ISSN : 2348-1927
Annals of Biological Sciences
Pharmacovigilance - An Overview
Pragya Dhiman*
iMedPub, Green Lane, London, UK
Received Date: February 25, 2021, Accepted Date: March 02, 2021, Published Date: March 11, 2021
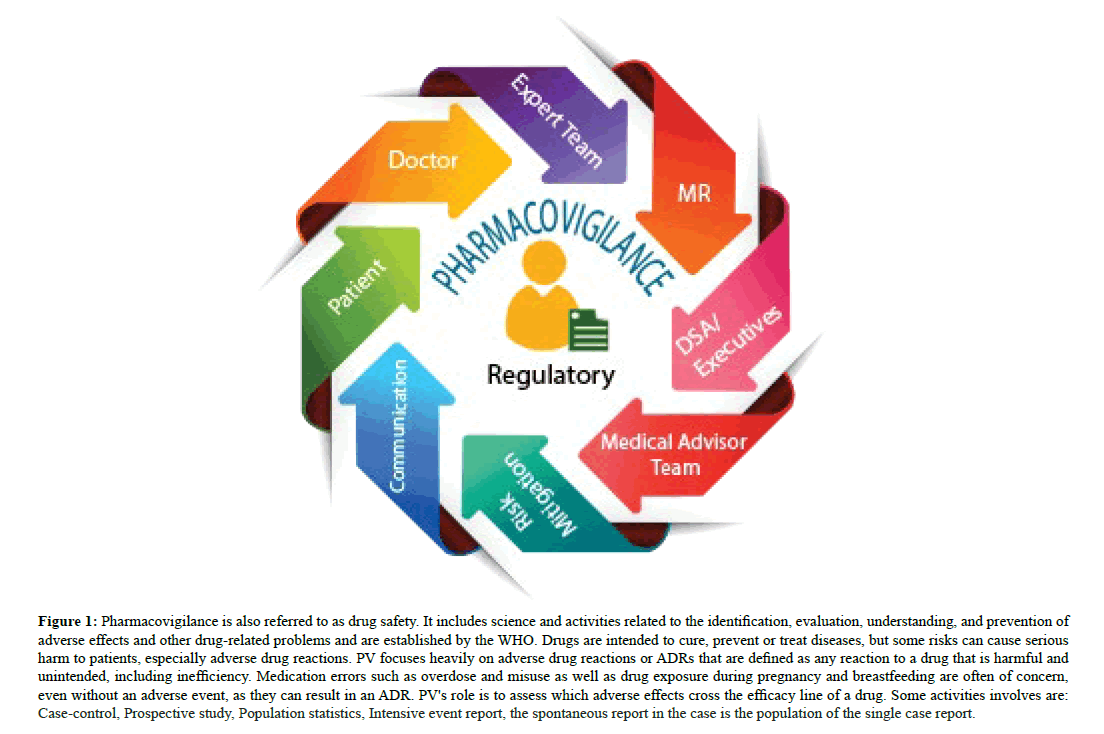
Figure 1: Pharmacovigilance is also referred to as drug safety. It includes science and activities related to the identification, evaluation, understanding, and prevention of adverse effects and other drug-related problems and are established by the WHO. Drugs are intended to cure, prevent or treat diseases, but some risks can cause serious harm to patients, especially adverse drug reactions. PV focuses heavily on adverse drug reactions or ADRs that are defined as any reaction to a drug that is harmful and unintended, including inefficiency. Medication errors such as overdose and misuse as well as drug exposure during pregnancy and breastfeeding are often of concern, even without an adverse event, as they can result in an ADR. PV's role is to assess which adverse effects cross the efficacy line of a drug. Some activities involves are: Case-control, Prospective study, Population statistics, Intensive event report, the spontaneous report in the case is the population of the single case report.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences