Periprostatic and Iliohypogastric Nerve Block for Pain in Transrectal Prostate Biopsy
1Department of Anesthesiology, University of Health Sciences, Dr. AY Oncology Training and Research Hospital, Ankara, Turkey
2Department of Urology, University of Health Sciences, Dr. AY Oncology Training and Research Hospital, Ankara, Turkey
- Corresponding Author:
- Guldeniz Argun
Department of Anesthesiology and Reanimation, University of Health Sciences, Dr. AY Oncology Training and Research Hospital
Ankara, Turkey
Tel: +905336230405
Fax: +903123464361
E-mail: guldargun@yahoo.com
Received date: September 17, 2018; Accepted date: October 5, 2018; Published date: October 12, 2018
Citation: Guldeniz A, Ismail S (2018) Periprostatic and Iliohypogastric Nerve Block for Pain in Transrectal Prostate Biopsy. J Anaesthesiol Crit Care Vol.1 No.3:13
Abstract
Objectives
We aimed to investigate the effect of Periprostatic Nerve Block (PNB) and Iliohypogastric Nerve Block (INB) on pain control during transrectal ultrasound-guided prostate biopsy.
Methods
In total, 90 patients who underwent transrectal ultrasound-guided prostate biopsy due to suspected prostate cancer were randomized into three groups (30 patients each). Group 1 received intrarectal prilocaine–lidocaine cream (control group). Group 2 received PNB and intrarectal prilocaine–lidocaine cream. Group 3 received INB and intrarectal prilocaine-lidocaine cream. Visual Analog Scale (VAS), a subjective scale, and Electromyography (EMG), an objective scale, were used to evaluate pain degrees during rectal probe insertion and prostate biopsy. Mean Motor Unit Potential (MUP) durations and amplitudes were recorded using EMG.
Results
The VAS scores during rectal probe insertion in Group 3 (INB) were significantly less than those in the other groups. The VAS scores during prostate biopsy in Group 3 were significantly less than those in the other groups. Mean MUP durations and amplitudes were significantly lesser in Group 3 than the other groups, during both procedures.
Conclusion
INB and intrarectal prilocaine–lidocaine cream application provided significantly better pain control than PNB and intrarectal prilocaine–lidocaine cream application.
Keywords
Iliohypogastric nerve block; Periprostatic nerve block; Transrectal ultrasound-guided prostate biopsy; Pain
Introduction
Transrectal ultrasound-guided prostate biopsy is the most widely used method to accurately diagnose patients with suspected prostate cancer. Although current guidelines recommend the use of periprostatic block for pain control during transrectal prostate biopsy, other anesthesia methods are still in practice. Pudendal block, intraprostatic analgesia, sedoanalgesia are among these anesthesia methods. Pudendal block can only prevent pain due to the application of rectal probe. Applications of sedoanalgesia involve the risks of general anesthesia and require follow-up of the patient. Intraprostatic block application is also painful. Particularly, transrectal probe insertion and multiple punctures of the prostatic tissue, which are painful procedures for patients. In current urology practices, Periprostatic Nerve Block (PNB) is the most commonly preferred method for pain control [1]. Combining PNB with an intrarectal topical anesthetic is also thought to be more efficient in achieving pain control [2]. In recent years, developments in ultrasound technology have provided enabling the direct visualization of the peripheral nerves [3]. In parallel to these developments, Iliohypogastric Nerve Block (INB) has been used for pelvic operations such as cesarean sections, inguinal hernia repairs in adults and groin surgeries in children [3,4].
Many studies on pain management during transrectal ultrasoundguided prostate biopsy, during rectal ultrasound probe insertions, rectal manipulations and biopsy procedures have been described in the literature. Various local anesthesia methods have been evaluated for their advantage. However, to the best of our knowledge, there are no original articles comparing the superiority of PNB to INB. Visual Analog Scale (VAS), a subjective scale and Electromyography (EMG), an objective scale, were used to evaluate pain degrees. Although VAS is commonly used in studies to evaluate pain degree, in some patients, pain cannot be accurately assessed. Hence, the current study was designed to incorporate EMG as an objective scale.
Materials and Methods
Patients and study design
The current study was designed as a prospective, blind and observational study and was approved by the local ethics committee at University of Health Sciences, Dr. AY Ankara Oncology Training and Research Hospital (ethics committee decision number: 2017-06/02). Written consent was obtained from patients or their legal representatives.
In total, 90 consecutive patients who underwent transrectal ultrasound-guided prostate biopsy, with the intention to accurately diagnose suspected prostate cancer, at the urology clinic between June and August 2017 were included in this study. Abnormal digital rectal examination and/or prostate-specific antigen (PSA; >4.0 ng/mL) levels indicated prostate biopsy requirement. Patients with previous biopsy attempts; radical prostatectomy; chronic pain syndrome; hemorrhagic diathesis; recto-anal pathology; treatment with monoamine oxidase inhibitors, tricyclic antidepressants, or anticoagulants; epilepsy history; lidocaine allergy; spinal cord trauma; and urge urinary symptoms were excluded from the study. Among the eligible patients, those aged between 50 and 75 years, who provided written informed consent, were recruited in the study.
Patients were categorized into three groups based on the block type used. Group 1 (control group) comprised 30 patients who received only intrarectal prilocaine–lidocaine cream. In Group 2, 30 patients received periprostatic nerve block following intrarectal prilocaine–lidocaine cream application. In group 3, 30 patients received iliohypogastric nerve block following intrarectal prilocaine–lidocaine cream application.
Prior to the procedures, EMG probes were placed on perineal surface in all patients and intrarectal prilocaine–lidocaine cream was applied and allowed to absorb for 10 min. In Group 1, the rectal probe was inserted and prostate biopsy was subsequently performed. In Group 2, following intrarectal prilocaine–lidocaine cream application, PNB was performed by injecting 10 mL of 1% lidocaine into the periprostatic nerve plexus at the prostate base just lateral to the seminal vesicle and prostate junction on each side, with an 18 cm spinal needle (Becton Dickinson Co. New Jersey, USA) under transrectal ultrasound guidance. The accuracy of the block was determined by local anesthetic fluid collection on transrectal ultrasound. Next, the rectal probe was inserted, and prostate biopsy was subsequently performed. PNB was performed by the same urologist. In Group 3, INB was performed following intrarectal prilocaine–lidocaine cream application and was assisted by transrectal ultrasound guidance (Leopard 2001, Bruel & Kjaer, Denmark). The 2 cm medial and superior positions of the anterior superior iliac spine were marked. A 22 gauge, 100 mm insulated needle (Stimuplex D.B. Braun Medical-Freiburg, Germany) was advanced between the facials of the internal oblique and the transversus abdominis muscles, and a 10 mL of 1% lidocaine was administered under real-time ultrasound guidance. INB was bilaterally performed by the same anesthetist. The rectal probe was then inserted and prostate biopsy was subsequently performed. All biopsies in the groups were performed by the same urologist.
Transrectal ultrasound was performed using a 7.5 MHz transducer (Leopard, Bruel & Kjaer, Denmark), with the patient being in the left lateral position. An 18G Tru-cut core needle biopsy gun was used and twelve core biopsies were routinely performed in all patients under transrectal ultrasound guidance. All patients involved in the study were asked, by the same nurse, to express the degree of pain during rectal probe insertion and prostate biopsy. Additionally, the outputs of EMG recordings, such as Motor Unit Potential (MUP) durations and amplitudes, were noted for each patient during rectal probe insertion and prostate biopsy. As prophylaxis, all patients were orally administered 500 mg ciprofloxacin twice a day.
Statistical Analysis
Power analysis determined that the study required 90 patients, with at least 30 patients in each group for a gain of 80.4% power at alpha error of 0.05, beta error of 0.20, and effect size of 0.50. All groups were controlled for conformity to normal distribution by Kolmogorov–Smirnov test. Since only MUP amplitudes during prostate biopsy were normally distributed, analysis of variance with Bonferroni correction was used for comparing this parameter among the three independent groups. Since parametric test prerequisites were not provided for the other parameters, Kruskal–Wallis test was used for comparing these parameters among the three independent groups. Spearman correlation was used to determine the correlation between the EMG parameters and VAS scores. A P value of <0.05 was deemed as significant. The Statistical Package for the Social Science (SPSS Inc, Chicago, Illinois, USA) version 22.0 was used for statistical analysis.
Results
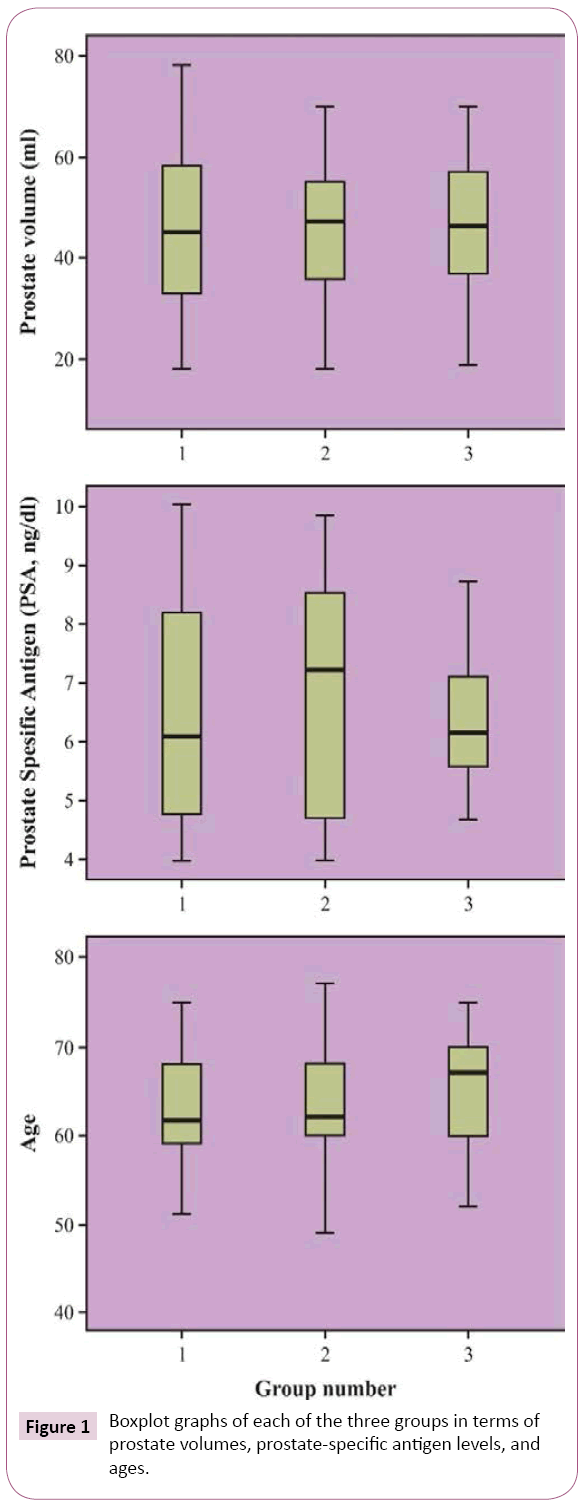
The mean patient age was 63.28 ± 7.01 (range, 43–77) years. The mean prostate volume was 45.19 ± 14.95 (range, 18–78) mL, and the mean PSA level was 6.62 ± 1.71 (range, 4–10) ng/mL (Table 1). There were no significant differences among the groups with regard to the prostate volumes, PSA levels and ages (Figure 1).
| Group 1 (n:30) | Group 2 (n:30) | Group 3 (n:30) | Total (n:90) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median | Min | Max | Mean ± SD | Median | Min | Max | Mean ± SD | Median | Min | Max | Mean ± SD | Median | Min | Max | |
| Age | 62.47 ± 6.53 | 61.4 | 51 | 75 | 62.17 ± 8.01 | 61.71 | 43 | 77 | 65.20 ± 6.21 | 66.29 | 52 | 75 | 63.28 ± 7.01 | 63.33 | 43 | 77 |
| VAS during probe insertion | 3.80 ± 1.12 | 3.78 | 2 | 6 | 2.70 ± 1.14 | 2.67 | 1 | 5 | 2.07 ± 0.94 | 2 | 1 | 4 | 2.86 ± 1.28 | 2.79 | 1 | 6 |
| MUP time during probe insertion (ms) | 13.37 ± 2.23 | 13.5 | 9 | 18 | 12.37 ± 1.54 | 12.67 | 9 | 14 | 10.60 ± 1.81 | 10.45 | 7 | 14 | 12.11 ± 2.19 | 12.34 | 7 | 18 |
| MUP amplitude during probe insertion (μV) | 549.63 ± 26.27 | 546.5 | 501 | 598 | 450.53 ± 30.61 | 457 | 401 | 498 | 394.77 ± 24.42 | 399.4 | 314 | 437 | 464.98 ± 69.80 | 457 | 314 | 598 |
| VAS during biopsy | 4.97 ± 0.85 | 4.91 | 4 | 7 | 3.43 ± 0.85 | 3.43 | 2 | 5 | 2.70 ± 0.75 | 2.68 | 1 | 4 | 3.70 ± 1.24 | 3.63 | 1 | 7 |
| MUP time during biopsy (ms) | 15.40 ± 2.25 | 15.33 | 11 | 21 | 13.63 ± 1.27 | 13.82 | 10 | 15 | 11.70 ± 1.53 | 11.75 | 9 | 15 | 13.58 ± 2.29 | 13.53 | 9 | 21 |
| MUP amplitude during biopsy (μV) | 642.73 ± 26.82 | 642.67 | 593 | 688 | 575.83 ± 37.64 | 642.67 | 507 | 663 | 503.90 ± 30.97 | 512.5 | 420 | 543 | 574.16 ± 65.25 | 572 | 420 | 688 |
| Prostate volume (mL;) | 45.20 ± 17.34 | 45 | 18 | 78 | 44.80 ± 14.08 | 47.33 | 18 | 70 | 45.57 ± 13.66 | 46 | 19 | 70 | 45.19 ± 14.95 | 46 | 18 | 78 |
| PSA (ng/dL) | 6.61 ± 1.83 | 6.16 | 4 | 10 | 6.81 ± 1.93 | 7.2 | 4 | 9.8 | 6.45 ± 1.34 | 6.15 | 4.7 | 10 | 6.62 ± 1.71 | 6.24 | 4 | 10 |
| VAS: Visual Analog Scale, MUP: Motor Unit Potential, PSA: Prostate Specific Antigen, Min: Minimum, Max: Maximum | ||||||||||||||||
Table 1: Demographic and clinical characteristics of patients in the three groups.
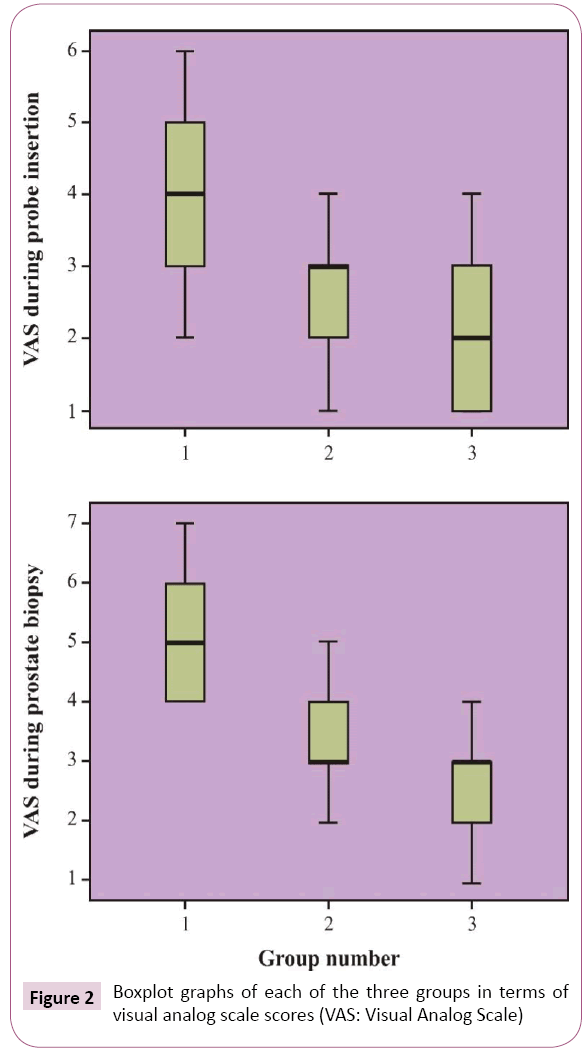
The mean VAS score during rectal probe insertion was 3.80 ± 1.12, 2.70 ± 1.14, and 2.07 ± 0.94 for the control, PNB, and INB groups, respectively. The mean VAS score during prostate biopsy was 4.97 ± 0.85, 3.43 ± 0.85, and 2.70 ± 0.75 for the control, PNB, and INB groups, respectively. Pain control was found to be significantly better during both prostate biopsy and rectal probe insertion in the INB group than in the PNB group (p=0.032 and p=0.002, respectively). The control group was found to have the highest degree of pain (p<0.001) (Figure 2).
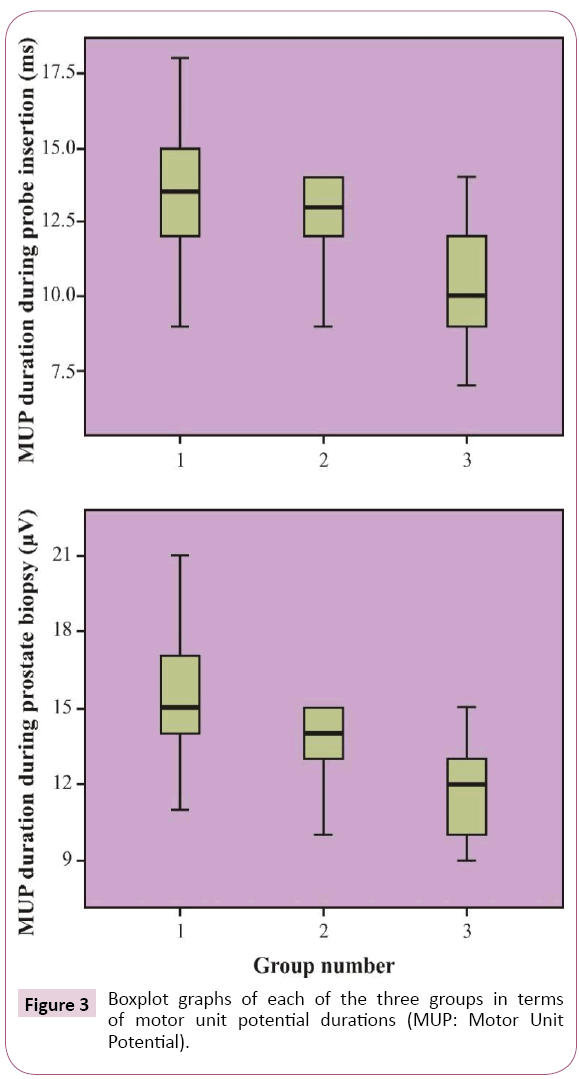
In EMG analysis, mean MUP durations during rectal probe insertion were 13.37 ± 2.23, 12.37 ± 1.54, and 10.60 ± 1.81 milliseconds (ms) in the control, PNB, and INB groups, respectively; during biopsy, these durations were 15.40 ± 2.25, 13.63 ± 1.27, and 11.70 ± 1.53 ms in these groups, respectively. Durations during both procedures were the smallest in the INB group than in the other groups (p<0.001). Patients in the control group had the highest MUP duration (p<0.001) (Figure 3).
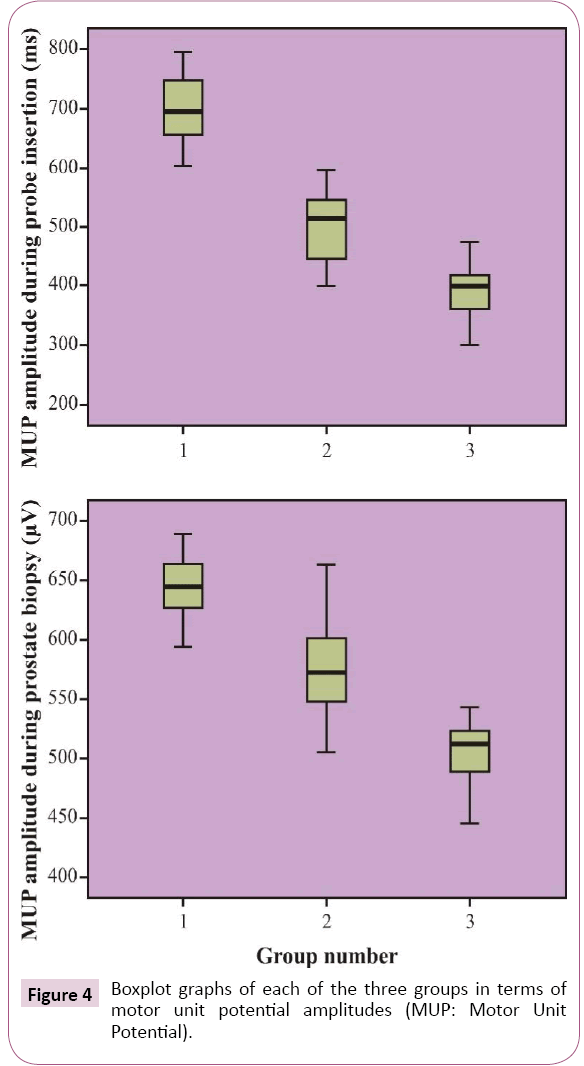
Mean MUP amplitudes during rectal probe insertion were 549.63 ± 26.27, 450.53 ± 30.61, and 394.77 ± 24.42 microvolt (μV) and during prostate biopsy were 642.73 ± 26.82, 575.83 ± 37.64, and 503.90 ± 30.97 μV in the control, PNB, and INB groups, respectively. All MUP amplitudes were the lowest in the INB group (p<0.001) and were at the highest levels in the control group (p<0.001) (Figure 4).
INB provided the greatest relaxation of the pelvic floor muscles during rectal probe insertion and biopsy. According to Spearman correlation, decreases in the EMG parameters were found to be consistent with decreases in the VAS scores (Tables 2 and 3).
| VAS | MUP time | MUP amplitude |
|---|---|---|
| During insertion of probe | ||
| r | 0.263* | 0.477** |
| p | 0.012 | 0 |
| During biopsy sampling | ||
| r | 0.426** | 0.671** |
| p | 0 | 0 |
VAS: Visual Analog Scale, MUP: Motor Unit Potential
*Correlation is significant at the 0.05 level (2-tailed)
**Correlation is significant at the 0.01 level (2-tailed)
Table 2: Participant demographics.
| MUP time | MUP amplitude |
|---|---|
| During insertion of probe | |
| r | 0.450* |
| p | 0 |
| During biopsy sampling | |
| r | 0.644* |
| p | 0 |
MUP: Motor Unit Potential
*Correlation is significant at the 0.01 level (2-tailed)
Table 3: Electromyography findings and visual analog scale scores recorded during prostate biopsy.
Discussion
Transrectal ultrasound-guided prostate biopsy is the standard method for diagnosing prostate cancer. In recent years, studies have shown that sextant biopsy is inadequate and a minimum of 10–12 core biopsies are more effective in providing an accurate diagnosis [5]. However, without anesthesia, an increased number of core biopsies result in increased cumulative and associated pain scores [6]. Pain during prostate biopsy is caused by anal discomfort due to the ultrasonic rectal probe and biopsy needle insertion into the prostate gland capsule. The biopsy needle does not cause pain on penetration in the rectal wall at the dentate line due to reduced nerve conduction at this site. Therefore, a majority of the pain associated with prostate biopsy is due to the stimulation of the periprostatic nerves localized in the capsule. Partly, pain is also due to rectal manipulation and prostate biopsy procedures. For this purpose, the use of topical creams, PNB, intraprostatic local anesthesia, sedation, daily anesthesia, and peripheral nerve block have previously been described in the literature.
The most commonly used anesthesia methods in prostate biopsy include PNB and intrarectal anesthetic gel application [1]. In prospective randomized studies, PNB is superior with regard to pain control and is considered to be the gold standard anesthesia method [7,8]. Despite the ability of PNB to ensure pain control of the rich nerve fibers of the autonomic innervation running basolateral with the prostatic vascular pedicles, its effect on the extrinsic nerve fibers of the automatic and sensory innervations of the prostate are not well-known [9,10]. As a result, the direct contact of the biopsy needle and these nerves in the prostatic capsule and stroma may result in inadequate pain management [11,12]. Biopsy needle intervention and ultrasound probe maneuver are attributed to pain during prostate biopsy [9]. In a randomized trial, lidocaine injection during PNB resulted in greater pain than needle penetrations and rectal probe maneuvers [13]. Furthermore, for patients undergoing repeated biopsies, fibrosis due to the prostate biopsy is a disadvantage of repeated periprostatic injections.
Ultrasonography-guided INB has been successfully used in obstetric and inguinal surgeries in adults [3,14,15]. This technique has the advantage in that it can be used with fewer analgesic agents and has greater success rates in pediatric patients than those in other techniques [16]. INB has less side effects, such as motor block of the lower limbs and urinary retention, and because INB is limited to the lower abdominal wall and inguinal region, hemodynamic changes are rarely observed [17]. To the best of our knowledge, there is only one study regarding the use of INB in prostate biopsies. This procedure was demonstrated to be superior compared with intrarectal prilocaine–lidocaine cream application; however, PNB was not performed on any patients in this study. As a result, PNB and INB were not compared regarding pain management [17]. According to the results of the current study, during rectal probe insertion, the VAS scores in the INB group were significantly lesser than those in the PNB group (2.07 ± 0.94 and 2.70 ± 1.14, respectively). During prostate biopsy, the VAS scores in the INB group were also significantly lesser than those in the PNB group (2.70 ± 0.75 and 3.43 ± 0.85, respectively). Mean MUP durations were significantly smaller in the INB group than those in the PNB group during both rectal probe insertion (10.60 ± 1.81 vs. 12.37 ± 1.54 ms) and prostate biopsy (11.70 ± 1.53 vs. 13.63 ± 1.27 ms). Furthermore, the INB group had lower mean MUP amplitudes than the PNB group during both rectal probe insertion (394.77 ± 24.42 vs. 450.53 ± 30.61 μV) and prostate biopsy (503.90 ± 30.97 vs. 575.83 ± 37.64 μV), and the difference was significant.
In male patients with Chronic Prostatitis/Chronic Pelvic Pain Syndrome (CP/CPPS), a relationship between hypersensitivity of the pelvic floor muscles and pelvic pain has been described [18]. According to the EMG values observed during biofeedback applications in male patients with CP/CPPS, the incidence of abnormal pelvic floor muscles was higher in patients with more severe pain [19]. This result demonstrates that pelvic pain can be objectively evaluated using EMG.
Recent studies have determined that although intrarectal lidocaine application is adequate during probe insertion, it does not provide the same benefit in decreasing pain during biopsy [2]. In the current study, we observed that the use of INB significantly reduced pain during rectal probe insertion in and declined pain during prostate biopsy.
The purpose of the current study was to compare the effects of PNB and INB, performed prior to prostate biopsy, on pain control. We aimed to make a more accurate assessment using an objective scale, EMG, as well as a subjective scale, VAS, which is frequently used in the literature. The main limitation of our study was the small patient population. Given that VAS score is not completely reproducible, to increase accuracy, the same number of biopsies (standard 12 core biopsies) were performed on each patient, and the same person evaluated VAS score to avoid inter-observer variability. Additionally, the use of EMG provided objective assessment.
Since, it was considered unethical to not administer anesthesia to patients in the control group, intrarectal prilocaine–lidocaine cream was applied. To ensure that the study was designed homogeneously, patients in both the PNB and INB groups underwent intrarectal prilocaine–lidocaine cream application; as a result, we could not compare the efficacy of PNB and INB alone. This situation represents one of the limitations of this study.
As mentioned previously, an additional limitation of the current study is the small patient population. As such, a large prospective randomized study is warranted to further validate these results.
All three anesthesia methods mentioned in this study carry a risk of complications. Here, we did not assess the patients for any long-term complications as it was beyond the scope of our study; this decision was an additional limitation of the study.
On the basis of our results, we believe that INB may be a more effective method to reduce involuntary contractions of the pelvic floor muscles during prostate biopsy. INB may also reduce pain during the prostate biopsy in urology practice.
Conclusion
We thus propose the SPAASMS score card as a reliable clinical tool for a rapid measurement of persistent pain. Further refinement of structure and component of SPAASMS; tested on larger number of patients may be regarded in the future.
Acknowledgement
This study was funded by the Private Practice Research Fund of Townsville. The authors thank Ms Helen Karami for collection of Data; Senior Clinical Psychologists Frank McDonald; and Dana Corden for comments and suggestions. We also thank Sameer Mitra for assistance with information technology. The authors have no conflicting financial interests.
References
- Nash PA, Bruce JE, Indudhara R, Shinohara K (1996) Transrectal ultrasound guided prostatic nerve blockade eases systematic needle biopsy of the prostate. J Urol 155: 607-9.
- Raber M, Scattoni V, Roscigno M, Rigatti P, Montorsi F (2005) Perianal and intrarectal anesthesia before transrectal biopsy of the prostate: a prospective, randomized study assessing lidocaine- prilocaine cream versus placebo. BJU Int 96: 1264-7.
- Sakalli M, Ceyhan A, Uysal HY, Yazici I, Basar H (2010) The efficacy of ilioinguinal and iliohypogastric nerve block for postoperative pain after caesarean section. J Res Med Sci 15: 6-13.
- Seyedhejazi M, Daemi OR, Taheri R, Ghojazadeh M (2013) Success rate of two different methods of ilioinguinal-iliohypogastric nevre block in children inguinal surgery. Afr J Paediatr Surg 10: 255-8.
- Bingqian L, Peihuan L, Yudong W, Jinxing W, Zhiyong W (2009) Intraprostatic local anesthesia with periprostatic nerve block for transrectal ultrasound guided prostate biopsy. J Urol 182: 479-83.
- Kaver I, Mabjeesh NJ, Matzkin H (2002) Randomized prospective study of periprostatic local anesthesia during transrectal ultrasound-guided prostate biopsy. Urology 59: 405-8.
- Lynn NN, Collins GN, Brown SC, O'Reilly PH (2002) Periprostatic nerve block gives better analgesia for prostatic biopsy. BJU Int 90: 424-6.
- Rodriguez A, Kyriakou G, Leray E, Lobel B, Guillé F (2003) Prospective study comparing two methods of anaesthesia for prostate biopsies: apex periprostatic nerve block versus intrarectal lidocaine gel: review of the literature. Eur Urol 44: 195-200.
- Leibovici D, Zisman A, Siegel YI, Sella A, Kleinmann J, et al. (2002) Local anesthesia for prostate biopsy by periprostatic lidocaine injection: a double-blind placebo controlled study. J Urol 167: 563-5.
- Benoit G, Merlaud L, Meduri G, Moukarzel M, Quillard J, et al. (1994) Anatomy of the prostatic nerves. Surg Radiol Anat 16: 23-9.
- Davies MR (1997) Anatomy of the nerve supply of the rectum, bladder and internal genitalia in anorectal dysgenesis in the male. J Pediatr Surg 32: 536-41.
- Hollabaugh RS Jr, Dmochowski RR, Steiner MS (1997) Neuroanatomy of the male rhabdosphincter. Urology 49: 426-34.
- Schostak M, Christoph F, Müller M, Heicappell R, Goessl G, et al. (2002) Optimizing local anesthesia during 10-core biopsy of the prostate. Urology 60: 253-7.
- Vallejo MC, Steen TL, Cobb BT, Phelps AL, Pomerantz JM, et al. Efficacy of the bilateral ilioinguinal-iliohypogastric block with intrathecal morphine for postoperative cesarean delivery analgesia. Sci World J 2012; 2012: 107316.
- Aveline C, Le Hetet H, Le Roux A, Vautier P, Cognet F, et al. (2011) Comparison between ultrasound-guided transversus abdominis plane and conventional ilioinguinal/iliohypogastric nerve blocks for day-case open inguinal hernia repair. Br J Anaesth 106: 380-6.
- Abdellatif AA (2012) Ultrasound-guided ilioinguinal/iliohypogastric nerve blocks versus caudal block for postoperative analgesia in children undergoing unilateral groin surgery. Saudi J Anaesth 6: 367-72.
- Hizli F, Argun G, Ozkul F, Güven O, Arik AI, et al. (2015) Novel approach for pain control in patients undergoing prostate biopsy: iliohypogastric nerve block with or without topical application of prilocaine-lidocaine: a randomized controlled trial. Urol J 12: 2014-9.
- Segura JW, Opitz JL, Greene LF (1979) Prostatosis, prostatitis or pelvic floor tension myalgia? J Urol 122: 168-9.
- Hetrick DC, Ciol MA, Rothman I, Turner JA, Frest M, et al. (2003) Musculoskeletal dysfunction in men with chronic pelvic pain syndrome type III: a case control study. J Urol 170: 828-31.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences