Response of Commercial Cotton Varieties to Xanthomonas citri pv. malvacearum at Early Developmental Stages in South Texas
Enrique G. Medrano1* , Greta L. Schustera2 and Srinadh Kodalia2
1Department of Agriculture, Insect Control and Cotton Disease Research,College Station, Texas, USA
2Department of Agriculture, Agribusiness,and Environmental Sciences, Texas A and M University-Kingsville, Texas, USA
- *Corresponding Author:
- Enrique G. Medrano
Department of Agriculture,
Insect Control and Cotton Disease Research College Station,
Texas,
USA
Email:gino.medrano@usda.gov
Received Date: September 03, 2021 Accepted Date: September 17, 2021 Published Date: September 24, 2021
Citation:Medrano EG, Schustera GL,Kodalia S, (2021) Response of Commercial Cotton Varieties to Xanthomonas citri pv.Malvacearum at Early Developmental Stages in South Texas. Res J Plant Pathol Vol.4 No.4:01.
Abstract
Cotton is the most profitable non-food crop in the world. Xanthomonas citri pv. malvacearum (Xcm) is a Gram-negative prokaryotic pathogen that causes bacterial blight of cotton. Bacterial blight has been controlled for more than 50 years by breeding for host plant resistance. However, recent sporadic blight outbreaks in the US commercially grown varieties have raised concerns among growers and scientists about the possible development of resistant Xcm strains. The objective of the study was to evaluate seven commercial cotton varieties for resistance towards Xcm when inoculated at three cotton growth stages. The varieties consisted of one resistant (NG 5711), two susceptible (NG 3406 and DP 1725), and four partially resistant cotton (NG 3729, DP 1646, DP 1845 and DP 1948). All varieties were inoculated with Xcm at match head, candle, and pink flower stages at a concentration of 106 colony forming units per ml. The inoculation mixture consisted of Xcm, Silwet L-77 (0.25% v/v), and deionized water. Disease incidence and severity data were collected seven and fourteen days post-inoculation. A statistically significant difference was observed among the varieties both for disease incidence and severity. As predicted, susceptible varieties had significantly greater disease expression than the resistant varieties (P<0.05). Between partially resistant varieties, disease expression was greater in variety NG 3729 and lowest in DP 1948. Notably, varieties considered partially resistant were found to be nearly twice (40%) as susceptible to infection as labeled (25%). Amongst the possible reasons may be due to a higher Xcm virulence from the recently field-isolated strain used, the plants harbored an incomplete suite of resistance genes and/or environmental conditions that were conducive for infections. In any event, the importance of maintaining a full resistance gene package to minimize disease was shown. The data demonstrated the vitality of a continuous evaluation of commercial cotton varieties for resistance towards bacterial blight to identify and control the spread of epidemics.
Keywords
Bacterial blight; Xanthomonas; Prokaryotic pathogen; Seedling blight
Introduction
Cotton (Gossypium hirsutum L) is a profitable non-food crop in the world. The U.S. is the third largest producer and the largest exporter of cotton in the world. The U.S. produced about 20 million bales of cotton in 2019, of which production in Texas accounts for 7 million bales [1]. Cotton production is an economic factor in several regions across the United States. Cotton, as an industry, has a value of about 7 billion dollars with millions of people involved in production, ginning and baling. Primarily grown for its fiber, cotton crop has other uses such as feed-in animal production, and as seed oil-in many industries.
Bacterial blight is a disease in cotton resulting from the infection caused by the bacterium Xanthomonas citri pv. malvacearum (Xcm) that attacks plant tissues during all developmental stages. Different plant parts exhibit varying symptoms as a result of the infection during different stages of development. Typical disease symptoms include: Seedling blight; Angular leaf spots; Vein blight; Boll rot [2]. Leaves exhibit characteristic symptoms as angular spots that are confined by veins and the magnitude of damage is genotype dependent [3]. Favorable conditions for Xcm can modify plant-pathogen connections by improving host plant susceptibility [4]. At present management tools to curtail disease development are unavailable, except the use of disease resistant varieties.
Cotton bacterial blight caused significant yield losses during late 20th century ranging from 10-77% in susceptible cultivars [3,5,6]. More recently, significant yield losses were recorded during the new sporadic outbreaks in Arkansas and Mississippi [7]. Cases were documented in Oklahoma and Texas and more recent epidemics occurred in mid-southern and southeast regions of the US [7-9].
The central hypothesis of this study was that bacterial blight symptoms on leaves and bolls would correspond to the resistance level of the varieties evaluated. The objectives of this study were:
• To evaluate the Disease Incidence (DI) and Disease Severity (DS) of bacterial blight in seven commercial cotton varieties.
• To determine the most susceptible growth stage to the disease in south Texas growing conditions.
Knowledge of bacterial blight and the variety performance in south Texas is crucial for the producers in the region as bacterial blight has the potential to cause severe economic losses.
The Xcm pathovar has 22 races in the U.S. according to the virulence phenotypes [7]. Race 18 is the most virulent and predominant race affecting cotton in the U.S. The effectiveness of the current, commercially available resistant varieties might be negatively impacted if races other than 18 become more virulent. Continuous screening of commercial cotton varieties under field conditions is essential. In this study, the commercial cotton varieties were evaluated for disease susceptibility and resistance after inoculating with Xcm at matchhead, candle and pink flower stages.
Cotton is among the earliest domesticated non-food crop in the world, domesticated independently in both old world and new world countries [10]. The genus Gossypium has about 50 species, of which the primary production area is limited to two of the new world tetraploid species, G. hirsutum and G. barbadense [11]. The Asiatic diploid species G. arboreum and G. herbaceum occupy a minimal area in India, Eastern Asia, and parts of Africa. Gossypium hirsutum represents about 90% of commercial cotton production in the world. In the US both upland cotton (G.hirsutum) and Pima (G.barbadense) are cultivated for commercial use with 95% of production from upland cotton [1]. All commercial cotton varieties used in this study were upland cotton (G. hirsutum).
Cotton is a crucial agriculturally produced raw material in the world supplying a significant portion of the world’s natural fiber [12]. Although the majority of the cotton value resides in the fiber, there are many additional byproducts captured through cotton production. The economic importance of cotton led to the popularity of cotton as an important commercial crop in over 100 countries around the world. Among all the cotton-producing countries India leads in production followed by China and the US [13]. Production in India, USA, and China accounts for more than 50% of the total cotton production in the world. The US had been a leading cotton exporter, accounting for about 1/3 of the global cotton trade [1].
Cotton production is continuously challenged by diseases such as leaf blight, damping off, anthracnose, cotton bacterial blight, etc. First reported in Alabama in 1891, bacterial blight of cotton was initially referred to as black rust [14]. The disease became more prevalent in the USA during the 1950’s and in India during 1970’s [15,16]. Cotton bacterial blight has caused severe yield losses ranging from 10-77% in susceptible cultivars [3,5,6]. Bacterial blight has caused significant yield losses during late 20th century and remains a potentially significant disease because of the variability of the pathogen and the appearance of new races. Blight in the US is predominantly caused by race 18 [17]. Seven different races were also recently documented in India [18]. As there are no corrective measures once the disease appears in the field, scientists around the world have formulated various management strategies including: variety selection; crop rotation; residue management; irrigation type. The development of resistant varieties offers the most economical means of controlling the disease in cotton [17-21].
Xanthomonas is a genus belonging to the phylum Proteobacteria, many of which cause plant diseases [4]. The genus Xanthomonas has about 27 species that cause severe infections in about 400 host plants, including a wide variety of commercially important crops such as rice, citrus, cotton and pepper [22]. Xanthomonas citri pv. malvacearum has undergone a series of taxonomic changes over time, based on further research done on the bacterium [2]. Xcm was first described as Bacterium vesicotarium, later on was classified as Pseudomonas malvacearum, Bacterium malvacearum, and Xanthomonas malvacearum [17,23]. In 2009, Ah-You et al. assigned the name Xanthomonas citri pv. malvacearum based on DNA analysis of the 16S-23S ribosomal intergenic spacer sequences [24].
Races are genetically distinct populations of individuals within the same species. A race of the pathogen is an informal rank in the taxonomic hierarchy, below the level of subspecies. Twentytwo Xcm races have been described worldwide with their relative prevalence varying by country [17,20]. Pathogenicity is considered one of the stable characters and serves as a reliable criterion for differentiating species (19). Among all the races, race 18 is the most aggressive and occurs in most of the cotton-producing areas including Texas [20].
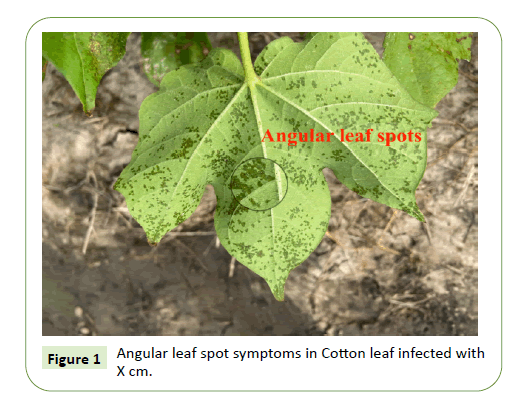
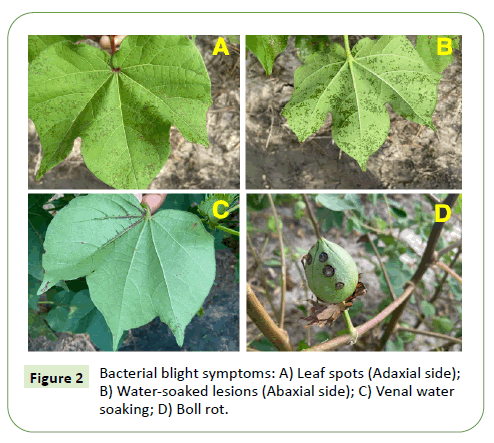
Bacterial blight on cotton can occur at all the growth stages and can express symptoms on all areal plant parts. Uniquely, the symptoms expressed by bacterial blight are expressed as angular leaf spots (on leaves), black arm lesions on stem and petioles, boll rot, and seedling blight (Figures 1 and 2).
The same isolate can cause all phases of the disease. Although infection can occur during the seedling stage, the classic foliar symptoms of bacterial blight are angular leaf spots on leaves that are confined by veins, with water soaking lesions eventually developing. Water-soaked lesions will progress to black, sunken spots that will ultimately result in defoliation. The yield losses from the disease are either a result of plant injury due to infection or damage to the bolls [7].
Bacterial blight resistance
Inherent resistant traits in cotton against Xcm were found in the plant and breeding for resistance has been going on since 1939 [25]. The first program to breed for resistance was initiated in the Republic of Sudan, where the disease was extremely destructive, later on resistant varieties of G. barbadence were developed [19,26]. Cotton varieties resistant to bacterial blight were released in the US in 1950’s. The uses of resistant varieties coupled with acid de-linting were effective in controlling the disease. The inheritance of resistance in G. hirsutam towards Xcm was further investigated by Innes et al. [27].
Resistance to bacterial blight varies considerably within the genus Gossypium since the annual diploid varieties of G. arboreum, and G. herbaceum cultivated for centuries in the Indian sub-continent were highly resistant to the disease [3]. The tetraploid genotypes G. hirsutum has the broadest spectrum of disease expression, varying from fully susceptible to resistant. Most of the resistant varieties have been developed to suit the geographically isolated races. Should races other than the more common ones in a region/ country become more pathogenic, there is a greater chance that this could lead to a more severe outbreak of the disease. It is therefore, vital to have a continuous screening program for the evaluation of bacterial blight of cotton in all the cotton-growing regions around the globe.
Materials and Methods
Field trails
Seven commercial cotton varieties were planted at Texas A and M University-Kingsville farm on April 4, 2019, and April 11, 2020, at the rate of 12 seed per meter. The cotton varieties used are from Next Gen (NG), and Delta Pine (DP). The seven commercial cotton varieties evaluated in the study are listed in Table 1. The range of resistance was tested by Dever et al. at Texas A and M AgriLife research Lubbock [28] (Table 1).
| Variety number represented in plot plan | Variety | Resistance* |
|---|---|---|
| 1 | NG 3406 B2XF | S |
| 2 | NG 5711 B3XF | R |
| 3 | NG 3729 B2XF | PR |
| 4 | DP 1646 B2XF | PR |
| 5 | DP 1845 B3XF | PR |
| 6 | DP 1725 B2XF | S |
| 7 | DP 1948 B3XF | PR |
*S = susceptible (> 90% incidence of bacterial blight in inoculated field trials). PR=partially resistant (10 to 25% incidence of blight typically in inoculated field trials). R=Resistant (<10% incidence of blight in inoculated field trials)
Table 1: Commercial cotton varieties evaluated during 2019 and 2020 field trials at TAMUK.
Experimental design
Two trials were planted in a Completely Randomized Block Design (CRBD). There are 28 rows per trial, with four rows for each variety. Each variety was replicated four times, where each replication is six meters in length with a 1.5-meter border between them. Each six-meter plot was further divided into two three-meter sections. The first 3-meter section was sprayed with water, and the other half in the same plot, was inoculated with the bacterial inoculum using a CO2 backpack sprayer (Table 2).
| Section | Spray fluid at match head stage | Spray fluid at pink flower stage |
|---|---|---|
| A | Xcm | Di water |
| B | Di water | Xcm |
| C | - | Xcm |
| D | - | Di water |
Table 2: Trial 3 Inoculations at Match head and pink flower stages.
Inoculum preparation
X cm (prospective race 18) was obtained from Dr. Thomas Isakeit (Texas A and M University, College Station). The samples were then cryopreserved in glycerol solution at a temperature of -75°C. Bacterial samples were plated onto Nutrient Agar (NA; (DifcoTM)) plates periodically to check the viability of the bacterium. To calculate the bacterial concentrations 10-fold serial dilution plating method was used. Concentration of the bacteria was adjusted to 106 cfu/ml by mixing 292 ml of bacterial culture grown overnight in nutrient broth (DifcoTM) mixture with 700 ml of deionized water.
The broth mixture was prepared by dissolving 8 gm of nutrient broth, 2 gm of yeast extract in one liter of deionized water. The mixture is then placed on a hot plate stirrer for 10 min, after which it was autoclaved at 121°C for 15 min. The mixture is then allowed to cool, and once it is at room temperature, the bacterium was added. The same protocol was followed in the preparation of NA plates, with the addition of 10 gm of agar for 1 liter deionized water.
Field inoculations
All the cotton varieties were inoculated with Xcm (prospective race 18) at matchhead, candle and pink flower stages. The inoculations were applied over the top canopy between 5:30 AM-7:30 AM at a concentration of 106 cfu/ml using a CO2 backpack sprayer at 20 Psi pressure. The concentration of the bacterial cultures was checked by dilution plating. The concentration of the inoculum was at a 1:10 ratio of bacterial broth to deionized water. An organosilicon surfactant (Silwet L-77; 0.25%v/v) was added to the inoculation solution, which was reportedly increases successful bacterial inoculation of the plant [29] (Table 3, Figures 3 and 4).
| Inoculum used | Trial 1 | Trial 2 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spray W- water | 2 | 5 | 3 | 4 | 7 | 1 | 6 | 2 | 5 | 3 | 4 | 7 | 1 | 6 | |
| Spray W-Bacteria | |||||||||||||||
| 5 ft border | |||||||||||||||
| Spray W- water | 7 | 4 | 2 | 6 | 3 | 5 | 1 | 7 | 4 | 2 | 6 | 3 | 5 | 1 | |
| Spray W-Bacteria | |||||||||||||||
| 5 ft border | |||||||||||||||
| Spray W- water | 6 | 7 | 1 | 5 | 2 | 4 | 3 | 6 | 7 | 1 | 5 | 2 | 4 | 3 | |
| Spray W-Bacteria | |||||||||||||||
| 5 ft border | |||||||||||||||
| Spray W- water | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Spray W-Bacteria |
Table 3:Research plot design (numbers 1-7 correspond to the seven varieties as presented in Table 1
Growth stages study
All the varieties in trial 1 (T1) were inoculated with Xcm at match head stage on, May 16, 2019, and on May 21, 2020. Plots were rated for DI and DS, 7 and 14 days after inoculation in 2019, 2020. All the varieties in trial 2 (T2) were inoculated with Xcm at the candle stage on May 29, 2019, and on April 4, 2020. Plots were rated for DI and DS, 7 and 14 days after inoculation in 2019, 2020. In trial 3, all the varieties were inoculated with Xcm at the pink flower stage on June 12, 2020. Each plot was divided into four sections for collecting data. Section A was sprayed twice (first at match head stage with Xcm and again at pink flower with Deionized (Di) water), section B was sprayed twice (once with Di water at match head and second with Xcm at pink flower stage), Section C was sprayed once with Xcm at pink flower stage, and section D was sprayed once with Di water. DI and DS data was collected seven and fourteen days post-inoculation.
In trial 4, the first row of each plot was inoculated with Xcm at the pink flower stage on June 22, 2020. Each plot was divided into four sections for collecting data. Section A was sprayed once with Xcm (at candle stage), section B was sprayed with Di water once at candle stage, section C was sprayed once with Xcm at pink flower stage, section D was sprayed once with deionized water at pink flower stage (Table 4).
| Section | Spray fluid at candle stage | Spray fluid at pink flower |
|---|---|---|
| A | Xcm | - |
| B | Di water | - |
| C | - | Xcm |
| D | - | Di water |
Table 4: Trial 4 Inoculations at Candle and pink flower stages.
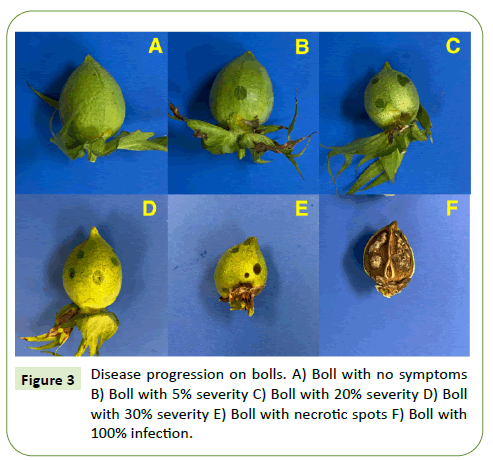
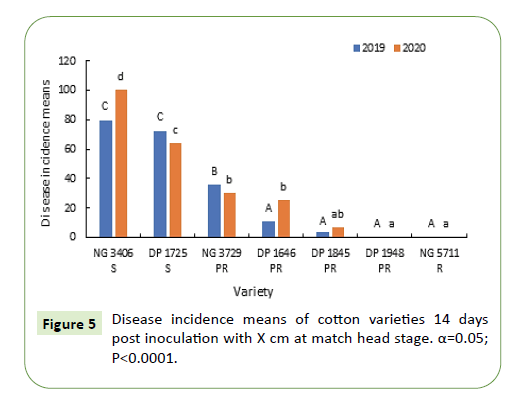
Boll rot symptoms were observed seven days after inoculation. The symptoms started as water-soaked dots on the surface of the bolls and later developed into necrotic spots on the bolls (Figure 5).
Disease evaluation
Bacterial blight symptoms initially appeared as water-soaked lesions on the underside of the leaf seven days after inoculation. As the infection progressed, the lesions developed into necrotic brown to black angular spots and were also seen on the upper leaf surface. Plots were rated for Disease Incidence (DI) and Disease Severity (DS) at seven and fourteen Days Post-Inoculation (DPI).
Disease incidence assessment
Disease incidence data were collected from the middle two rows over a distance of 7.5 ft in each row. The average number of plants per plot was 35. The disease incidence was calculated using the following formula.
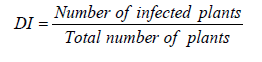
Disease severity assessment
All plants in the middle two rows exhibiting symptoms were rated for disease severity on a scale of 0-100 based on the area of the infected leaf [19].
Pathogen recovery
Leaf and boll samples that have the disease symptoms were randomly collected. The samples were then washed in 10% isopropyl alcohol and rinsed with Di water. Samples thus obtained were macerated in 2 ml deionized water. One ml from the sample was plated onto NA plates. After 3-4 days, bacterial growth was observed on the growth medium.
Statistical analysis
Disease incidence and disease severity were compared between the growth stages and varieties using Analysis of Variance (ANOVA) with a Generalized Linear Mixed Model (GLM) procedure of Statistical Analysis System (SAS) v.9.4. using the following model.
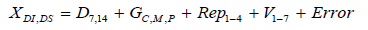
The dependent variable XI, S was used to denote Disease Incidence (DI) and Disease Severity (DS). The letter D represents Days Post Inoculation; the term G represents the Growth Stage. Rep denotes the replication; V represents the Varieties used for Evaluation. Experimental error is denoted by the term Error. A significance level of α=0.05 was used. A significant overall F test on the null hypothesis of variety equality was followed with mean separation using fishers LSD.
Results
Growth stage study
The objectives of this study were to evaluate the commercial cotton varieties for their susceptibility to Xcm at matchhead, candle and pink flower stages. All the seven commercial cotton varieties were inoculated with Xcm and water at the different flowering stages and disease incidence and severity were assessed seven and 14 days after inoculation.
Match head stage
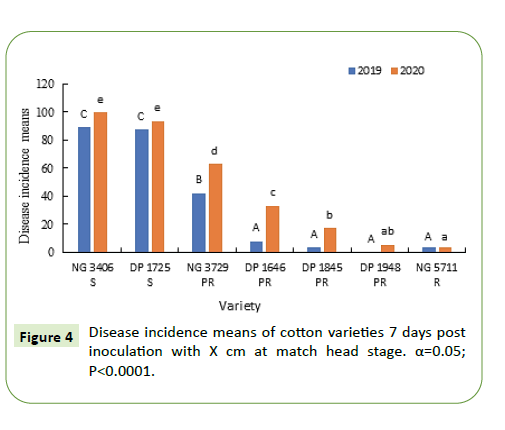
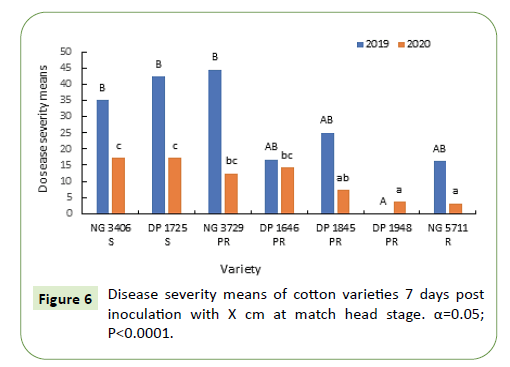
During 2019-20, seven days after inoculation, disease incidence in susceptible varieties NG 3406, DP 1725 was greater than 90% and was significantly greater (50-60%) than the partially resistant varieties. Disease incidence of partially resistant varieties was between 10-40% that is almost double of labeled specification. Between the partially resistant varieties NG 3729 had a significantly greater disease incidence during both the growing seasons. Resistant variety NG 5711 had the lowest disease incidence (0-5%) during both growing seasons (Figure 6).
Fourteen days after inoculation, disease incidence in susceptible varieties NG 3406, DP 1725 varied between 70% - 100% and was significantly greater (50-60%) than the partially resistant varieties. Disease incidence between partially resistant varieties varied from 5% to 40% with the exception of variety DP 1948, in which disease incidence was more similar to resistant variety NG 5711. Disease symptoms were not observed in varieties DP 1948 and NG 5711 (Figure 7).
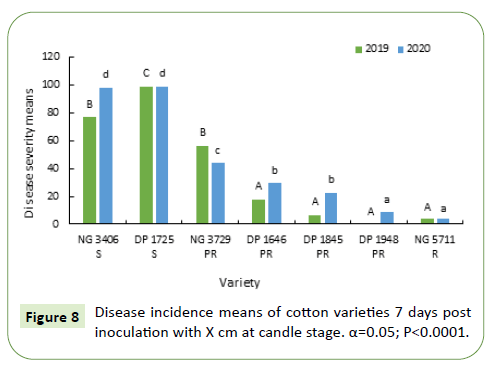
Seven days after inoculation, disease severity in most of the varieties was in the range of 15%-45% during 2019 and 10-15% during 2020. Disease severity of varieties DP 1948, NG 5711 was less than 5% during both growing seasons. Disease severity in susceptible varieties NG 3406, DP 1725 and partially resistant variety NG 3729 was significantly higher in 2019 and was similar to partially resistant varieties during 2020 (Figure 8).
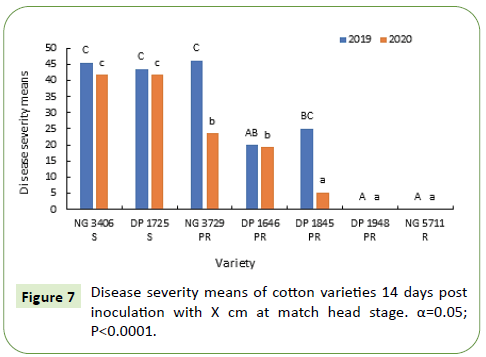
Fourteen days after inoculation, disease severity in susceptible varieties NG 3406, DP 1725, partially resistant variety NG 3729 varied between 20%-45% and was significantly greater than the resistant variety NG 5711. Disease symptoms were not observed in resistant variety NG 5711 and partially resistant variety DP 1948 (Figure 9).
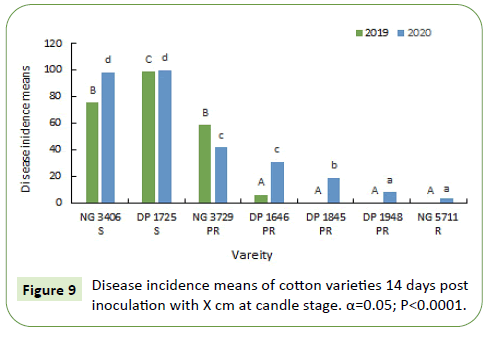
Candle stage disease incidence and severity data
During 2019-20, 7 and 14 days after inoculation, susceptible varieties NG 3406 and DP 1725 had a disease incidence between 75%-100%. Disease incidence of most of the partially resistant varieties varied from 10%-50%. Disease incidence in resistant variety NG 5711 and partially resistant variety DP 1948 was less than 5% (Figures 10 and 11).
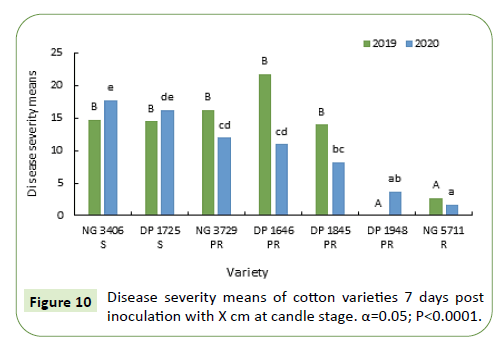
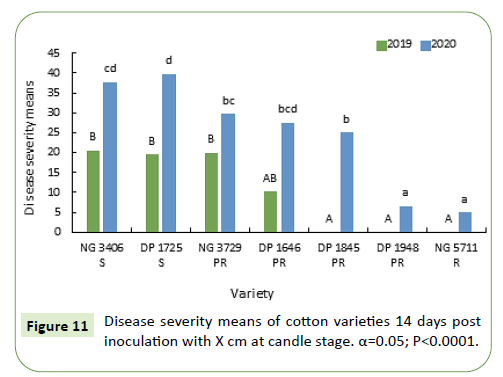
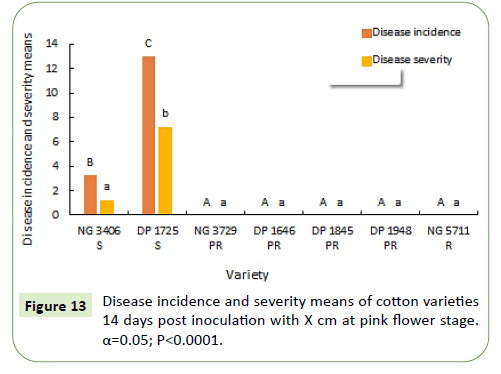
During 2019-20, 7 and 14 days after inoculation the disease severity means of susceptible varieties NG 3406, DP 1725 and partially resistant varieties NG 3729, DP 1646 and DP 1845 are similar, ranging from 15% to 25%. Resistant variety NG 5711 and partially resistant variety DP 1948 has less than 5% mean disease severity (Figures 12 and 13).
Boll rot study
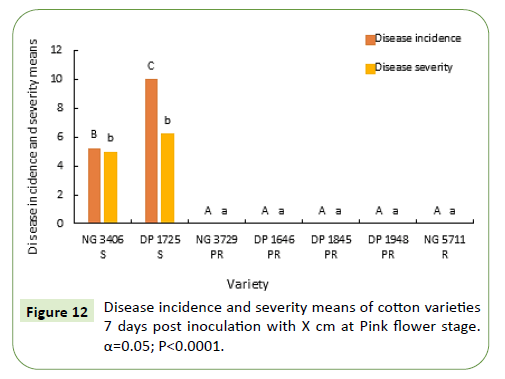
In addition to the objectives of this study, the seven commercial cotton varieties were evaluated for resistance towards boll rot in south Texas. Seven days after inoculation with Xcm, no boll rot symptoms were observed on resistant and partially resistant varieties. The low disease pressure can be attributed to the unfavorable environmental conditions during the growing season. In the susceptible varieties DP 1725 has a greater disease incidence but no significant difference was observed for disease severity.
Fourteen days after inoculation at pink flower stage, no boll rot symptoms were observed in the resistant and partially resistant varieties. Between the susceptible varieties DP 1725 had a significantly greater disease incidence and severity. The disease severity of susceptible variety NG 3406 was not statistically different from that of resistant and partially resistant varieties.
Discussion
Infection by Xcm is prevalent during favorable environmental conditions of high temperature, high relative humidity and high rainfall [3]. The average annual rainfall in south Texas ranges from 50 to 80 cm. Precipitation during the 2019 growing season was 23 cm and 21 cm for the 2020 season. During the 2019 growing season the average temperature was 29.5°C and the average relative humidity was 75%. In the 2020 season, the average temperature was 27°C and the average relative humidity was 74%. Generally, less than normal precipitation was recorded in both 2019 and 2020.
In 2019, disease incidence was significantly greater in the plants inoculated at candle stage. However, the disease severity was greater in the plants inoculated at match head stage. The increase in the disease incidence can be attributed to precipitation after administration of the inoculum. Between the cotton varieties evaluated susceptible varieties DP 1725 and NG 3406 had a greater disease incidence compared to the resistant varieties. Amongst the susceptible varieties DP 1725 had a significantly greater disease incidence; however, no significant difference was observed between DP 1735 and NG 3406 for disease severity. Between the partially resistant varieties evaluated variety NG 3729 had significantly greater disease incidence and severity, variety DP 1845 had significantly less disease incidence.
During 2020, there was no significant difference between the data collected on 7 and 14 days after inoculation for disease incidence. However, the disease severity was significantly greater at 14 days after inoculation. Plants inoculated at match head and candle stages had significantly greater disease incidence and severity compared to the plants inoculated at pink flower stage. Susceptible varieties NG 3406 and DP 1725 had a 50%-60% greater disease incidence and 20%-30% greater disease severity compared to the resistant variety. Resistant variety NG 5711 and partially resistant variety DP 1948 had significantly less than 5% disease incidence and severity. Between the partially resistant varieties NG 3729 had a significantly greater disease incidence and severity.
In this study, seven commercial cotton varieties were evaluated on the resistance to cotton bacterial blight in south Texas. All of the resistant varieties tested remained resistant during the study and the susceptible varieties remained susceptible. Varieties labeled as partially resistant developed symptoms when inoculated at candle and matchhead square stages, indicating that disease resistance increases as plants reach maturity. Flower susceptibility to Xcm also decreases as blooms reaches maturity. The level of infection drops in all the varieties depending on the time of bloom inoculation. Cotton plants inoculated at pink flower stage have expressed boll rot symptoms. However, boll rot symptoms were observed only in susceptible varieties with no disease expression in partially resistant and resistant varieties. The level of boll rot infection increased in the susceptible varieties as the bolls reached maturity. Cotton plants inoculated with Xcm during bloom have lower disease expression compared to the plants inoculated at candle and matchhead square stages.
Conclusion
Inoculation of cotton plants with Xcm was successful during all the growth stages tested. Therefore, cotton varieties can be tested throughout the growing season in south Texas. Notably, disease expression was greater for the plants inoculated at early stages. Under favorable environmental conditions for cotton bacterial blight development, the use of susceptible varieties can cause significant yield losses in south Texas. Therefore, the use of resistant and partially resistant varieties is advised to significantly lower disease incidence and severity.
Expectantly, the susceptible plants became infected and the resistant plants showed negligible disease symptoms. However, the infection level of cotton varieties that were considered to be partially resistant was nearly doubles than the expected infection rate. These results suggested that the resurgence of bacterial blight in the U.S. may be caused by a new strain of Xcm that has increased virulence and/or plants in the field did not contain the full suite of resistance genes. Regardless, our research demonstrated the importance of continuous evaluation of commercial cotton varieties to ensure plants contain the appropriate resistance genes to manage the bacterial blight disease in cotton.
Acknowledgement
We would like to thank Thomas Isakeit from Texas A and M for kindly providing the Xcm strain.
References
- Leslie Meyer (2020) USDA ERS - Cotton Sector at a Glance.
- Showmaker K C, Arick M A, Hsu C Y, Martin B E, Wang X, et al. (2017) The genome of the cotton bacterial blight pathogen Xanthomonas citri pv. malvacearum strain MSCT1. Stand Genomic Sci 12(1): 42.
- Jalloul A, Sayegh M, Champion A, Nicole M. (2015) Bacterial blight of cotton. In Phytopathologia Mediterranea 54(1): 3-20.
- An S Q, Potnis N, Dow M, Vorholter F J, He Y Q, et al. (2019) Mechanistic insights into host adaptation, virulence and epidemiology of the phytopathogen Xanthomonas. FEMS Microbiol Rev 44(1): 1-32.
- Hillocks R J. (1992) Bacterial blight 39-85. In R J Hillocks (ed.) Cotton diseases. CAB Int. Wallingford Oxon, UK.
- Umesha S, Kavitha R J, Mortensen C N. (2010) Molecular detection of Xanthomonas citri subsp. malvacearum in cotton seeds. Res J Biotech 5(1): 20-26.
- Phillips A Z, Berry J C, Wilson M C, Vijayaraghavan A, Burke J, et al. (2017) Genomics-enabled analysis of the emergent disease cotton bacterial blight. PLOS Genet 13(9): e1007003.
- Cox K L, Babilonia K, Wheeler T, He P, Shan L. (2019) Return of old foes - recurrence of bacterial blight and fusarium wilt of cotton. Curr Opin Plant Bio 50(1): 95-103.
- Kemerait B, Allen T, Lu S, Rothrock C, Faske T, et al. (2017) Identification and management of bacterial blight of cotton. Cotton Incorcporated Bulletin, Cotton Incorporated, Cary, North Carolina.
- Brubaker CL, Bourland FM, Wendel JF (1999) The origin and domestication of cotton. In: Smith CW, Cothren MTJ (Eds) Cotton: Origin, History, Technology and Production. John Wiley and Sons, New York.
- Lubbers, EL, Chee PW (2009) The worldwide gene pool of G. hirsutum and its improvement. In: Genetics and Genomics of Cotton. Springer, New York, 23-52.
- Morris D (1990) The importance of cotton to developing countries p. 135-146, In Economie des Filières en Régions Chaudes: Formation des Prix et Échanges Agricoles M. Griffon (Ed.), Acte du Xe Séminaire Äéconomie et de Sociologie, 11-15 September 1989, Montpellier 1990.
- Meyer L, Macdonald S (2015) Cotton and wool outlook. Eco Res Ser USDA, CWS,12: 1-16.
- Atkinson GF (1891) Black" rust" of cotton. Alaba Agri Exp Stat Bullet 27: 1-16.
- Schnathorst WC (1964) Longevity of Xanthomonas malvacearum in dried cotton plants and its significance in dissemination of the pathogen on seed. Phytopathol 54: 1009-1011.
- Verma JP (1986) Bacterial blight of cotton. In: Boca Raton, CRC Press FL.
- Delannoy E, Lyon BR, Marmey P, Jalloul A, Daniel JF, et al. (2005) Resistance of cotton towards Xanthomonas campestris pv. malvacearum. Ann Rev Phytopathol 43: 63-82.
- Kumar AS, Aiyanathan KEA, Nakkeeran S, Manickam S (2018) Documentation of virulence and races of Xanthomonas citri pv. malvacearum in India and its correlation with genetic diversity revealed by repetitive elements (REP, ERIC, and BOX) and ISSR markers. 3 Biotech 8: 479.
- Brinkerhoff LA (1963) Variability of Xanthomonas malvacearum: The cotton bacterial blight pathogen.Okla Agri Exp Station Tech Bullet 98.
- Braga WDS, Cunha RWSD, Suassuna ND, Coutinho WM (2016) Prevalence of race 18 of Xanthomonas citri subsp. malvacearum on cotton in Brazil. Trop Plant Pathol 41: 128-131.
- Issa W, Larbouga B, Vianney T, Issouf S, Leonard O (2019) Identification of resistance sources among traditional cotton genotypes from burkina faso against Xanthomonas citri pv. malvacearum. Agri Res Tech: Open Access J 23: 260-266.
- Ryan RP, Vorhölter FJ, Potnis N, Jones JB, Sluys MAV, et al. (2011) Pathogenomics of Xanthomonas: Understanding bacterium-plant interactions. Natur Rev Microbiol 9: 344-355.
- Doidge EM (1921) A tomato canker. Annal Appl Biol 7: 407-430.
- Ah-You N, Gagnevin L, Grimont PAD, Brisse S, Nesme X, et al. (2009) Polyphasic characterization of Xanthomonas pathogenic to members of the Anacardiaceae and their relatedness to species of Xanthomonas. Int J Syst Evol Microbiol 59: 306-318.
- Knight RL, Clouston TW (1939) The genetics of blackarm resistance: I. Factors B1 and B2. J Gene 38: 133-159.
- Knight RL (1946) Breeding cotton resistant to blackarm disease. Empir J Exp Agri 14: 161.
- Innes NL, Brown SJ, Walker JT (1974) Genetical and environmental variation for resistance to bacterial blight of upland cotton. Heredity 32: 53-72.
- Dever JK, Morgan V, Kelly CM, Wheeler TA, Stair K (2019) Texas A&M AgriLife Research.Texas High Plains Cotton Performance Tests in the Texas High Plains 2019.
- Wheeler TA, Sagaram US, Schuster GL, Gannaway JR (2007) Plant pathology and nematology:factors that influence bacterial blight screening. J Cotton Sci 11: 91-97.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences