleton (Figure 1).
It consists of four fused rings. A perhydrophenanthrene (rings A, B and C) is the completely saturated derivatives of phenanthrene while D is a 5 member cyclopentane ring. Steroids are any of a class of natural or synthetic organic compounds characterized by a molecular structure of 17 carbon atoms arranged in four rings. Steroids are important in biology chemistry and medicines. Different categories of Steroids are frequently distinguished from each other by names that relate to their biological source, example-phytosterols (found in plants), adrenal steroids and bile acids.
Steroids vary from one another in the nature of attached groups, the position of the groups and the configuration of the steroids nucleus. Small modifications in the molecular structures of Steroids can produce remarkable difference in their biological activities. A steroid is a very useful and essential compound for living organisms. They found in both plants as well as animal kingdom [2]. The general steroids having found to contain partially or completely hydrogenated 17 H cyclopantaphenanthrene nucleus.
They are very much potent and give their pharmacological action at very low concentration. In steroids methyl group generally present at C-10 and C-13 and alkyl side chain present at C-17. When hydroxy group at C-3 they called sterols which activity is slightly differ from steroids [3]. This compounds (steroids and sterols) are related with number of activity in body and also use as medicinal purpose like cardiac glycoside, birth control pills, in hormone replacement therapy, anti-inflammatory action in cancer like disease and also treatment of cancer.
Literature Review
Cortical steroids are used widely for their immunosuppressant and anti-inflammatory properties. They may be used in usually or in combination with other drugs and are described in both short and long courses depending on the condition being treated and the response of the patient [4]. The short course corticosteroids adverse effects include change in mood and behavior, vomiting and sleep disturbances etc.
The long course corticosteroids adverse effect includes hypothalamic pituitary adrenal Axis suppression. This leads to increase in susceptibility to infections including viral infection such as varicella zoaster, bacterial infection such as cellulitis, and fungal infection such as candida and tinea. Many of the side effects are reversible if the medication is stopped.
Historical development
The first therapeutic use of steroids occurred in the 18th century when English physician William Withering used digitalis, compound extracted from the leaves of the common foxglove (Digitalis purpurea), to treat edema. Studies of steroids commenced in the early 19th century with investigations of the unsaponifiable (i.e., remaining undissolved after heating with excess of alkali) material, largely cholesterol, of animal fat and gallstones and of acids obtainable from bile. This early work, with which many of the noted chemists of the time were associated, led to the isolation of cholesterol and some bile acids in reasonable purity and established some significant features of their chemistry.
Since the 1800’s scientists have studied the role of the adrenal glands. At that time, Englishman Thomas Addison discovered a fatal disease caused by lesions of these glands, underlining their importance to the human body. Addison’s disease is a condition in which the patient suffers from insufficient production of hormones (cortisone) by the adrenal glands. In 1896, William Osler found that one could treat this disease using extracts of adrenal glands from animals.
Insight into the complex polycyclic steroid structure, however, came only after the beginning of the 20th century, following the consolidation of chemical theory and the development of chemical techniques by which such molecules could be broken down step by step. Arduous studies, notably by the research groups of German chemists Adolf Windaus and Heinrich Wieland, ultimately established the structures of cholesterol; of the related sterols, stigmasterol and ergosterol; and of the bile acids. Investigation of ergosterol was stimulated by the realization that it can be converted into vitamin D. Only in the final stages of this work was the arrangement of the component rings of the nucleus clarified by results obtained by pyrolytic (heat induced bond breaking) dehydrogenation and X-ray crystallography.
The first clinical evidence that an extract of animal adrenocortical tissue could counteract human adrenal failure was demonstrated in 1930. In 1930's scientists were able to produce an artificial form of testosterone to help treat men who were unable to produce enough of the hormone for normal, healthy growth [5]. During World War II it was discovered that this form of artificial testosterone could treat malnourished soldiers and make them gain weight and performance.
In 1933, a team of American chemists succeeded in isolating cortisol within extracts from the adrenal glands of animals. For the first time, a patient suffering from rheumatoid arthritis was treated with the cortisone issued from these animal’s glands. Soon after, the team observed a remarkable improvement in the patient’s health. Afterwards, several other patients were treated with similar results. Although patients were not cured, their symptoms mostly disappeared. At that time, certain people in the medical world already underlined the frequent side effects of this treatment. With the foundations of steroid chemistry firmly laid, the next decade saw the elucidation of the structures of most of the physiologically potent steroid hormones of the gonads and the adrenal cortex. Added impetus was given to steroid research when American physician Philip S. Hench and American chemist Edward C. Kendall announced in 1949 that the hitherto intractable symptoms of rheumatoid arthritis were dramatically alleviated by the adrenal hormone cortisone [6]. New routes of synthesis of steroids were developed, and many novel analogs were therapeutically tested in a variety of disease states. From these beginnings has developed a flourishing steroid pharmaceutical industry and with it a vastly expanded fundamental knowledge of steroid reactions that has influenced many other areas of chemistry.
As chemical analyses of cortical extracts proceeded, mainly in the laboratories of Kendall at the Mayo clinic and Reichstein in Zurich, it became evident that there is not one cortical hormone, but that all are steroids. By 1940 it was understood that there are two categories: Those that cause sodium and fluid retention and those that counteract shock and inflammation. Structurally the presence or lack of oxygenation at C11 on the steroid skeleton was critical [7]. In 1948 the first patient with rheumatoid arthritis was treated with cortisone and soon thereafter other rheumatologic patients received cortisone or, to stimulate native cortisone production, ACTH. In 1950, despite these negative views, the doctors and chemists responsible for the discovery of cortisone received the Nobel Prize in medicine and physiology. During the 1950’s, the potential for corticosteroid therapy expanded. After its initial success in treating rheumatoid arthritis, cortisone was used to successfully treat a range of other diseases as well.
Oral and intra-articular administration of cortisone and hydrocortisone began in 1950-51. Several lines of research to produce cortisone semi-synthetically showed some success by 1952. Between 1954 and 1958 six synthetic steroids were introduced for systemic anti-imflammatory therapy. By 1960 all of the toxic effects of chronic corticosteroid administration had been described, as well as protocols to withdraw such drugs while minimising symptoms of cortical insufficiency.
1970 the introduction of methotrexate and other antimetabolites further circumscribed the dosages and indications for corticosteroids in the rheumatic diseases.
Knowledge of the biochemistry of steroids has grown at a comparable rate, assisted by the use of radioisotopes and new analytical techniques. The metabolic pathways (sequences of chemical transformations in the body), both of synthesis and of decomposition, have become known in considerable detail for most steroids present in mammals, and much research relates to control of these pathways and to the mechanisms by which steroid hormones exert their effects. The hormonal role of steroids in other organisms is also of growing interest.
The World Health Organization (WHO) announced the COVID-19 occurrence as a global pandemic in March 2020. Where, corticosteroids, i.e., dexamethasone, methylprednisolone, hydrocortisone and prednisone are used alone or in combination for the treatment of moderate, severe and critically infected COVID-19 atients who are hospitalized and require supplemental oxygen as per current management strategies and guidelines for COVID-19 published by the national institutes of health [8].
Types of steroid
Corticosteroids, are available in different forms including, tablets, injections, nasal sprays, lotions, gels and can be administered in numerous ways and corticosteroids, or creams. Corticosteroids are classified as glucocorticoids and mineralocorticoids (Table 1).
| Corticosteroids | |||
|---|---|---|---|
| Glucocorticosteroids | Mineralocorticosteroids | ||
| Natural | Synthetic | Natural | Synthetic |
| Cortisone Hydrocortisone |
Prednisone Prednisolone Triamsinolol Betamethasone Dexamethasone |
Aldesterone Deoxycorticosteroids |
Fludrocortisone |
Table 1: Classification of corticosteroids.
Glucocorticoids suppress inflammation and assist in the breakdown of fats and carbohydrates and protein. Mineralocorticoids regulate the balance of salt and water in the body (Table 2).
| Corticoids | Half-life | Compound | Gluco. | Mineralo. | Equiv. dose (anti-inflammatory) |
|---|---|---|---|---|---|
| Glucocorticoids | Short acting (biological t½<12 hr) | 1. Hydrocortisone | 1 | 1 | 20 mg |
| Intermediate acting (Biological t½ 12-36 hr) | 2. Prednisolone | 4 | 0.8 | 5 mg | |
| 3. Methyl-Prednisolone | 5 | 0.5 | |||
| 4. Triamcinolone | 5 | 0 | 4 mg | ||
| 5. Deflazacort | 3-4 | 0 | 6 mg | ||
| Long acting (Biological t½>36 hr) | 6. Dexamethasone | 25 | 0 | 0.75 mg | |
| 7. Betamethasone | 25 | 0 | 0.75 mg | ||
| Mineralocorticoids | 8. Desoxycortico-sterone Acetate (DOCA) | 0 | 100 | Equiv. Salt Retaini. Dose | |
| 9. Fludrocortisone | 10 | 150 | 2.5 mg (sublingual) | ||
| 10. Aldosterone | 0.3 | 3000 | 0.2 not used clinically |
Table 2: Relative activity of systemic corticosteroids.
Marketed steroids
Prednisone: Prednisone is a man-made anti-inflammatory glucocorticoids derived from cortisone. It is biologically non-reactive and it is converted to prednisolone in the liver (Figure 2).
Prednisone is an FDA approved, corticosteroid indicated as an anti-inflammatory or immunosuppressive agent to treat a broad range of diseases, including immunosuppressive, dermatologic, allergic states, ophthalmic, respiratory, hematologic, neoplastic, gastrointestinal and acute exacerbations of multiple sclerosis.
Mechanism of action: Prednisone decreases inflammation via suppression of the migration of polymorphonuclear leukocytes and reversing increased capillary permeability. It also suppresses the immune system by reducing the activity and the volume of the immune system. The antineoplastic effects may correlate with the inhibition of glucose transport, phosphorylation, or induction of cell death in immature lymphocytes [9]. It may have antiemetic effects by blocking the cerebral innervation of the emetic center via inhibition of prostaglandin.
Prednisone is a prodrug to prednisolone, which mediates its glucocorticoid effects. Prednisone is a synthetic glucocorticoid that has both anti-inflammatory and immunomodulating properties.
After cell surface receptor attachment and entry into the cell, prednisone enters the nucleus, binds, and activates specific nuclear receptors, resulting in altered gene expression and inhibition of proinflammatory cytokine production [10]. This agent decreases the number of circulating lymphocytes, inducing cell differentiation, and stimulates apoptosis in sensitive tumor cell populations.
The effects of glucocorticoids are su bject to mediation by mechanisms that alter DNA replication within the nucleus.
Pharmacology: Prednisone is a synthetic glucocorticoid used for its anti-inflammatory and immunosuppressive properties. Prednisone is a prodrug; it is metabolised in the liver by 11-β-HSD to prednisolone, the active drug. Prednisone has no substantial biological effects until converted via hepatic metabolism to prednisolone.
Pharmacokinetics: Prednisone is absorbed in the gastrointestinal tract and has a half-life of 2–3 hours. It has a volume of distribution of 0.4–1 L/kg. The drug is cleared by hepatic metabolism using cytochrome P450 enzymes. Metabolites are excreted in the bile and urine.
Adverse effect: Prednisone treatment to various diseases shows many adverse effects such as hyperglycemia, insomnia, increased appetite, hypertension, osteoporosis, cataracts, edema, adrenal suppression; delay wound healing, pain, nausea, vomiting, drowsiness, dizziness, headache, skin fragility, weight gain, increase risk of infections and fractures, hypertension, dyslipidemia.
Brand name: Deltasone, Rayos, Winpred.
Methylprednisolone
Methylprednisolone is a synthetic glucocorticoid, well known for its anti-inflammatory and immunosuppressive effects. Chemically, methylprednisolone is a synthetic pregnane steroid derived from hydrocortisone and prednisolone, belonging to a class of synthetic glucocorticoids (Figure 3).
Methylprednisolone has a higher affinity to glucocorticoid receptors than to mineralocorticoid receptors. The cellular functions of methylprednisolone are to regulate homeostasis, metabolism, development, cognition, and inflammation. Methylprednisolone is on the WHO's list of essential medicines for its effects against lymphoid leukemia.
Methylprednisolone exhibits pleiotropic effects on a variety of physiological mechanisms [11]. Methylprednisolone is dependent on its association with intracellular glucocorticoid receptors, and to a lesser extent, mineralocorticoid receptors.
By mimicking structurally and functionally like endogenous corticosteroid methylprednisolone act upon HPA axis in a similar fashion. In metabolism, methylprednisolone is similar to endogenous steroids but differ from affinity for glucocorticoids and mineralocorticoids receptors, affinity for protein binding, rate of elimination, and metabolic products.
Mechanism of action: Methylprednisolone diffuses passively across the cellular membrane and binds to the intracellular glucocorticoid receptor. This complex translocate into the nucleus, where it interacts with specific DNA sequences, resulting in either enhancement or suppression of transcription of particular genes [12]. The methylprednisolone-glucocorticoid receptor complex binds and blocks promoter sites of proinflammatory genes, promotes expression of anti-inflammatory gene products, and inhibits the synthesis of inflammatory cytokines, mainly by blocking the function of transcription factors, such as Nuclear Factor-Kappa-B (NF-kB). Like the rest of the corticosteroids, methylprednisolone also suppresses the synthesis of Cyclooxygenase (COX)-2, responsible for the production of prostaglandins in damaged tissue leading to the inflammation cascade.
By reversing capillary permeability, suppressing the migration of fibroblasts and polymorphonuclear leukocytes, controlling the rate of protein synthesis, and stabilizing lysosomes at the cellular level, methylprednisolone may control or prevent inflammation through these actions as well.
Methylprednisolone inhibits cell mediated immunologic functions, especially those dependent on lymphocytes. Glucocorticoid administration results in neutrophilic leukocytosis, smaller elevations in monocytes, dramatic reductions in circulating eosinophils, and lesser reductions in lymphocytes. The use of methylprednisolone and other glucocorticoids results in a reduced ability of leukocytes to adhere to vascular endothelium and exit from the circulation. Glucocorticoids impair a variety of T cell functions, and moderate to high doses induce T cell apoptosis while keeping B cell function and antibody production preserved.
Tissue specific responses to steroids can occur by the presence in each tissue of specific protein regulators controlling the interaction between the hormone receptor complex and particular DNA response elements. This activity leads to a wide array of gene expression and physiological responses by corticosteroids.
Pharmacokinetics: Methylprednisolone is approved for oral and parenteral administration. Methylprednisolone for oral administration is available in a tablet formulation in 2 mg, 4 mg, 8 mg, 16 mg or 32 mg strengths. Both methylprednisolone acetate and methylprednisolone succinate are approved for intramuscular injection. Depo-Medrol is additionally approved for intralesional, intra-articular and soft tissue injections. Depo-Medrol is available as sterile aqueous solution in 20 mg/mL, 40 mg/mL, or 80 mg/mL strengths. Solu-Medrol is the only derivative of methylprednisolone that is approved for intravenous infusion, as the sterile powder is soluble in water and can be mixed with a diluent. Strengths vary from 40 mg to 2 g.
Synthetic glucocorticoids are similar to endogenous steroids in metabolism, but differ in affinity for glucocorticoid and mineralocorticoid receptors, affinity for protein binding, rate of elimination, and metabolic products.
Oral methylprednisolone is readily absorbed from the gastrointestinal tract with a bioavailability of 89.9%. In contrast to endogenous GCs, methylprednisolone does not bind to the glycoprotein transcortin (Corticosteroid Binding Globulin, CBG) but does have moderate protein binding to albumin. Thus, pharmacokinetics of methylprednisolone is linear and shows no dose dependency. Patients exhibiting low albumin concentrations are at risk for adverse effects during glucocorticoid therapy. Oral methylprednisolone has a moderate distribution into tissue at 1.38 L/kg.
Pharmacodynamics: Methylprednisolone is a synthetic Glucocorticoid (GCs) that exhibits pleiotropic effects on a variety of physiological mechanisms. However, they have been prescribed extensively for their effects on inflammation and immunity. The effect of synthetic glucocorticoids, such as methylprednisolone, is dependent on its association with intracellular Glucocorticoid Receptors (GRs), and to a lesser extent, Mineralocorticoid Receptors (MRs). GRs are widely distributed in contrast to MRs that shows a restricted tissue distribution. By this mechanism, the ligand bound receptors translocates to the nucleus and modulate gene expression.
Methylprednisolone is primarily eliminated by hepatic metabolism and renal excretion of metabolites; with renal excretion of unchanged methylprednisolone at only 1.3%–9.2%. Methylprednisolone can be interconverted with methylprednisone.
Adverse effects: Methylprednisone treatment to various diseases shows many adverse effects such as flushing, aspergillus infection, zygomycetes infection, growth retardation in children, psychological effects, iatrogenic cushing syndrome, weight gain, myopathy, osteoporosis, increased risk of infection and hypertension,
Brand name: Depo-medrol, Depo-medrol with Lidocaine, Hybrisil, Medrol, Medroloan Suik, Readysharp-p40, Readysharpp80.
Dexamethasone
Dexamethasone is a glucocorticoid medication used to treat rheumatic problems, a number of skin diseases, severe allergies, asthma, chronic obstructive lung disease, croup, brain swelling, eye pain following eye surgery, superior vena cava syndrome (a complication of some forms of cancer), and along with antibiotics in tuberculosis (Figure 4).
Dexamethasone has a wide variety of uses in the medical field. As a treatment, dexamethasone has been useful in treating acute exacerbation of multiple sclerosis, allergies, cerebral edema, inflammation, and shock. Patients with COVID-19, asthma, atopic and contact dermatitis, and drug hypersensitivity reactions have benefited from dexamethasone.
Mechanism of action: Dexamethasone is a potent glucocorticoid with very little, if any, mineralocorticoid activity. Dexamethasone’s effect on the body occurs in a variety of ways. It works by suppressing the migration of neutrophils and decreasing lymphocyte colony proliferation. The capillary membrane becomes less permeable, as well. Lysosomal membranes have increased stability. There are higher concentrations of vitamin A compounds in the serum, prostaglandin, and some cytokines (interleukin-1, interleukin-12, interleukin-18, tumor necrosis factor, interferon-gamma, and granulocyte-macrophage colony-stimulating factor) become inhibited? Increased surfactant levels and improved pulmonary circulation have also been shown with dexamethasone. Dexamethasone is metabolized by the liver and excreted in the urine mainly.
Pharmacokinetics: According to the manufacturer's labeling, the pharmacokinetics of oral dexamethasone is dose-proportional between the dose range of 0.5 to 40 mg.
Absorption: Dexamethasone median time to peak concentrations (Tmax) is 1 hour (range: 0.5 to 4 hours). A high fat, high calorie diet decreased Cmax by 23% of a single 20 mg dose of dexamethasone.
Distribution: Dexamethasone is about 77% bound to human plasma proteins in vitro.
Elimination: The mean terminal half-life of dexamethasone is 4 hours (18%), and oral clearance is 15.7 L/hr following a single dose of dexamethasone.
Metabolism: Dexamethasone is metabolized by CYP3A4.
Excretion: Renal excretion of dexamethasone is less than 10% of total body clearance. Less than 10% of dexamethasone is excreted in the urine.
Adverse effects: Although dexamethasone is generally well tolerated, it does have its drawbacks as a medication. The most frequently reported adverse effect by patients is the presence of insomnia after use. Other frequent adverse effects include acne, indigestion, fluid retention, electrolyte imbalances, weight gain, increased appetite, anorexia, nausea, vomiting, acne, agitation, and depression. There have been reports of adrenal suppression, arrhythmias, spermatogenic changes, glaucoma, hypokalemia, pulmonary edema, pseudotumor cerebri and increased intracranial pressure. Steroid-induced osteonecrosis of the femoral head (long-term treatment).
Hepatotoxicity: Likelihood score: A well-established cause of liver injury when given in high doses, due to reactivation of hepatitis B or a hepatocellular injury after high dose treatment.
Brand name: Baycadron, Ciprodex, Decadron, Dexamethasone Intensol, Dextenza, Dioptrol, Hexadrol, Hidex 6-day Taper, Maxidex, Maxitrol, Neofordex.
Fludrocortisone
Fludrocortisone is a synthetic adrenal steroid with high mineralocorticoid activity that is a commonly used drug to treat adrenocortical insufficiency. Fludrocortisone, sold under the brand name Florinef, among others, is a corticosteroid used to treat adrenogenital syndrome, postural hypotension, and adrenal insufficiency. In adrenal insufficiency, it is generally taken together with hydrocortisone. Fludrocortisone is taken by mouth and is most commonly used in its acetate form (Figure 5).
Mechanism of action: Fludrocortisone acetate is an inactive pro-drug requiring hydrolyzation by esterases or pseudo-esterases in the liver and other body fluids. Fludrocortisone is a synthetic adrenal steroid with high mineralocorticoid activity and is practically devoid of glucocorticoid effect. As an analog, its mechanism of action and the permissive effects on α-adrenoreceptors are similar to the endogenous mineralocorticoid. High lipid solubility allows fludrocortisone to easily penetrate the plasma membrane and bind with its cytoplasmic receptor.
Upon binding, receptor complex translocates to the nucleus and initiates the transcription of responsive genes to exert its effects. The primary sites of fludrocortisone's effect are the distal convoluted tubules and collecting ducts, where it enhances Na+ and water ostatic hypotension management is due to potentiating the pressor effects of various endogenous vasoconstrictors such as norepinephrine and angiotensin II.
Pharmacology: Fludrocortisone is a corticosteroid and acts as a powerful mineralocorticoid, along with some additional but comparatively very weak glucocorticoid activity. Relative to cortisol, it is said to have 10 times the glucocorticoid potency but 250 to 800 times the mineralocorticoid potency. Fludrocortisone acetate is a prodrug of fludrocortisone, which is the active form of the drug. Plasma renin, sodium, and potassium are checked through blood tests to verify that the correct dosage is reached.
Adverse effects: Most of the side effect of fludrocortisone is related to mineralocorticoid activity. If this drug is used along with glucocorticoid or other related drugs, the adverse effect increases. Some of the more common or significant side effects appear in the list below:
Cardiovascular system: Hypertension (dose dependent iatrogenic has been described as an adverse effect on children using fludrocortisone for CAH, which correlates with plasma renin activity, fluid retention, and edema (May cause swelling of lower limbs), congestive heart failure. Nervous System: Headache, increased intracranial pressure, vertigo, change in behavior, convulsion Gastrointestinal: May impair gastric protective barrier and cause stomach ulcer, perforation. Endocrine: Menstrual abnormalities, cushingoid features, growth delay in a child, acute adrenal insufficiency in times of stress.
Musculoskeletal: Can cause muscle weakness, can reduce muscle mass, increase risk of osteoporosis and pathological fracture, vertebral compression, necrosis of femoral head.
• Dermatologic: Increased sweating, poor wound healing,
hirsutism, thinning of the skin, severe allergic reaction.
• Ophthalmic: Cataracts, glaucoma.
• Metabolic: Elevated blood and urine glucose, weight gain.
• Electrolytes disturbance: Severe hypokalemia, metabolic
alkalosis.
Brand name: Florinef
Hydrocortisone
Hydrocortisone is the name for the hormone cortisol when supplied as a medication. Uses include conditions such as adrenocortical insufficiency, adrenogenital syndrome, high blood calcium, thyroiditis, rheumatoid arthritis, dermatitis, asthma, and COPD. It is the treatment of choice for adrenocortical insufficiency. It can be given by mouth, topically, or by injection. Stopping treatment after long-term use should be done slowly (Figure 6).
Pharmacodynamics: Hydrocortisone binds to the glucocorticoid receptor leading to downstream effects such as inhibition of phospholipase A2, NF-kappa B, other inflammatory transcription factors, and the promotion of anti-inflammatory genes. Hydrocortisone has a wide therapeutic index and a moderate duration of action. Patients should stop taking the medication if irritation or sensitization occurs.
Pharmacodynamics: Hydrocortisone is a corticosteroid, acting specifically as both a glucocorticoid and as a mineralocorticoid. That is, it is an agonist of the glucocorticoid and mineralocorticoid receptors.
Hydrocortisone has low potency relative to synthetic corticosteroids. Compared to hydrocortisone, prednisolone is about 4 times as potent and dexamethasone about 40 times as potent in terms of anti-inflammatory effect. Prednisolone can also be used as cortisol replacement, and at replacement dose levels (rather than anti-inflammatory levels), prednisolone is about eight times more potent than cortisol.
Pharmacokinetics: Most cortisol in the blood (all but about 4%) is bound to proteins, including Corticosteroid Binding Globulin (CBG) and serum albumin. Free cortisol passes easily through cellular membranes, explaining its 100% bioavailability after oral administration. Inside cells it interacts with corticosteroid receptors.
Mechanism of action: The short term effects of corticosteroids are decreased vasodilation and permeability of capillaries, as well as decreased leukocyte migration to sites of inflammation. Corticosteroids binding to the glucocorticoid receptor mediates changes in gene expression that lead to multiple downstream effects over hours to days.
Glucocorticoids inhibit neutrophil apoptosis and demargination; they inhibit phospholipase A2, which decreases the formation of arachidonic acid derivatives; they inhibit NF-Kappa B and other inflammatory transcription factors; they promote anti-inflammatory genes like interleukin-10.
Lower doses of corticosteroids provide an anti-inflammatory effect, while higher doses are immunosuppressive. High doses of glucocorticoids for an extended period bind to the mineralocorticoid receptor, raising sodium levels and decreasing potassium levels.
Adverse effects: Data regarding acute overdoses of glucocorticoids are rare.
Chronic high doses of glucocorticoids can lead to the development of cataract, glaucoma, hypertension, myopathy, hyperlipidemia, peptic ulcer, mood changes, psychosis, dermal atrophy, hypertrichosis, immune suppression and growth suppression.
Brand name: Ala-cort, Ala-scalp, Alcortin, Alkindi, Anusol HC, Aquanil HC, Casporyn HC, Cipro, Cipro HC, Colocort, Cortaid, Cortane-B, Cortef, Cortenema, Cortisporin, Cortizone-10, Dermacort, Dermarest Eczema, Dermazene, Home Papkit, Hydroskin.
Cortisone
Cortisone is a pregnene (21-carbon) steroid hormone. It is a naturally occurring corticosteroid metabolite that is also used as a pharmaceutical prodrug; it is not synthesized in the adrenal glands. Cortisol is converted by the action of the enzyme corticosteroid 11-beta-dehydrogenase isozyme 2 into the inactive metabolite cortisone, particularly in the kidneys. Cortisone is converted back to the active steroid cortisol by the action of the enzyme 11β-Hydroxysteroid dehydrogenase type 1, particularly in the liver (Figure 7).
Cortisone can be administered as a prodrug, meaning it has to be converted by the body (specifically the liver, converting it into cortisol) after administration to be effective. It is used to treat a variety of ailments and can be administered intravenously, orally, intra-articularly (into a joint), or transcutaneously. Cortisone suppresses various elements of the immune system, thus reducing inflammation and attendant pain and swelling. Risks exist, in particular in the long-term use of cortisone.
Mechanism of action: The short term effects of corticosteroids are decreased vasodilation and permeability of capillaries, as well as decreased leukocyte migration to sites of inflammation. Corticosteroids binding to the glucocorticoid receptor mediates changes in gene expression that lead to multiple downstream effects over hours to days. 3 glucocorticoids inhibit neutrophil apoptosis and demargination; they inhibit phospholipase A2, which decreases the formation of arachidonic acid derivatives; they inhibit NF-Kappa B and other inflammatory transcription factors; they promote anti-inflammatory genes like interleukin-10. Lower doses of corticosteroids provide an anti-inflammatory effect, while higher doses are immunosuppressive. High doses of glucocorticoids for an extended period bind to the mineralocorticoid receptor, raising sodium levels and decreasing potassium levels.
Pharmacodynamics: Corticosteroids bind to the glucocorticoid receptor, inhibiting pro-inflammatory signals, and promoting anti-inflammatory signals. The duration of action is moderate as it is generally given once daily. Corticosteroids have a wide therapeutic window as patients may require doses that are multiples of what the body naturally produces. Patients taking corticosteroids should be counselled regarding the risk of hypothalamic-pituitary-adrenal axis suppression and increased susceptibility to infections.
Adverse effects: Data regarding acute overdoses of glucocorticoids are rare. Chronic high doses of glucocorticoids can lead to the development of cataract, glaucoma, hypertension, water retention, hyperlipidemia, peptic ulcer, pancreatitis, myopathy, osteoporosis, mood changes, psychosis, dermal atrophy, allergy, acne, hypertrichosis, immune suppression, decreased resistance to infection, moon face, hyperglycemia, hypocalcemia, hypophosphatemia, metabolic acidosis, growth suppression, and secondary adrenal insufficiency. Overdose may be treated by adjusting the dose or stopping the corticosteroid as well as initiating symptomatic and supportive treatment.
Brand name: Celestone, Cortone Acetate, Cotolone, Decadron, Deltasone.
Betamethasone
Betamethasone is a systemic corticosteroid used to relieve inflammation in various conditions, including but not limited to allergic states, dermatologic disorders, gastrointestinal diseases, and hematological disorders (Figure 8).
It is also used for a number of diseases including:
Rheumatic disorders such as rheumatoid arthritis and systemic lupus erythematosus, skin diseases such as dermatitis and psoriasis and allergic conditions such as asthma and angioedema, development of the baby's lungs, crohn's disease, cancers such as leukemia, adrenocortical insufficiency. For the treatment of skin irritation, itching, and eczema topical cream of betamethasone medication can be used. It is also used for the local psoriasis. Betamethasone is also used prior to delivery of a preterm baby to help prepare the lungs for breathing.
Mechanism of action: Betamethasone can act through nongenomic and genomic pathways. 3messenger the genomic pathway is slower and occurs when glucocorticoids activate glucocorticoid receptors and initiate downstream effects that promote transcription of anti-inflammatory genes including Phosphoenolpyruvate Carboxykinase (PEPCK), IL-1 receptor antagonist, and Tyrosine Amino Transferase (TAT). On the other hand, the non-genomic pathway is able to elicit a quicker response by modulating T-cell, platelet and monocyte activity through the use of existing membrane-bound receptors and second messengers.
Pharmacology: Corticosteroids bind to the glucocorticoid receptor inhibiting pro-inflammatory signals, while promoting anti-inflammatory signals. Corticosteroids have a wide therapeutic window as patients may require doses that are multiples of what the body naturally produces. Patients who require long-term treatment with a corticosteroid should be counselled regarding the risk of hypothalamic-pituitary-adrenal axis suppression and increased susceptibility to infections.
Adverse effects: Some of the common side effects of betamethasone include:
• Euphoria, depression, adrenal suppression, hypertension,
groupings of fine blood vessels becoming prominent under the
skin, excessive hair growth (hypertrichosis).
• Serious side effects include: Increased risk of infection, muscle
weakness and severe allergic reactions and psychosis.
• Long-term use of betamethasone can leads to HPA axis
suppression. Suddenly stoppage of betamethasone
medication can leads to danger for long term use.
Prolonged use of this medicine on extensive areas of skin, result in enough corticosteroid being absorbed to have side effects on other parts of the body; for example, by causing a decrease in the production of natural hormones by the adrenal glands. When injected into the epidural space or the spine, it may cause serious side effects like loss of vision, stroke, and paralysis.
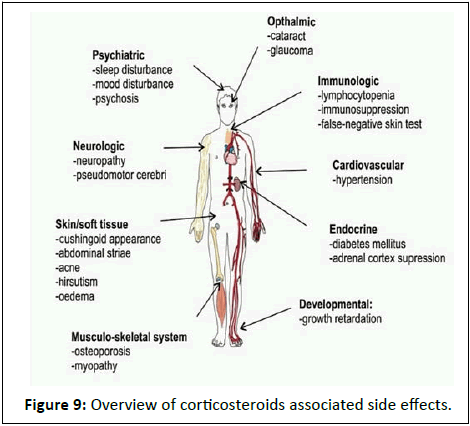
Brand names: Betaderm, Betaloan Suik, Beteflam, Celestoderm, Celestone Soluspan, Dermacinrx Therazole Pak, Diprolene, Diprosalic, Diprosone, Dovobet, Enstilar, Fucibet, Lotriderm, Lotrisone, Luxiq, Marbeta, Rivasone, Rolene, Rosone. With the short course as well as long course corticosteroid therapy leads to induction of the various adverse effects such as psychiatric, ophthalmic, cardiovascular etc. (Figure 9).
Important findings of adverse effect
Dexamethasone seems like one of the most powerful corticosteroid, even short term of the dexamethasone treatment to the various diseases leads to the contribution in the potential adverse effects such as neuromuscular weakness, psychiatric symptoms and even iatrogenic cushing syndrome. Dexamethasone have effect on psychiatric symptoms where there is very less data existing in the recovering methods of the psychiatric symptoms.
Corticoticosteroids affect on different body parts with various adverse effects such as body, blood, bone, eye, skin, gastroinstestinal, adrenal gland, muscle, cardiovascular, infectionous, psychiatric etc. (Table 3).
| Affected body part | Side effect |
|---|---|
| Body | Moon facies/buffalo hump/truncal obesity |
| Blood | Increased blood sugar |
| Bone | Decreased bone density/ avascular necrosis (most commonly in head of femur) |
| Eye | Cataracts/glaucoma |
| Skin | Skin fragility/bruising/hirsutism |
| Gastrointestinal | Gastritis |
| Adrenal gland | Multiple systemic effects, blood pressure changes, water retention, lack of stress response |
| Muscle | Atrophy of proximal limb muscles |
| Cadiovascular | Potential increased risk of heart attack, increased blood pressure, myocardial infarction and cerebrovascular disease |
| Infection | Bacterial, fungal and viral infection |
| Psychiatric | Mania/depression/aggressiveness, Anxiety/insomnia/restlessness |
Table 3: Affected body part and respective side effects due to corticosteroids.
Recently a large scale global statistical analysis revealed that while males and females are at equivalent risk of SARS-CoV-2 infection, male sex is associated with the higher risk of severe diseases that means male patients are more likely to receive the dexamethasone treatment and suffer from ONFH. Excessive use of dexamethasone can promote apoptosis through multiple signaling mechanisms. Induced osteoporosis can be caused by the suppression of the cononial wnt signal.
Dexamethasone can down regulate the expression of matrix marker biglycan and lack of biglycan contributes to the reduction in tribecular bone volume, mineral deposition rate and bone formation rate. A cumulative doses of prednisolone >100 mg or dexamethasone >140 mg and previous history of peptic ulceration leads to the advance cancer itself. Dexamethasone treatment can cause skeleton muscle atrophy which leading to severe muscle weakness, inactivity and reduced quality of life for the patient.
Recent study has found that dexamethasone increase synthesis of catecholamine by inducing the transcription of the rate limiting enzyme tyrosine hydroxylase and excessive catecholamine level induces vasoconstriction. Increasing calcium influx in vascular smooth muscle is also a way in which dexamethasone causes hypertension.
Hypersensitivity to hydrocortisone is well documented. Reports include urticaria, angioedema, bronchospasm, and anaphylaxis. A case of urticaria and branchospasm with dexamethasone has also been reported.
Bensley, et al. claim that cardiomyocyte proliferation can be inhibited by premature birth, which may adversely affect heart growth, cardiac function, functional reserve, and repair ability throughout postnatal life. Therefore, dexamethasone treatment may bring about more serious damage to the heart in preterm infants.
Studies found that in a group of asthmatic patients who received prednisolone >40 mg per day, two third had detectable hip flexor weakness when formally tested. In contract, weakness was detectable in only 3% receiving 30 mg/day or less. Animal studies also suggest that dexamethasone can cause more atrophy and weakness than hydrocortisone and prednisolone.
All long term oral corticosteroid therapies are independent of the dose, as even low doses of oral corticosteroid elevate the risk of comorbidity and complications.
Recent evidence suggested that dexamethasone treatment in adults could result in hypertension, pathologic cardiac remodeling, cardiac hypertrophy associated with maladaptive remodeling and ultimate ventricular dysfunction. Cardiac hypertrophy, myocardial fibrosis, hypoxia and ventricular dysfunction via angiotensin-2 signaling pathway can caused by the excessive treatment of dexamethasone.
Facial and upper trunk flushing has been reported with intra corticular injection of triamcinolone acetonide 40 mg which is equivalent to methylprednisolone 40 mg. Short courses of dexamethasone leads to the a vascular bone necrosis with a total cumulative dose of about 200 mg following neurosurgery.
It has been reported that dexamethasone makes the gastric mucosa susceptible to ulceration, but the mechanism of ulcerogenic action remains unclear. Specifically the earlier study indicated that dexamethasone dimishes gastro protection and damages the mucosa by inhibiting the activity of prostaglandin synthase and peroxides respectively.
Results
Prednisolone and budesonide are the most used corticosteroids in children in long course of corticosteroid. Weight gain was the most common side effects. This was not however significant different to that reported for short corticosteroid treatment.
The risk of these adverse effects was greater with prednisolone than budesonide in children. The risk of growth retardation is the greater with prednisolone than IV methylprednisolone which is the second most frequent observed side effect in children. Also the risk was significantly greater with boys than girls. Cushingoid features are the 3rd most frequently observed side effect caused by oral corticosteroid which has greater risk with prednisolone than budesonide (Table 4).
| Adverse effect | More risky drug |
|---|---|
| HPA suppression | Dexamethasone>Prednisolone |
| Weight gain | Prednisolone>Budesonide |
| Growth retardation | Prednisolone>IV methylprednisolone |
| Cushing’s syndrome | Prednisolone>Budesonide |
Table 4: Adverse effect and more risky drug.
There is an increased risk of hypokalemia if high doses of corticosteroids are described with beta 2 adrenoreceptor agonist or carbenoxolone. By thinning the circumference smooth muscle and making the bowel wall more vulnerable dexamethasone could increase the risk of bowel perforation. In a controlled trial in 40 terminal cancer patients of methylprednisolone 32 mg per day for 2 weeks, appetite increased in 77%, mood in 71% and activity in 68% of patients.
An excessive prednisone, dexamethasone and other glucocorticoids therapy can cause sodium retention, hypokalemia, and hypertension by influencing these symptoms in different ways. The resulting hypertension can increase the risk of bleeding, in particular of gastric bleeding, which might even become life threatening. Hypokalemia can cause severe heart problems. The most common effects of long term dexamethasone and other glucocorticoids treatment of adrenal insufficiency treatment affect the HPA axis and cause disorders, such as iatrogenic cushing syndrome (moon face, buffalo hump, central obesity), adrenal insufficiency, and growth inhibition. The appearance of a cushingoid habitus is extremely variable; some patients seem able to tolerate 30 mg/day prednisolone, while others become cushingoid on less than one-half of this dose. Additional problems of suppression of the HPA axis represent reduced general steroid hormone production due to a decreased adrenocorticotropic hormone.
With higher doses (>0.4 mg/day), Clark, et al., showed fluticasone administration to asthmatic children via a large volume spacer caused adrenal suppression as measured by urinary cortisol. Budesonide however at the some dose did not have this effect. In a similar test in asthmatic adults comparing fluticasone and budesonide administered via metered dose inhalers, only 8.3% patients had low urinary cortisol levels with budesonide, whilst 58% of patients on fluticasone were seen to have low urinary cortisol levels.
Remarkably, even high dose inhalative treatment with glucocorticoids may results in adrenal insufficiency with a threshold dose that for beclomethasone dipropionate may lie between 800-1200 microgram/day. Dexamethasone inhibit the small intestinal growth via both increase degradation and decreases synthesis of protein. Dexamethasone treatment can be associated with the neuropsychiatric diseases and neurotoxicity. Peripheral administration of the dexamethasone induces biphasic effects on anxiety related behaviours, anxiolytic effects at low and anxiogenic effects at high doses. The risk of HPA axis suppression during long course corticosteroid was greater with dexamethasone than prednisolone in leukemia patients. Also separation was greater with oral corticosteroids than inhaled corticosteroids in asthmatic patients.
Recent evidence, however suggest that even oral dosages as low as 6 mg prednisone per day for 6 months may cause significant bone loss and may increase the rate of osteoporotic fracture within 1 year. A series of investigations by Walkovits studied impact of prednisone at dosage of 80 mg per day on 12 healthy volunteers of these volunteers 9 where observed using constructured logs to experience emotional or behavioural alterations of sub clinical nature: Depression, mood elevation, irritability, anger, insomnia, excessive talkativeness andincreased apetate.
The systemic glucocorticoid usage for more than one year, even at low dosage (equivalent to less than 5 mg/day prednisone), induces skin atrophy, ecchymosis and erosions in about 5% of patients. Catabolic effects of corticosteroids cause atrophy, striae and delayed wound healing. With the treatment of using 7.5 mg or more of prednisolone the risk of coronary heart disease, heart failure and even sudden death has been reported to be increased. High dose corticosteroid treatment makes patients vulnerable to virus, bacteria, fungus and parasite infection, and increases the risk of reactivation of latent infection such as tuberculosis. The treatment of rheumatoid arthritis by using 5–10 mg/day of prednisone or equivalent over two years was associated with an increase of mean body weight of 4%–8%.
Glucocorticoid-induced myopathy is more common with the use of dexamethasone, betamethasone and triamcinolone, than with the use of prednisone and prednisolonemost commonly observed adverse effect associated with long course oral corticosteroids were weight gain, growth retardation and cushingoid features. Weight gain was the most common side effects. This was not however significant different to that reported for short corticosteroid treatment. The risk of these adverse effects was greater with prednisolone than budesonide in children. In adult patients was associated with longer duration of corticosteroid use, even with low dose. The risk of growth retardation is the greater with prednisolone than IV methylprednisolone which is the second most frequent observed side effect in children. Also the risk was significantly greater with boys than girls. Cushingoid features are the 3rd most frequently observed side effect caused by oral corticosteroid which has greater risk with prednisolone than budesonide.
In cancer patient receiving chemotherapy, adrenal suppression and adrenal insufficiency having reported after dexamethasone use. Changes in growth hormone, insulin like growth factor, melatonin and parathyroid hormone levels are associated with dexamethasone induced endocrine disorder. Major risk factors for candidemia or aspergillosis is prednisone treatment.
Several studies from the Fred Hutchinson cancer research centre and university of Washington, demonstrate an increased risk of invasive mold infections including aspergillus and zygomycetes as well as an increased risk of death from the infections in bone marrow transplant patients receiving high dose of prednisolone or methylprednisolone (>2 mg/kg/day). Prednisone doses higher than 20 mg per day decrease the production of liutinising hormone, leading to a secondary hypogonadism state.
The secondary hypogonadism decreases testosterone production, further decreasing bone formation and increasing bone resorption. Prednisone as low as 5 mg for as little as 2 months can leads to cataracts. Cutaneous complications such as cushing syndrome, skin atrophy, striae, ecchymoses and changes in mechanical properties of the skin can be caused by prednisone or methylprednisolone.
Discussion
Steroids commonly known as corticosteroids come in a variety of forms and can be administered in numerous ways. Generally corticosteroids are classified as glucocorticoids and mineralocorticoids.
The first clinical evidence that an extract of animal adrenocortical tissue could counteract human adrenal failure was demonstrated in 1930. The WHO announced the COVID-19 occurrence as the global pandemic in March 2020, where, corticosteroids, e.g. dexamethasone, methylprednisolone, hydrocortisone, and prednisone are used alone or in combination for the treatment of moderate, severe and critically infected COVID-19 patients who are hospitalized. Corticosteroids work to reduce inflammation of suppressed immune system by obstructing various phases of the immune system's up regulation.
In this review we are discussing the some of the severe adverse effects causing due to the various corticosteroid drug taken for short time of treatment as well as for long time of treatment of various diseases. Corticosteroids used for the treatment of various diseases leads to the induction of the various adverse effects of both short course corticosteroid as well as long course corticosteroids due to the wide expression of the receptor in the body which leads to the to the functional and anatomical alterations which may responsible for observed adverse effects. Various corticosteroids such as Prednisone, prednisolone, dexamethasone, methylprednisolone, fludrocortisone etc. Induces the various adverse effects. Dexamethasone seems like one of the most powerful corticosteroids in which the dexamethasone treatment whether short term or long term leads to the occurrence of various adverse effects including neuromuscular weakness, psychiatric symptoms and iatrogenic cushing syndrome.
Dexamethasone drug used for the treatment of the cancer chemotherapy which leads to the induction of the adverse effects like adrenal suppression and adrenal insufficiency. It is 7 times more potent than the prednisolone where 2 mg is dexamethasone is approximately equivalent to the 15 mg of prednisolone.
On comparing high dose ICS given via the same device, fluticasone propionate and beclomethasone dipropionate have more of effects on the HPA axis than budesonide. Thus it can be concluded that fluticasone is more potent at inhibiting the HPA axis than budesonide and also this suppression is dose related. Fluticasone is more effective in suppressing the adrenal vs. budesonide because of higher lipophilic properties and longer half-life of fluticasone. Fluticasone is able to distribute effectively due to it's highly lipophilic nature, and it's long half-life means it is able to exert it's adrenal suppressive effects for an extended period.
Fluticasone and budesonide in COPD patients increased the risk of pneumonia. Two recent studies have further cemented the association of inhaled corticosteroids and increased risk of pneumonia. The study revealed that patients taking fluticasone and salmeterol are more likely to be admitted with pneumonia or pneumonia related events compared to those on budesonide and formoterol. All corticosteroids are associated with an increased risk of serious pneumonia infections with fluticasone the most commonly associated ICS type and having a dose related risk.
The prolonged exposure of body to the hydrocortisone leads to the hormonal disorder called cushing's syndrome. Intranasal corticosteroids may contribute to the development of serious side effects such as adrenal cortical hypofunction, cushing's syndrome and growth retardation.
HPA axis suppression, bone metabolism and susceptibility to infections are the major adverse effects caused by the various corticosteroids such as prednisone, prednisolone and dexamethasone. Corticosteroids like cortisone and hydrocortisone also induces the some of the adverse effects like fluid retention, mood swings, psychological symptoms and nausea, heartburn, headache, glaucoma, delayed in wound healing etc.
In long course of corticosteroid treatment in children weight gain, growth retardation and cushing’s syndrome are the most observed adverse effects. Prednisolone and budesonide are the most used corticosteroids in children in long course of corticosteroid. Weight gain was the most common side effects. This was not however significant different to that reported for short corticosteroid treatment. The risk of these adverse effects was greater with prednisolone than budesonide in children. The risk of growth retardation is the greater with prednisolone than IV methylprednisolone which is the second most frequent observed side effect in children. Also the risk was significantly greater with boys than girls. Cushing symptoms are the 3rd most frequently observed side effect caused by oral corticosteroid which has greater risk with prednisolone than budesonide.
Methylprednisolone shows the different adverse effects such as infections like aspergillus, zygomycetes and growth retardation in children etc. Association of corticosteroid treatment with depressive and manic syndrome is relatively well documented. Also there is a need for additional studies in the various clinical populations so that frequency and severity of this disturbance is very specific populations can be more clearly lucidated.
Conclusion
This article represents a comprehensive review of the hazardous and adverse effects of the corticosteroids. Corticosteroid remains an important part of the immunosuppressive and anti-inflammatory medications. These corticosteroids can be prescribed for the treatment of various conditions and diseases, for this reason, specific effects of these drugs including their severity and associated effects are better understood.
The use of corticosteroid such prednisone, prednisolone, methylprednisolone, dexamethasone, budesonide etc. were associated to the development of the morphological changes including cushing syndrome, skin changes, adrenal suppression, delayed wound healing, ophthalmic, myopathy, cardiovascular and psychiatric adverse effects. These effects are due to the wide expression of receptor in the body which leads to the functional and anatomical alteration which might be responsible for observed adverse effects.
Most of the adverse effects were found with the dexamethasone, prednisone, and methylprednisolone treatment while in children prednisolone and budesonide were the most used long course corticosteroids responsible for the weight gain, growth retardation and cushing’s syndrome.
References
- Benedek TG (2011) History of the development of corticosteroid therapy. Clin Exp Rheumatol 29:5-12
[Google Scholar] [PubMed]
- Patten SB, Neutel CI (2000) Corticosteroid-induced adverse psychiatric effects. Drug Saf 22:111-122
[Crossref] [Google Scholar] [PubMed]
- Schacke H, Docke WD, Asadullah K (2002) Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther 96:23-43
[Crossref] [Google Scholar] [PubMed]
- Volmer T, Effenberger T, Trautner C, Buhl R (2018) Consequences of long-term oral corticosteroid therapy and its side-effects in severe asthma in adults: A focused review of the impact data in the literature. Eur Respir J 52:1800703
[Crossref] [Google Scholar] [PubMed]
- Pandya D, Puttanna A, Balagopal V (2014) Systemic effects of inhaled corticosteroids: An overview. Open Respir Med J 8:59-65
[Crossref] [Google Scholar] [PubMed]
- Rollema C, van Roon EN, Ekhart C, van Hunsel FP, de Vries TW (2022) Adverse drug reactions of intranasal corticosteroids in the Netherlands: An analysis from the Netherlands pharmacovigilance center. Drugs Real World Outcomes 9:321-331
[Crossref] [Google Scholar] [PubMed]
- Twycross R (1994) The risks and benefits of corticosteroids in advanced cancer. Drug Saf 11:163-178
[Crossref] [Google Scholar] [PubMed]
- Aljebab F, Choonara I, Conroy S (2017) Systematic review of the toxicity of long-course oral corticosteroids in children. PloS one 12:e0170259
[Crossref] [Google Scholar] [PubMed]
- Poetker DM, Reh DD (2010) A comprehensive review of the adverse effects of systemic corticosteroids. Otolaryngol Clin North Am 43:753-768
[Crossref] [Google Scholar] [PubMed]
- Chen F, Hao L, Zhu S, Yang X, Shi W, et al. (2021) Potential adverse effects of dexamethasone therapy on COVID-19 patients: Review and recommendations. Infect Dis Ther 10:1907-1931
[Crossref] [Google Scholar] [PubMed]
- Oray M, Abu Samra K, Ebrahimiadib N, Meese H, Foster CS (2016) Long-term side effects of glucocorticoids. Expert opinion on drug safety 15:457-465
- Ocejo A, Correa R (2021) Methylprednisolone. StatPearls Publishing, StatPearls, Treasure Island.
[Google Scholar] [PubMed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences