ISSN : 0976 - 8688
Der Pharmacia Sinica
Pharmacological Activities of Some 5-Substituted-3-phenyl-NïÃÂâ-(substituted-2-oxo-2H-pyrano [2, 3-b] quinoline-3-carbonyl)-1H-indole-2-carboxyhydrazides
S M Basavarajaiah1,2* and BHM Mruthyunjayaswamya2
1Department of Studies and Research in Chemistry, Gulbarga University, Gulbarga–585106, India
2Department of Chemistry, Vijaya College, RV Road, Basavanagudi, Bengaluru-560 004, India
- *Corresponding Author:
- Basavarajaiah SM
Department of Studies and Research in Chemistry, Gulbarga University, Gulbarga–585106, India
E-mail: drsmbasu@gmail.com
Received Date: August 12, 2021; Accepted Date: September 02, 2021; Published Date:September 09, 2021
Citation: Basavarajaiah SM, Mruthyunjayaswamya BHM (2021) Pharmacological Activities of Some 5-Substituted-3-phenyl-Nβ-(substituted- 2-oxo-2H-pyrano [2, 3-b] quinoline-3- carbonyl)-1H-indole-2-carboxyhydrazides. Der Pharmacia Sinica, Vol.12 No.5: 011.
Abstract
Indoles are amongst the most important class of heteroaromatics in organic chemistry, being generally found in biologically active natural products. Indole motifs have become noteworthy compounds because of their ample assortment of applications in medicine, synthetic chemistry, coordination chemistry, as well as in the field of industrial chemistry. Ethyl-3-oxo-3-{2-[(5-substituted-3- phenyl-1H-indol-2-yl) carbonyl] hydrazinyl} propanoates 5a-b were synthesized according to the literature method. These on further reaction with substituted- 2-hydroxy-3-formylquinolines 3a-e yielded 5-substituted-Nβ-(2-oxo-2H-pyrano [2, 3-b] quinoline-3-carbonyl)-3-phenyl-1H-indole- 2- carbohydrazide 6a-j. Spectral techniques were used to confirm the structures of the all synthesized compounds. All these compounds have been screened for their pharmacological activities such as anti-inflammatory and analgesic activities.
Keywords
Heterocyclic compounds; Indole; Pharmacological activities; Pyranoquinoline- 2-one; Quinoline
Introduction
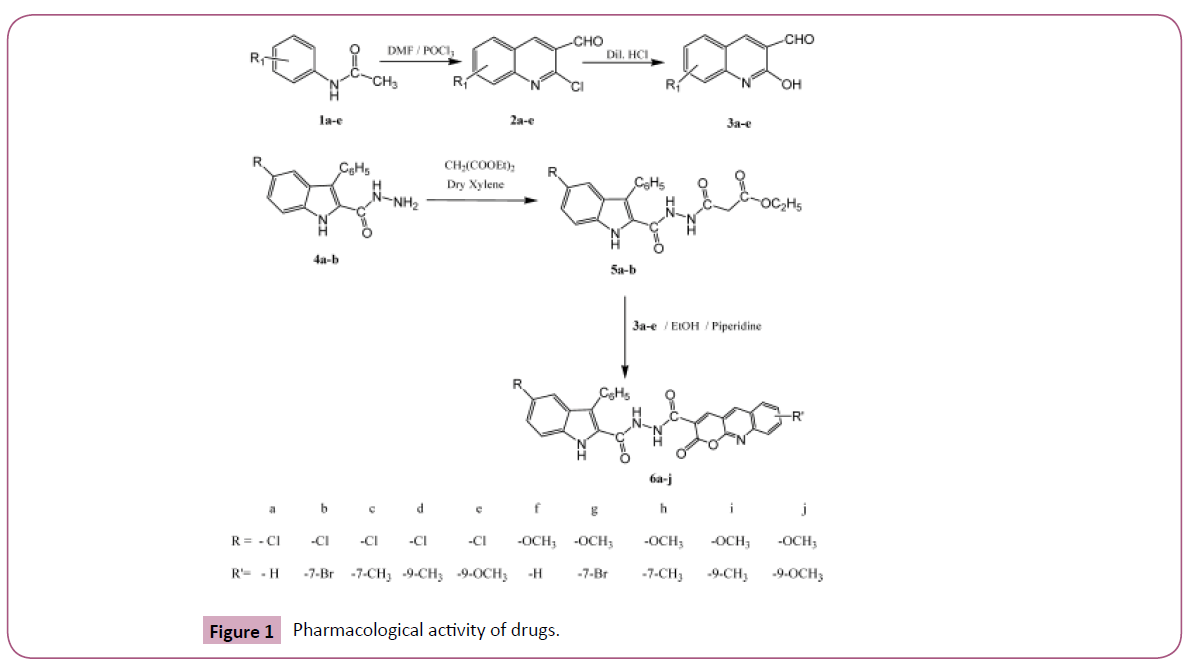
Heterocyclic moieties endow with as model scaffold on which pharmacophore can be efficiently attached to create novel drugs. Indoles are amongst the most imperative class of heteroaromatics in medicinal chemistry, which are everpresent in natural artefacts. The foresaid analogues have become noteworthy compounds because of their wide variety of applications in drug design and development. Numerous indole derivatives accounted in the literature are identified to acquire diverged biological potentials viz., antimalarial activity [1], anti-TB evaluation [2] and anti-inflammatory [3]. Quinolines analogues have been engrossed the interest of the many chemists because of their existence in various natural products exhibiting considerable biological activities [4-8]. Many indolo[2, 3-c] isoquinolines investigated from our laboratory have been found to possess bactericidal and fungicidal activities[9-11]. Earlier we have disclosed the synthesis of 3, 5-disubstituted-Nβ- (2-oxobenzopyran-3-carbonyl)- 1H-indole-2-carbohydrazide [12] by making use of ethyl 3-oxo-3-{2-[(5-substituted-3-phenyl-1Hindol- 2-yl)carbonyl] hydrazinyl}propanoates [13] 5a, b, which in turn were prepared by the reaction of 5-substituted-3-phenyl- 1H-indole-2-carbohydrazides 4a, b and diethylmalonate. In vision of these findings and in persistence of our research work on heterocyclic compounds, [14-23] we hereby account the pharmacological activities of some 5-substituted-Nβ-(2-oxo- 2H-pyrano[2, 3- b] quinoline-3-carbonyl)-3-phenyl-1H-indole- 2-carbohydrazides 6a-j by ethyl 3-oxo-3-{2-[(5-substituted-3- phenyl-1H-indol-2-yl) carbonyl] hydrazinyl} propanoates 5a, b and substituted- 2-hydroxy-3-formylquinolines 3a-e as starting materials wherein substituted-2H-pyrano[2, 3-b]quinoline moiety attached to β-nitrogen of 5-substituted-3-phenyl-1Hindole- 2-carbohydrazide at its 3-positon via carbonyl group (Figure 1).
Materials and Methods
Melting points were determined in open capillary tubes and are uncorrected. IR spectra were recorded in KBr discs (νmax in cm-1) on Perkin- Elmer FT-IR (Spectrum ONE) spectrophotometer, 1H NMR spectra on a Bruker AMX (400 MHz) spectrophotometer using DMSO-d6 as solvent using TMS as an internal standard (chemical shifts in d) and mass spectra on a mass spectrometer JEOL sx-102 (FAB) instrument (m/z in %). Compounds were checked for their purity by TLC on silica gel 60G F254 plates and iodine vapors were used as visualizing agent. Elemental analysis carried out using Flash EA1112 series elemental Analyser.
General methods
The starting materials ethyl 3-oxo-3-{2-[(5-substituted-3- phenyl-1H-indol-2-yl) carbonyl] hydrazinyl} propanoates [13] 5a, b and substituted-2-hydroxy- 3-formyl quinolines [8, 9] 3a-e were papered according to reported methods. Synthesis and antimicrobial activities of all the 5-Substituted-Nβ-(2-oxo- 2H-pyrano[2, 3-b] quinoline-3-carbonyl)-3-phenyl-1H-indole-2- carbohydrazides 6a-j were already reported by us [15].
Synthesis of Substituted-2-chloro-3-formyl quinolines 2a-e: Dimethyl formamide (0.006 mol) was cooled to 0°C in a flask equipped with a drying tube and phosphorous oxychloride (0.006 mol) was added dropwise with stirring. To this solution acetanilide (1a-e) (0.001 mol) was added in small portions and after 5 min the reaction mixture was heated for 16 h on boiling water bath. The reaction mixture was poured into ice water and stirred for 30 min. The solid separated was filtered, dried and recrystallized from ethyl acetate to get substituted-2-chloro-3- formyl quinolines 2a-e in good yield.
Synthesis of Substituted-2-hydroxy-3-formyl quinolines 3a-e: A mixture of 2-chloro-3-formylquinolines 2a-e (0.001 mol) and aqueous hydrochloric acid 3.5 mL (4 N) was heated under reflux conditions for 1 h and then allowed to cool to room temperature. The reaction mixture was poured on to crushed ice and the solid separated filtered, washed with water, dried and recrystallized from aqueous acetic acid to get substituted-2-hydroxy-3-formyl quinolines 3a-e in good yield.
Synthesis of 5-Substituted-Nβ-(2-oxo-2H-pyrano[2, 3-b] quinoline- 3-carbonyl)-3-phenyl-1H-indole-2-carbohydrazides 6a-j: A mixture of compounds ethyl 3-oxo-3-{2-[(5-substituted-3-phenyl-1Hindol- 2-yl)carbonyl] hydrazinyl}propanoates 5a, b (0.001 mol) and various substituted-2-hydroxy-3-formylquinolines 3a-e (0.001 mol) in ethanol (10 mL) was refluxed for 5 h in the presence of catalytic amount of piperdine. Excess of ethanol was removed by distillation. Crystalline residue obtained was filtered, washed with little amount of ethanol, dried and crystallized from suitable solvent to afford 5-substituted-Nβ-(2-oxo-2H-pyrano[2,3-b] quinoline-3-carbonyl)-3-phenyl-1H-indole-2-carbohydrazides 6a-j in good yield. The compounds 6a, 6b, 6c, 6d, 6e and 6g were recrystallized from ethanol and the compounds 6f, 6h, 6i and 6j were recrystallized from isopropyl alcohol.
5-Chloro-Nβ-(2-oxo-2H-pyrano[2, 3-b] quinoline-3-carbonyl)-3- phenyl-1H-indole-2-carbohydrazide (6a): Colourless crystals in 71% yield, mp 212°C: IR (KBr) in cm-1: 1149 (COC), 1597 (CN), 1668, 1695, 1733 (CO/CO/CO), 3065, 3201, 3386 (NH/NH/NH). 1H NMR in d: 7.14-7.92 (m, 14H, ArH), 9.42 (s, 1H, indole NH), 9.71 (s, 1H, CONH), 10.20 (s, 1H, CONH). FAB-MS m/z (in %): 508, 510 (33%, 12%), 311, 313 (48%, 19%), 255, 257 (100%, 31%), 225, 227 (24%, 9%), 190 (36%). Anal. Calcd for C28H17N4O4Cl: C, 66.08; H, 3.34; N, 11.01; Found: C, 65.91; H, 3.42; N, 10.89.
5-Chloro-Nβ-(7-bromo-2-oxo-2H-pyrano[2, 3-b] quinoline-3- carbonyl)-3- phenyl-1H-indole-2- carbo hydrazide (6b): Pale yellow crystals in 69% yield, mp185°C: IR (KBr) cm-1: 1158 (COC), 1593 (CN), 1663, 1701, 1731 (CO/CO/CO), 3050, 3211, 3305 (NH/NH/NH). 1H NMR in δ : 6.80-7.84 (m, 13H, ArH), 9.58 (s, 1H, indole NH), 9.95 (s, 1H, CONH), 10.19 (s, 1H, CONH). Anal. Calcd for C28H16N4O4ClBr: C, 57.19; H, 2.72; N, 9.53; Found: C, 56.98; H, 2.58; N, 9.34.
5-Chloro-Nβ-(7-methyl-2-oxo-2H-pyrano[2, 3-b] quinoline-3- carbonyl)-3-phenyl-1H-indole-2-carbohydrazide (6c): Colourless crystals in 75% yield, mp 145°C: IR (KBr) cm-1: 1154 (C–O–C), 1564 (CN), 1667, 1689, 1713 (CO/CO/CO), 3060, 3195, 3281 (NH/ NH/NH). 1H-NMR in δ: 1.74 (s, 3H, CH3), 7.12-7.80 (m, 13H, ArH), 9.48 (s, 1H, indole NH), 9.84 (s, 1H, CONH), 10.28 (s, 1H, CONH). Anal. Calcd for C29H19N4O4Cl: C, 66.60; H, 3.64; N, 10.71; Found: C, 66.48; H, 3.52; N, 10.55.
5-Chloro-Nβ-(9-methyl-2-oxo-2H-pyrano[2, 3-b] quinoline-3- carbonyl)-3-phenyl-1H-indole-2-carbohydrazide (6d): Colourless crystals in 64% yield, mp 191°C: IR (KBr) cm-1: 1162 (C–O–C), 1579 (CN), 1668, 1695, 1723 (CO/CO/CO), 3061, 3187, 3258 (NH/ NH/NH). 1H NMR in δ: 1.98 (s, 3H, CH3), 7.21-7.91 (m, 13H, ArH), 9.18 (s, 1H, indole NH), 9.70 (s, 1H, CONH), 10.18 (s, 1H, CONH). Anal. Calcd for C29H19N4O4Cl: C, 66.60; H, 3.64; N, 10.71; Found: C, 66.41; H, 3.48; N, 10.61.
5-Chloro-Nβ-(9-methoxy-2-oxo-2H-pyrano[2, 3-b] quinoline-3- carbonyl)-3-phenyl-1H-indole-2-carbohydrazide (6e): Brown crystals in 71% yield, mp 231°C: IR (KBr) cm-1: 1162 (COC), 1578 (CN), 1671, 1692, 1719 (CO/CO/CO), 3081, 3188, 3280 (NH/NH/ NH). 1H NMR in δ: 2.28 (s, 3H, –OCH3), 7.32—7.98 (m, 13H, ArH), 9.61 (s, 1H, indole NH), 10.0 (s, 1H, CONH), 10.36 (s, 1H, CONH). Anal. Calcd for C29H19N4O5Cl: C, 64.62; H, 3.53; N, 10.40; Found: C, 64.40; H, 3.35; N, 10.25.
5-Methoxy-Nβ -(2-oxo-2H-pyrano[2, 3-b] quinoline-3-carbonyl)- 3-phenyl- 1H-indole-2-carbohydrazide (6f): Pale yellow crystals in 69% yield, mp 189°C: IR (KBr) cm-1: 1166 (COC), 1578 (CN), 1681, 1698, 1719 (CO/CO/CO), 3083, 3105, 3187 (NH/NH/NH). 1H NMR in δ: 2.15 (s, 3H, –OCH3), 7.01-7.78 (m, 14H, ArH), 9.65 (s, 1H, indole NH), 9.91 (s, 1H, CONH), 10.28 (s, 1H, CONH). FABMS m/z (in %): 504 (42%), 307 (21%), 251 (100%), 221(15%), 190 (41%). Anal. Calcd for C29H20N4O5: C, 69.05; H, 3.97; N, 11.11; Found: C, 68.80; H, 3.75; N, 11.05.
5-Methoxy-Nβ -(7-bromo-2-oxo-2H-pyrano[2, 3-b] quinoline- 3-carbonyl)- 3-phenyl-1H-indole-2-carbohydrazide (6g): Brown crystals in 72% yield, mp 225 °C: IR (KBr) cm-1: 1154 (C–O–C), 1599 (CN), 1678, 1698, 1728 (CO/CO/CO), 3058, 3108, 3281 (NH/ NH/NH). 1H NMR in δ: 2.18 (s, 3H, –OCH3), 6.91-7.68 (m, 13H, ArH), 9.80 (s, 1H, indole NH), 10.28 (s, 1H, CONH), 10.57 (s, 1H, CONH). Anal. Calcd for C29H19N4O5Br: C, 59.69; H, 3.26; N, 9.60; Found: C, 59.48; H, 3.14; N, 9.39.
5-Methoxy-Nβ -(7-methyl-2-oxo-2H-pyrano[2, 3-b] quinoline-3- carbonyl)- 3-phenyl-1H-indole-2-carbo hydrazide (6h): Colourless crystals in 64% yield, mp 251 °C: IR (KBr) cm-1: 1162 (C–O–C), 1580 (CN), 1661, 1680, 1723 (CO/CO/CO), 3061, 3188, 3251 (NH/ NH/NH). 1H NMR in δ: 1.71 (s, 3H, CH3), 2.20 (s, 3H, –OCH3), 7.14-7.78 (m, 13H, ArH), 9.76 (s, 1H, indole NH), 10.19 (s, 1H, CONH), 10.56 (s, 1H, CONH). Anal. Calcd for C30H22N4O5: C, 69.50; H, 4.25; N, 10.81; Found: C, 69.36; H, 4.15; N, 10.60.
5-Methoxy-Nβ-(9-methyl-2-oxo-2H-pyrano[2, 3-b] quinoline-3- carbonyl)- 3-phenyl-1H-indole-2-carbohydrazide (6i): Colourless crystals in 71% yield, mp 165 °C: IR (KBr) cm-1: 1166 (C–O–C), 1578 (CN), 1668, 1685, 1719 (CO/CO/CO), 3083, 3187, 3248 (NH/ NH/NH).1H NMR in δ: 1.74 (s, 3H, CH3), 2.18 (s, 3H, –OCH3), 7.10- 7.79 (m, 13H, ArH), 9.36 (s, 1H, indole NH), 9.78 (s, 1H, CONH), 10.08 (s, 1H, CONH). Anal. Calcd for C30H22N4O5: C, 69.50; H, 4.25; N, 10.81; Found: C, 69.70; H, 4.05; N, 10.58.
5-Methoxy-Nβ -(9-methoxy-2-oxo-2H-pyrano[2, 3-b] quinoline-3- carbonyl)-3-phenyl-1H-indole-2-carbohydrazide (6j): Colourless crystals in 69% yield, mp 158 °C: IR (KBr) cm-1: 1162 (C–O–C), 1578 (C_N), 1661, 1678, 1723 (CO/CO/CO), 3057, 3186, 3281 (NH/NH/NH).1H NMR in δ: 2.19 (s, 3H, –OCH3), 2.41 (s, 3H, – OCH3), 7.21-7.79 (m, 13H, ArH), 10.00 (s, 1H, indole NH), 10.25 (s, 1H, CONH), 10.58 (s, 1H, CONH). Anal. Calcd for C30H22N4O6: C, 67.42; H, 4.12; N, 10.49; Found: C, 67.31; H, 4.19; N, 10.68.
Pharmacological Activities
Ant-inflammatory activity by paw-edema method
In vivo anti-inflammatory activity was assessed by carrageen an induced rat hind paw oedema method. Albino rats of either sex weighing between 150-200 g were divided into groups of six animals each. The first group served as the control and received vehicle only (Tween-80, 1%), second group of animals were administered with standard drug Indomethacin 25 mg/ kg body weight, orally. The animals of the other groups were treated with synthesized compounds at a dose of 25 mg/kg body weight, orally. A mark was made on both the hind paws just below the tibio-tarsal junction so that every time the paw could be dipped in the mercury column of plethysmo graph upto the mark to ensure constant paw volume. The normal paw volume was measured for both the legs, after 30 min. of above treatment an inflammation was induced in the left hind paw by injecting 0.1 ml of carrageen an (1%, w/v) in the planter tissue of the paw of all animals. The right paw served as a reference to non-inflammed paw for comparison. The initial paw volume was measured plethysmo graphically within 30 sec. of the injection. The relative increase in the paw served as a reference to noninflammed paw for comparison. The relative increase in the paw volume was measured in control, standard and treated group, for 4 hr after carrageen an injection. The percent increase in paw volume over the initial reading was also calculated. This increase in paw volume in animals treated with standard drug and the synthesized indole and thiazole derivatives were compared with the increase in paw volume of control animals. Thus, percent inhibition of paw volume was calculated using the formula
% inhibition = (1-Vt / Vc) x 100.
Where, Vt and Vc are mean relative changes in the paw volume of the test and control respectively.
All the newly synthesized indole derivatives were screened for their anti-inflammatory activity as compared with standard drug indomethacin and 1% Tween-80 was used as a control. The results of anti-inflammatory testing of all the tested compounds are summarized in the following Table 1.
Analgesic activity by tail flick method
Tail flick method was pursued for the testing of analgesic activity using the analgesiometer. Albino mice of either sex weighing between 25-30 g were randomly distributed into groups consisting of six animals in each group. The first group served as a control group and animals were administered with vehicle (Tween-80, 1%) orally. The second group was administered with standard drug analgin at a dose of 25 mg/kg body weight, orally. The animals of the other groups were treated with indole derivatives at a dose of 25 mg/kg body weight, orally. The reaction time was noted at 0, 30, 60 and 90 min. of time intervals after the drug administration. Percent protection against tail flicking was calculated using the formula:
% Protection = (1-Wc/Wt) x 100
Where Wc and Wt are the mean time for the tail flicking in the test and control groups, respectively.
All the newly synthesized indole derivatives were screened for their analgesic activity as compared with standard drug analgin and 1% Tween-80 was used as a control. The results of analgesic testing of all the tested compounds are summarized in the following Table 2.
Results and Discussions
Pharmacological activities
Ant-inflammatory activity: All the indole derivatives were screened for their in vivo anti-inflammatory activity as compared with standard drug indomethacin and 1% Tween-80 was used as a control [24].
From Table 1 of the results of anti-inflammatory evaluation of indole derivatives, it is clearly indicates that the compounds 6b, 6c, 6e and 6g have displayed excellent anti-inflammatory activity as compared with that of standard drug indomethacin. The compounds 6a, 6d and 6j showed moderate anti-inflammatory activity as compared with that of standard drug indomethacin. The remaining compounds 6f, 6h and 6i were less active when compared with that of standard drug indomethacin. Under these conditions, the standard drug indomethacin used exhibited 73.02% anti-inflammatory activity and control 1% Tween-80 used as a control did not show any anti-inflammatory activity.
| Group | Dose | Mean values (± SE) of oedema | % inhibition | |||
|---|---|---|---|---|---|---|
| (Compound) | mg/kg b.w | Volume at different intervals | at 2hr | |||
| 1 | 1 ml | 0.305 | 0.295 | 0.285 | 0.275 | - |
| (Control) | (± 0.014) | (± 0.024) | (± 0.022) | (± 0.019 | ||
| 2 | 25 | 0.195** | 0.145 | 0.081*** | 0.091 | 72.47 |
| (Indomethacin) | (± 0.010) | (± 0.012) | (± 0.009) | (± 0.011) | ||
| 6a | 25 | 0.308 | 0.209** | 0.224 | 0.128* | 36.8 |
| (± 0.008) | (± 0.016) | (± 0.016) | (± 0.016) | |||
| 6b | 25 | 0.188** | 0.119 | 0.088*** | 0.108 | 65.27 |
| (± 0.015) | (± 0.019) | (± 0.021) | (± 0.015) | |||
| 6c | 25 | 0.218** | 0.125 | 0.118 | 0.104 | 60.51 |
| ( ± 0.016) | ( ± 0.016) | ( ± 0.021) | ( ± 0.019) | |||
| 6d | 25 | 0.241** | 0.199 | 0.192 | 0.217 | 32.63 |
| ( ± 0.011) | ( ± 0.021) | ( ± 0.018) | ( ± 0.012) | |||
| 6e | 25 | 0.213 | 0.154 | 0.104*** | 0.112 | 63.35 |
| ( ± 0.011) | ( ± 0.013) | ( ± 0.009) | ( ± 0.011) | |||
| 6f | 25 | 0.287 | 0.264 | 0.255 | 0.231 | 10.52 |
| ( ± 0.020) | ( ± 0.021) | ( ± 0.021) | ( ± .014) | |||
| 6g | 25 | 0.247** | 0.231 | 0.118** | 0.108 | 61.88 |
| ( ± 0.021) | ( ± 0.012) | ( ± 0.021) | ( ± 0.017) | |||
| 6h | 25 | 0.288 | 0.241** | 0.238 | 0.224* | 12.8 |
| ( ± 0.008) | ( ± 0.016) | ( ± 0.016) | ( ± 0.016) | |||
| 6i | 25 | 0.281 | 0.261** | 0.241 | 0.220* | 18. 10 |
| ( ± 0.008) | ( ± 0.016) | ( ± 0.016) | ( ± 0.016) | |||
| 6j | 25 | 0.26 | 0.247 | 0.238 | 0.221 | 32.77 |
| ( ± 0.018) | ( ± 0.016) | ( ± 0.021) | ( ± 0.019) | |||
| No. of animals for each group=6, Control= 1% Tween-80 Significance levels *P< 0.05, **P<0.01, ***P<0.001 compared with respective control (ANOVA followed by Dunnet’s test). Each value represents ±SE (n=6).
|
||||||
Table 1. Anti-inflammatory activity of indole compounds.
Analgesic activity: All the indole derivatives were screened for their analgesic activity as compared with standard drug analgin and 1% Tween-80 was used as a control [25].
From the Table 2, it is clearly indicates that the compounds 6b, 6c, 6e and 6g revealed good analgesic potential as evaluated with that of standard drug analgin. The compounds 6a, 6d and 6j showed sensible analgesic activity as compared with that of standard drug analgin. The rest of the compounds 6f, 6h and 6i were less active when compared with that of standard drug. Under these conditions the standard drug analgin used exhibited 63.18% analgesia, after 60 min drug administration and 1% Tween-80 used as a control did not show any analgesic activity.
| Group (Compound) |
Dose mg/kg b.w |
Average (± SE) reaction time (sec.) Time after drug treatment (min.) |
% Analgesia at 60 min |
|||
|---|---|---|---|---|---|---|
| 0 | 30 | 60 | 90 | |||
| 1 (Control) |
1 ml | 3.245 | 3.26 | 3.271 | 3.257 | - |
| ( ± 0.219) | ( ± 0.223) | ( ± 0.230) | ( ± 0.212) | |||
| 2 (Analgin) |
25 | 3.895** | 5.278** | 8.884** | 9.057 | 63.18 |
| ( ± 0.219) | ( ± 0.223) | ( ± 0.208) | ( ± 0.218) | |||
| 6a | 25 | 3.385 ** | 5.212 | 5.200* | 5.12 | 37.09 |
| (± 0.219) | ( ± 0.223) | ( ± 0.2108) | ( ± 0.2108) | |||
| 6b | 25 | 3.715 ** | 5.001 | 7.982** | 8.015 | 59.02 |
| (± 0.145) | (± 0.223) | (± 0.281) | (± 0.108) | |||
| 6c | 25 | 3. 724** | 4.648 | 6. 227* | 6. 725** | 47.47 |
| (± 0.219) | (± 0.223) | (± 0.210) | (± 0.208) | |||
| 6d | 25 | 3.254 | 4. 628** | 5.228 | 5.275 | 37.43 |
| (± 0.219) | (± 0.223) | (± 0.2108) | (± 0.2108) | |||
| 6e | 25 | 3.754 ** | 5.218 | 7.041** | 6.188 | 53.58 |
| (± 0.070) | (± 0.223) | (± 0.218) | (± 0.208) | |||
| 6f | 25 | 3. 445* | 3. 828 | 3.791 | 3. 788 | 13.78 |
| (± 0.219) | (± 0.293) | (± 0.2108) | (± 0.2108) | |||
| 6g | 25 | 3.919** | 4.068 | 5.557* | 4.725 | 41.11 |
| (± 0.104) | (± 0.211) | (± 0.108) | (± 0.201) | |||
| 6h | 25 | 3.281 | 3.289 | 4.117 | 3. 725 | 21.65 |
| (± 0.219) | (± 0.223) | (± 0.2108) | (± 0.2108) | |||
| 6i | 25 | 3.318 | 3.541 | 3.451 | 3.657 | 5.22 |
| (± 0.019) | (± 0.224) | (± 0.219) | (± 0.2108) | |||
| 6j | 25 | 3.215 | 3.954 | 5.951* | 6.117 | 35.3 |
| (± 0.104) | (± 0.423) | (± 0.108) | (± 0.109) | |||
| No. of animals for each group=6, Control= 1% Tween-80 Significance levels *P< 0.05, **P<0.01, ***P<0.001 compared with respective control (ANOVA followed by Dunnet’s test). Each value represents ±SE (n=6). | ||||||
Table 2: Analgesic activity of indole compounds.
Conclusion
All the indole derivatives were evaluated for their in vivo pharmacological activities as compared with standard drug indomethacin and analgin respectively with 1% Tween-80 as a control. The results of anti-inflammatory and analgesic activities of indole derivatives, it is clear that the compounds 6b, 6c, 6e and 6g have exhibited good pharmacological activities as compared with that of standard drugs.
Acknowledgement
The authors are thankful to Directors, CDRI, Lucknow and IISc, Bangalore for spectral data. Authors are also thankful to Chairman, Department of Chemistry, Gulbarga University, Gulbarga-585106 for providing laboratory facilities. Author acknowledges The Director, Skanda Life Sciences Pvt. Ltd. Chandana layout, Sunkadakatte, Bengaluru, Karnataka 560091 for providing facilities to carry out biological evaluation.
References
- Onyeibor O, Croft SL, Dodson HI, Wright CW. (2005) Synthesis of some cryptolepine analogues, assessment of their antimalarial and cytotoxic activities, and consideration of their antimalarial mode of action, J. Med. Chem 48:2701-2709.
- Guuzel O, Terzioglu N, Capan G , Salman A.(2006) Synthesis and biological evaluation of new 5-methyl-N-(3-oxo-1-thia- 4-azaspiro[4.5]-dec-4-yl)-3-phenyl-1H-indole-2-carboxamide derivatives. Arkivoc 12:98-110.
- Kalgutkar AS, Crews BC, Saleh S, Prudhommae D, Marnett LJ. (2005) Indolyl esters and amides related to indomethacin are selective COX- 2 inhibitors. Bioorg. Med. Chem 13(24):6810-6822.
- Mital A, Singh NV, and Ramachandran U. (2006) Synthesis and antimycobacterial activities of certain trifluoromethylaminoquinoline derivatives, Arkivoc 10: 220-227.
- Rajkumar U, Rajkumar V, Maske V, Shingare MS. (2006) Synthesis and antibacterial activities of α-hydroxyphosphonates and α-acetyloxyphosphonates derived from 2-chloroquinoline-3- carbaldehyde. Arkivoc 11: 196-204.
- Klingenstein R, Melnyk P, Rutger LS, Ryckebusch A, Korth C. (2006) Synthesis and in vitro and in vivo antimalarial activity of N1-(7-chloro- 4-quinolyl)-1,4-bis(3-aminopropyl)piperazine derivatives. J. Med. Chem 49: 5300-5308.
- Srivastav A, Singh R M. (2005) Vilsmeier-Haack reagent: A facile synthesis of 2-chloro-3-formylquinolines from N-arylacetamides and transformation into different functionalities. Indian J. Chem 44B:1868-1875.
- Srivastava A, Chandra A, Singh RM. (2005) Thiophene-fused quinoline analogues: Facile synthesis of 3-amino-2-cyanothieno [2, 3-b] quinolines from 2-chloro-3-cyanoquinolines. Indian J. Chem 44B:2077-2081.
- Hiremath, SP, Saundane A R, Swamy HKS, Mruthunjayaswamy BHM. (1993) Indian J. Chem 3B:37-42.
- Hiremath, S P, Saundane AR, Mruthunjayaswamy BHM. (1993) Indian J. Heterocyclic Chem 30:603-609.
- Hiremath, SP, Saundane AR, Mruthunjayaswamy BHM. (1992) J. Indian Chem. Soc 72:735-741.
- Mruthunjayaswamy BHM, Shanthaveerappa B K. (2000) Synthesis and pharmacological evaluation of 3, 5-disubstituted indole-2-[Nβ- (substituted benzopyran-2'-one-3'-carboxyl)]carboxy hydrazides and 2H-3-(various substituted indol-3' -yl)methyl-1 ,3-benzothiazoles. Indian J. Chem 39B:433-438.
- Mruthunjayaswamy, B. H. M.; Shanthaveerappa, B. K. (1997) Indian J. Heterocyclic Chem 6:263-270.
- Basavarajaiah, S M, Mruthyunjayaswamy BHM. (2009) Synthesis and anti-microbial activity of some new-5-substituted-N1-[(1E)-(2-hydroxyquinolin- 3-yl) methylene]-3-phenyl-1H-indole-2-carbohydrzide derivatives. Heterocyclic Communications 3(15):217-226.
- Basavarajaiah SM, Mruthyunjayaswamy BHM. (2009) Synthesis and antimicrobial activity of some 5-substituted-3-phenyl-Nβ- (substituted-2-oxo-2H-pyrano [2, 3-b] quinoline-3-carbonyl)-1Hindole- 2-carboxyhydrazide. Chem. Pharma. Bull 57(6):557-560.
- Basavarajaiah, SM, Mruthyunjayaswamy BHM. (2016) Synthesis and antimicrobial activity of novel 5-substituted-N-(substituted- 2H-[1, 3] oxazino [6, 5-b] quinolin-3(4H)-yl)-3-phenyl-1H-indole-2- carboxamides. Indian J. Chem 55B: 1511-1519.
- Sheshandrakumar KN, Srividya J, Narayanaswamy BJ, Umesha K, Basavarajaiah SM. (2017) Synthesis and evaluation of biological activity of some new 3, 7-substituted 2H-pyrano/thiopyrano [2, 3-b] quinolin-2-ones. Indian J. Heterocyclic. Chem 27(3): 281-288.
- Basavarajaiah SM, Mruthyunjayaswamy BH M. (2018) Synthesis and antimicrobial activity of some 5-chloro-3-phenyl-1H-indole-2- carbonyl azide derivatives. Indian J. Chem 57B:390-399.
- Basavarajaiah SM, Sheshandrakumara KN, Sudharshan NR, Lokesh B. (2018) Design, synthesis and evaluation of antimicrobial activity of some novel 3- (4-substituted phenyl)-2-(2-substituted1h-indol- 3-yl)-3, 4-dihydroimidazo [4, 5-b] indoles. International Journal of Creative Research Thoughts 6(2):1156-1161.
- Basavarajaiah SM, Mruthyunjayaswamy BHM. (2020) Pharmacological activities of 5-substituted-N-(substituted- 2H-[1, 3]oxazino[6, 5-b]quinolin-3(4H)-yl)- 3-phenyl-1H-indole-2- carboxamides, International Journal of Creative Research Thoughts 8(7):232-247.
- Basavarajaiah SM, Mruthyunjayaswamy BH M. (2020) Pharmacological activities of 6-substituted-3-(5-chloro-3-phenyl-1H-indole-2yl)-3, 4-dihydro-4-substituted-4-substituted-phenacyl-2H-1, 3-benzoxazin- 2-ones. Inter. J. Sci. Res 9(7):518-521.
- Basavarajaiah SM, Raviraj P, Nagesh GY. (2021) A Comprehensive Review on the Biological Interest of Quinoline and its Derivatives, Bioorg. Med. Chem 32:115973.
- Basavarajaiah SM, Nagesh GY. (2021). A Comprehensive Review on the Biological Interest of Quinoline and its Derivatives, Synth. Commu 51(8): 1133-1159.
- Da Silva J, Bijlsma, J. (2000) Rheum Dis Clin North Ame 26:859-890.
- Turner, R. A. (1965) “Screening Methods in Pharmacology”, Satya Publishers, India.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences