ISSN : 2348-9502
American Journal of Ethnomedicine
Phytochemical Screening and GCMS Studies of the Medicinal Plant Pavetta indica Linn
1Assistant Professor, Department of Chemistry, Vetri Vinayaha College of Engineering and Technology, Tholurpatti, Thottiam, Trichirappalli – 621215, Tamilnadu, India.
2Assistant Professor, Department of Chemistry, SNS College of Technology, Coimbatore – 641035, Tamilnadu, India.
3Associate Professor, Department of Chemistry, St. Joseph’s College, Trichirappalli – 620002, Tamilnadu, India
Abstract
Objective: The plant Pavetta indica Linn.is variable shrub (or) small tree belonging to the family of Rubiaceae, reported to have medicinal properties. The leaves and roots of this plant are used in poultices for boils and itches, to cure hemorrhoidal pain, constipation, jaundice etc. The present work is aimed at the phytochemical screening and GCMS Studies for the presence of secondary metabolites like alkaloids, flavonoids, terpenoids, steroids, tannins, etc.
Methods: The Phytochemical screening of the leaf extracts were carried out applying the standard methods and tests. It shows the presence of metabolites like alkaloids, carbohydrate, tannins, steroidal glycosides, steroids, flavonoids, etc. The ethanolic extract was subjected to GCMS studies.
Results: The phytochemical screening reveals that the both ethanolic and methanolic extracts of Pavetta indica Linn. contains the phytoconstituents - alkaloids, carbohydrate, tannins, steroidal glycosides, steroids, flavonoids, etc. The GCMS analysis of ethanolic extracts indicates the presence of 36 phytoconstituents belonging to the types of acids, alkanes, amines, esters and phenolic compounds. Conclusion: The phytochemical screening and GCMS analysis of the extracts are in good agreement with the presence of alkaloids; four alkaloids are reported to be present by the GCMS studies. The medicinal properties of Pavetta indica Linn. may are attributed to the presence of alkaloids.
Keywords
Medicinal values, GCMS studies, Pavetta indica Linn., Phytochemical screening.
Introduction
Pavetta indica Linn [1,2] (Tamil: Kattu thirani, Panna pavadai, Sirukonnai, Pavattai) is a shrub or small tree belongs to the family of Rubiaceace. The leaves very variable elliptic – oblong to elliptic – lanceolate and obovate – oblong, glossy – green flowers are white. The roots are said to possess purgative, aperient, diuretic and tonic properties and are prescribed in visceral obstructions, jaundice, headache, urinary diseases and dropsical affections. The phytochemical investigation [3], chemical composition of essential oil [4] and physio – phytochemical screening [5] had been reported to this plant. The plant was studied and found to possess anti – inflammatory potential [6], analgestic [7], antimicrobial [8] , antipyretic activities [9]. The aim of the present study was to identify the phytocomponents of the ethanolic and methanolic extracts of the plant leaves applying the GCMS and the phytochemical screening techniques.
MATERIALS AND METHODS
The leaves of Pavetta indica Linn. were collected from Elamanur region (Near Trichy) from the month of July at 5.00pm.They were identified and authenticated by the Rapinet Herbarium, St. Joseph college (Autonomous)Trichy -02, Tamilnadu, India.
Sample preparation
The leaves of Pavetta indica Linn. were shade dried and pulverized well. About 20g of the plant leaves were soaked in 100ml of ethanol and methanol. It was left for 24 hours in order to extract the phytoconstituents- alkaloids, carbohydrate, tannins, steroidal glycosides, steroids, flavanoids, acids and others. The above extracts were filtered using Whatmann No.1filter paper the residue was removed.
Phytochemical Screening [10,11]
The phytochemical screening of the leaf extracts were carried out applying the standard methods and tests as prescribed by J B Harbone [12]. Hence, the presence or absence of various phytoconstituents were determined. The experimental procedures and the results are given in the Table No -1.
Table 1. Details of Phytochemical Screening of the extracts of Pavetta indica Linn.
| S. No | Name of the Test | Experimental Procedure | Phytoconstituent |
|---|---|---|---|
| 1 | a) Mayer's test | 0.5 ml of extract was treated with Mayer's reagent (potassiomercuric iodide solution) to gave cream colored precipitate. |
Alkaloids |
| b) Dragondraff test | 0.5 ml of extract was treated with Dragendroff’s reagent (potassium bismith iodide). Formation of orange or orange red precipitate was obseved. |
Alkaloids | |
| c) Wagner test | 0.5 ml of extract was treated with Wager’s reagent (solution of iodine with KI) and it gave an brown or reddish brown precipitate. | Alkaloids | |
| 2 | a) Molisch test | 0.5ml of extract was treated with 1ml of alpha – napthol and con. H2SO4, which gave purple coloation. |
Carbohydrates |
| b) Fehling test | 0.5 ml of extract to which equal quantity of Fehling solution – A (copper sulphate) & B (potassium ammonium tartate) were added. The content was heating to give brick red precipitate was obtained. |
Carbohydrates | |
| 3 | Foam test | Dilute 1ml of alcohol in 0.5 ml of extract. The mixture was diluted to 20 ml of distilled water. It was shaken well for 15min. The formation of foam was observed. | Saponins |
| 4 | Lead acetate test | 0.5 ml of alcoholic or aqueous extracts was treated with lead acetate solution. White precipitate was observed. | Tannins |
| 5 | Ferric chloride test | 0.5 ml of alcoholic extracts was treated with 2 drops of neutral ferric chloride. brownish green coloration was observed | Pseudo Tannin (Condensed tannin) |
| 6 | Ammonia test | 0.5 ml of extract was treated with aqueous ammonia solution. It was exposed to air which gradually develops a green color. | Chlorogenic acid |
| 7 | NaOH test | 0.5 ml of extract was treated with aqueous sodium hydroxide solution formation of blue violet coloration | Anthocyanin |
| 8 | Libermann's Burchard test | 0.5ml of extract was dissolved in 2ml chloroform. The mixture was treated with acetic acid, acetic anhydride and conc. H2SO4 gave bluish green coloration. | Steroidal glycosides |
| 9 | H2SO4 test | 0.5 ml of extract was treated with 80% H2SO4, gave deep yellow color. | Saponins glycosides |
| 10 | Ammonia test | 0.5 ml of extract was mixed 2ml of ammonia. The mixture was observed under UV and visible lights - formation of fluorescence. | Flavonoids |
| 11 | Shinoda's test | 0.5 ml of alcoholic extract was treated with magnesium foil and conc. HCl. It gave intense cherry red coloration | Flavones |
| 12 | NaOH test | 0.5 ml of alcoholic extract was treated with 10% Sodium hydroxide solution, yellow coloration was observed. | Coumarin |
| 13 | Salkowaski test | 0.5 ml of extract was dissolved in 1ml of chloroform. The mixture was treated with conc. H2SO4. It gave red coloration. | Steroids |
Gas Chromatography and Mass spectrometry [13]
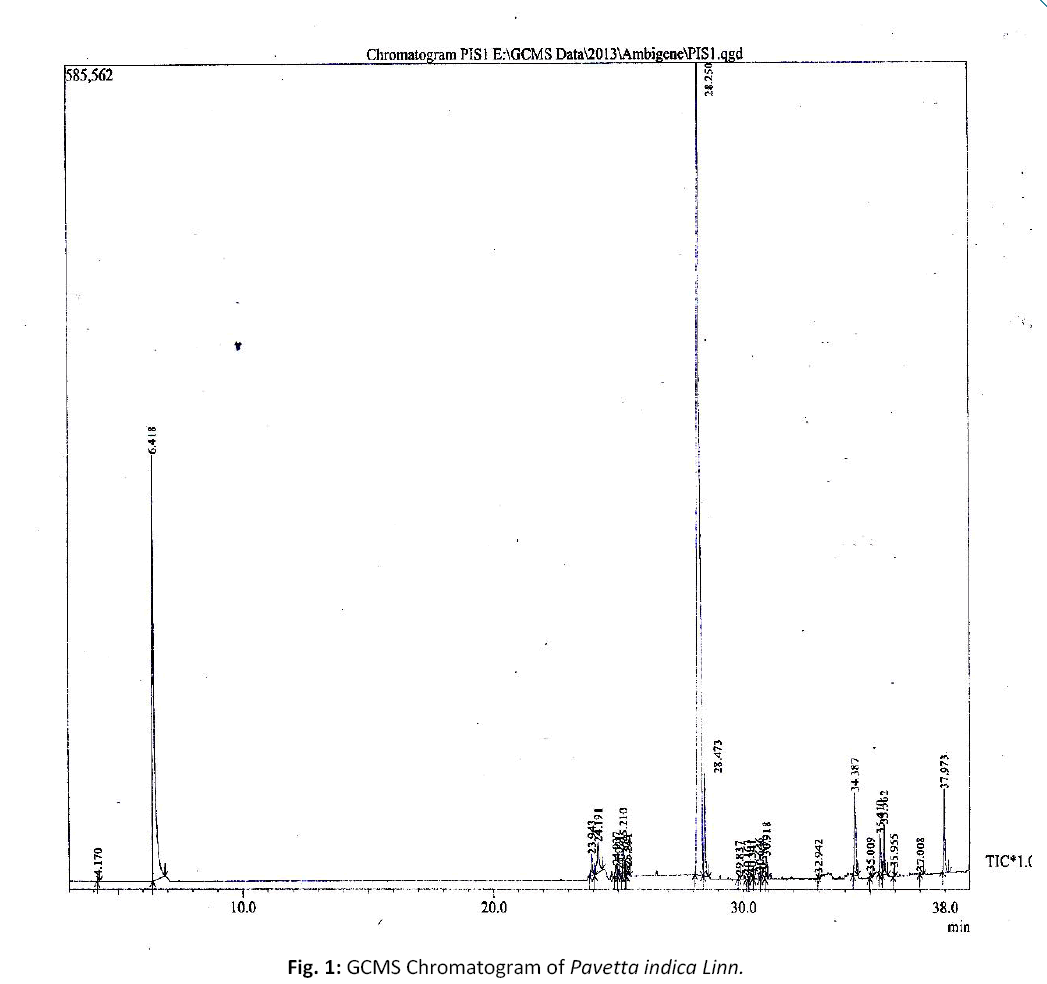
The ethanolic extract was subjected to GC-MS analysis of the instrument GCMS (Schimadz U Japan) with Elite – DB – 5M Column and the GCMS solution version 2.53SV3 software. Initially oven temperature was maintained at 30°C for 2 minutes and the temperature was increased gradually up to 200ºC at 10.0/35.0 min and 4.0 μL of sample was injected for analysis. Helium gas 99.9% of purity was used as carrier gas as well a elution. The flow rate of helium gas set to 1.5ml /min. The temperature was maintained at 230ºC. The sample injector with split ratio was 20 throughout the experiment periods. The mass spectroscopic analysis was done at 70 eV. The spectra were recorded for mass range 40 – 1000 m/z for about 35 minutes. The separated compounds were identified by comparing their mass spectra with the mass spectral data of the compounds present in the data bank. The GCMS chromatogram is attached in Figure No. 1.
RESULTS AND DISCUSSION
Phytochemical screening
The results of the phytochemical screening of the plant Pavetta indica Linn. and its GCMS profiling are presented here. The plant Pavetta indica Linn .was analysed qualitatively for the phytochemically active compounds and the results are given in the Table No: 2. The ethanolic and methanolic extracts of the leaves of Pavetta indica Linn. showed the presence of phytochemically active compounds such as alkaloids, carbohydrate, tannins, steroidal glycosides, steroids, flavonoids. The following metabolites were analysed to be absent in the ethanolic and methanolic extracts saponins, sapanin glycosides, cumarin, anthocyanin and flavones. The details are given in the Table No: 2.
Table 2. Details of Phytochemical Screening of the extracts of Pavetta indica Linn.
| S. No | Phytochemical constituents |
Name of the test | Methanol Extract |
Ethanol Extract |
|---|---|---|---|---|
| 1 | Alkaloids | Mayer’s test Dragondraff test Wagner test | + + + |
+ + + |
| 2 | Carbohydrates | Molish test Fehling test Benedicts test |
+ - - |
+ + - |
| 3 | Saponins | Foam test | - | - |
| 4 | Tannins | Lead Acetate test | + | + |
| 5 | Pseudo tannins | Ferric chloride. | Condensed Tannin |
Condensed Tannin |
| 6 | Chlorogenic acid | Ammonia test | + | + |
| 7 | Anthocyanin | NaOH test | - | - |
| 8 | Steroidal Glycosides | Liebermann’s Burchard test |
+ | + |
| 9 | Saponins glycosides | H2SO4 test | - | - |
| 10 | Flavonoids | Ammonia test | + | + |
| 11 | Flavones | Shinoda’s test | - | - |
| 12 | Coumarin | Sodium chloride test | - | - |
| 13 | Anthracene glycoside |
Ammonia test | - | - |
| 14 | Steroids | Salkowaski test | + | + |
Note: + = Present - = Absent
GCMS Study
GCMS analysis was carried out on the ethanolic extracts of Pavatta indica Linn. showed as many as 36 compounds to present. The lists of compounds are given in Table No – 3. The GCMS analysis was done using the instrument GCMS (Schimadz U QP2010 with GCMS solution version 2.53 software. The sample volumes was 4.0μL. The sample of ethanolic extract was run for 35 minutes. The chromatogram (Figure No:1) showed prominent peaks in the retention time ranging 4.0 – 38.0minutes.
Table 3. Phytoconstituents of Pavetta indica Linn. by GCMS Study.
| S. No | RT | Name of the compound | Molecular Formula | Molecular Weight | % Peak area | Compound Nature |
|---|---|---|---|---|---|---|
| 1 | 6.418 | Methane, sulfinylbis- (CAS) Dimethyl sulfoxide | C2 H6 O S | 78 | 20.52 | Organo sulphur |
| 2 | 6.418 | Propane, 2-chloro- (CAS) 2- Chloropropane | C3 H7 Cl | 78 | 20.52 | Haloalkane |
| 3 | 6.418 | n-Butyric-D7 acid | C7 H8 | 92 | 20.52 | Fatty acid |
| 4 | 24.191 | 1,2-Benzenedicarboxylic acid, dimethyl ester (CAS) Methyl phthalate | C10 H10 O4 | 194 | 1.84 | Aromatic di carboxylic acid |
| 5 | 24.191 | Methyl o- (Bromochloroacetyl)benzoate | C10 H8 Br Cl O3 | 296 | 1.84 | Ester |
| 6 | 25.210 | Docosane (CAS) n-Docosane - | C22 H46 | 310 | 1.18 | Alkane |
| 7 | 25.210 | Nonane, 5-methyl-5-propyl | C13 H28 | 184 | 1.18 | Alkane |
| 8 | 25.210 | 3-Bromodecane | C10 H21 Br | 220 | 1.18 | Haloalkanes |
| 9 | 25.210 | 4-Heptanone, 3-methyl- (CAS) 3- Methyl-4-heptanone | C8 H16 O | 128 | 1.18 | Ketone |
| 10 | 25.210 | Hexadecane (CAS) n-Hexadecane | C16 H34 | 226 | 1.18 | Alkane hydrocarbon |
| 11 | 28.250 | 1,2-Benzenedicarboxylic acid, diethyl ester (CAS) Ethyl phthalate | C12 H14 O4 | 222 | 59.63 | Phthalate ester |
| 12 | 28.250 | 2,4-Imidazolidinedione, 1-[[(5-nitro-2- furanyl)methylene]amino]- (CAS) Upiol | C8 H6 N4 O5 | 238 | 59.63 | Hetero cyclic compound |
| 13 | 28.250 | Phthalic acid, allyl ethyl ester (CAS) Ethylallylphthalate | C13 H14 O4 | 234 | 59.63 | Phthalate ester |
| 14 | 28.250 | 1,3-dioxolane, 2-phenyL-2- (phenylmethyl)- | C16 H16 O2 | 240 | 59.63 | Dioxy ether |
| 15 | 28.250 | tartronic acid, (p-ethoxyphenyl)-, diethyl ester | C15 H20 O6 | 296 | 59.63 | Ester |
| 16 | 34.38 | Phthalic acid , butyl ester with ester butyl glycolate (CAS) 1,2- Benzenedicarboxylicacid , 2- butoxy – 2- oxoethyl butyl ester (CAS) butyl (butoxycarbonyl)methyl phthalate | C18H24 O6 | 336 | 3.52 | Ester |
| 17 | 35.41 | Hydrazine, (phenylmethyl ) - (CAS) Benzylhydrazine - 95% | C8H10 O | 122 | 3.52 | Amino Compound |
| 18 | 35.41 | Headecanoic acid (CAS) Palmitic acid | C16 H32 O2 | 256 | 1.19 | Fatty acids |
| 19 | 35.41 | Octadecanoic acid (CAS) Stearic acid , n-OCtadecanoic acid | C18 H36 O2 | 284 | 1.19 | Fatty acids |
| 20 | 35.410 | 10-bromo-7-hydroxy-11-iodolaurene | C15 H18 Br I O | 420 | 1.19 | Alcohol |
| 21 | 35.410 | 3,6,9-trimethyl-7-nitro-2,3- dihydronaphtho[1,8-bc]pyran | C15 H15 N O3 | 257 | 1.19 | Hetro cyclic compound |
| 22 | 35.410 | 3,4-Hexanediol, 2,5-dimethyl- (cas) 2,5-dimethyl-3,4-hexandiol | C8 H18 O2 | 146 | 1.19 | Alcohol - |
| 23 | 35.410 | Tetradecanoic acid (CAS) Myristic acid | C14 H28 O2 | 228 | 1.19 | Fatty acids |
| 24 | 35.410 | Decanoic acid (CAS) Capric acid | C10 H20 O2 | 172 | 1.19 | Saturated fatty acids |
| 25 | 35.562 | butyl-2-ethylhexyl phthalate | C20H30O4 | 334.44 | 1.52 | Ester |
| 26 | 35.562 | 2-methyl-6-beta-d- ribofuranosylimidazo[1,2-c]pyrimidin- 5(6H)-one | C12 H15 N3 O5 | 281 | 1.52 | Hetero cyclic compound |
| 27 | 35.562 | 3-methylhomoadamantane Tricyclo[4.3.1.13,8]undecane, 3- methyl- (CAS) | C12 H20 | 164 | 1.52 | Alkane |
| 28 | 35.562 | (3R*,4S*)-3-(2-Nitro-4- methoxyphenyl)-4-(4- hydroxyphenyl)hexane |
C19 H23 N O4 | 329 | 1.52 | Hrtro cyclic compound |
| 29 | 37.97 | Butanoic acid, 2-hydroxy-, methyl ester (CAS) methyl 2-hydroxybutyrate | C5 H10 O3 | 118 | 2.67 | Ester |
| 30 | 37.973 | 4-p-chorophenyl-2-dimethylamino-5- nitrosothiazole | C12 H13 N3 O S | 247 | 2.67 | Hetero cyclic compound |
| 31 | 37.973 | 1-Propanamine (CAS) n-Propylamine | C3 H9 N | 59 | 2.67 | Amine |
| 32 | 37.973 | Formamide, N-methyl- (CAS) N- methylformamide Methylformamide | C2 H5 N O | 59 | 2.67 | Amide |
| 33 | 37.973 | 1-germa-2-silabutane ( ethylsilyl) germane | C2 H10 GE SI | 136 | 2.67 | Alkane |
| 34 | 37.973 | N-[1,2,2,2-tetrafluoro-1- (trifluoromethyl)ethyl]sulfimide- trimethylamine adduct | C6 H9 F7 N2 O2 S | 306 | 2.67 | Sulfamide |
| 35 | 37.973 | Ethyl 2-(1'-hydroxy-1'-methylethyl)- 5,6,6-trimethyl-3,4-heptadienoate | C15 H26 O3 | 254 | 2.67 | Alkane |
| 36 | 37.973 | 3-Fluoro-2-methoxy-3- (trifluoromethyl)nonan-4-one | C11 H18 F4 O2 | 258 | 2.67 | Haloketone |
Based on the percentage peak area the compounds 1,2- benzene dicarboxylic acid, diethylester(CAS) Ethyl phthalate, 2,4- Imidazolidinedione, 1-[[(5-nitro-2-furanyl) methane]amino]-(CAS)upiol, phalic acid, allyl ethyl ester(CAS) Ethylallylphthalate, 1, 3-dioxoline, tartronic acid, (PEthoxyphenyl) diethyl ester were found to be significantly in higher quantities with the peak areas ranging from 59.63 to 60%.
The compounds methane, sulfinyl bis – (CAS) dimethyl sulfoxide, propane, 2- chloro-(CAS) 2-chloropropane, n-butyric D7acid were observed to be in moderate quantities with the peak area ranging from 20 to21%. The following compounds 1, 2- benzenedicarboxylic acid, phthalic acid, butyl ester, di isobutyl benzene – 1, 2 – dicarboxylate, hydrazine, hexadecanoic acid, palmitic acid, octadecanoic acid, strearic acid, 3, 4 – hexanediol, tetradecanoic acid, myristic acid, decanoic acid, capric acid, 1- propanamine, n-propylamine, formamide, nonane, 3-bromodecane, 4-heptane were quatified to be in lower amounts with the peak area ranging from 1 to 5%. The data of GCMS studies are given in the Table No: 3.
CONCLUSION
The results of the phytochemical screening revealed that both ethanolic and methanolic extracts of Pavetta indica Linn. contained the phytoconstituents - alkaloids, carbohydrate, tannins, steroidal glycosides, steroids, flavonoids, etc.
The GCMS analysis of ethanolic extracts indicated the presence of 36 phytoconstituents belonging to the types of acids, alkanes, amines, esters and phenolic compounds. Hence, the medicinal plant Pavetta indica Linn had been found to possess significant phytoconstituents that might be attributed to the medicinal characteristics.
ACKNOWLEDGEMENT
We acknowledge Dr. M. Karthikeyan, Principal and Mr. D. Johnson, Head of General Engineering, Vetri Vinayaha College of Engineering and Technology for their constant encouragement in doing this part of my research work.
We place on record once sincere acknowledgement Rev. Fr. Andrew Francis, Principal, St. Joseph’s College (Autonomous) for permitting us to do laboratory facilities in the Department of Chemistry.
We gratefully acknowledge the timely help offered by L. Rakesh Sharma and S. Saraswathi doing the GCMS studies at the Ampigen Laboratories, Tanjavur- 613501, Tamilnadu, India.
REFERENCES
- The Wealth of India, “A dictionary of Indian raw materials and industrials products, Raw materials” 1991, Vol – 7, N – Pe, pp.282.
- Bur Kill, H.M,”The Useful Plants of western tropical, Africa”, 1985, Vol – 4.
- Ramamoorthy J, Venkatramanan S, Meena R, Satkar Prasad and Devi A.P, “Phytochemical investigation of pavetta indica”, Indian Journal of Chemical Science, 2011,9(1), pp.397 – 402.
- K Prasad, K.Moulekhi and G Bisht “Chemical composition of the essential oil of Pavetta indica Linn.L leaves”, Research Journal of Phytochemistry, 2011,5,pp.66 – 69.
- Ramamoorthy J “Physio – Phytochemical screening and Diuretic activity of leaves of Pavetta indica Linn.Linn” Journal of Pharmaceutical Science and Research, 2010 Aug – 1.
- Subhash C , Mandal et al “Evaluation of anti – inflammatory potential of Pavetta indica Linn.Linn. leaf extract (family: Rubiaceae) in rats”, Phytotherapy Research, Vol 17 issue 7, pp817 – 820 / D O I 10.1002/ Ptr.1095.
- Golwala D K et al, “Analgestic activity of Ethanolic leaf Extract of Pavetta indica”, International Journal of Pharmaceutical Science and Drug Research 2009; I(2) pp.119 – 120.
- Vinod Kumar Gupta, Charanjeet Kaur, Aritra Simlai and Amit Roy , “Antimicrobial activity of Pavetta indica Linn.leaves”, Journal of Applied Pharmaceutical Science, 2013 April, Vol – 3 (04), pp.078 – 082 / DOI : 10.7324/JAPS.2013.3414
- Lakshmi S , Mohana et al,” Antipyretic Activity of Pavetta indica Linn.Linn (Rubiaceae) Leaf Extract”, Selected issues in ethnopharmacology / Division of Pharmacognosy and Phytochemistry, Department of Pharmaceutical Technology , Jadavapur University, Kolkata – 700032, India / Subhash mandal @ yahoo.com . asok_kenny@yahoo.com.
- Evans W C, Trease and Evans, Pharmacology, 15th Edition, Reed Elsevier India Pvt Ltd., New Delhi, 2006.
- Harbone J B, A Guide to Modern Techniques of Plant Analysis, 3rd Edition, Kluwer Academic Publisher U.S.A.,1998.
- Harbone J B, Phytochemical Methods, 2nd Edition1928.
- Ganesh S and Jannet Vennila J., Phytochemical Analysis of Acanthus ilicifolius and Avicennia officinalis by GC- MS, Research Journal of Phytochemistry, 2011,5(1) pp60 – 65.
- F.W Mclafferty, Interprewtation of Mass Spectra, 2nd Edition, W. A. Benjamin. Inc. Publishers, New York,1984.
- Prasad K, Moulekhi K and Bisht G., “Chemical composition of the Essential oil of Pavetta indica Linn. Leaves”, Research Journal of Phytochemistry, 2011, 5(1), pp.66-69.
- Prasad K and Bisht G., “Evaluation of Nutritive, Antioxidant and Mineral Composition of Pavetta indica Linn. Leaves”, Research Journal of Phytochemistry, 2011, 5(1), pp.54-59.
- N.Bidyanada Sing and N. Saravanan, the effect of Pavetta indica Linn.in CCl4 induced Hepatotoxicity in Rats / Pharmacie Globale (IJCP), 2012, 6(04).
- Natarajan. P, Thanga thirupathi A, Ramarajan S and Jaya S.,”Preliminary Study of Antidiabetic activity of Methanolic extract of Pavetta indica Linn. in diabetic rats”. Asian Journal of Pharmacetical and clinical Research,2013, 6(1)
- Sujatha S and Gowri Prakash, “Bioactive Screening and antimicrobial activity of flowers from the medicinal plant Pavetta indica Linn.”,International Journal of Current Microbiology and Applied Sciences,2013,5,pp.211-221, https://www. IJCMAS.com
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences