Multiple Arterial Thrombosis in Nephrotic Syndrome: Clinical Nephrology Case Report
G Prasanna Babu*, Edwin Fernando M, Thirumalvalavan S and Thirumalvalavan K
Department of Nephrology, Government Stanley Medical College, Chennai, India
- *Corresponding Author:
- G Prasanna Babu
Department of Nephrology,
Government Stanley Medical College,
Chennai, - India,
E-mail: prsn71188@gmail.com
Received date: May 29, 2024, Manuscript No. IPJRM-24-19114; Editor assigned date: May 31, 2024, PreQC No. IPJRM-24-19114 (PQ); Reviewed date: June 14, 2024, QC No. IPJRM-24-19114; Revised date: March 07, 2025, Manuscript No. IPJRM-24-19114 (R); Published date: March 14, 2025, DOI: 10.36648/IPJRM.8.1.51
Citation: Babu GP, Fernando EM, Thirumalvalavan S, Thirumalvalavan K (2025) Multiple Arterial Thrombosis in Nephrotic Syndrome: Clinical Nephrology Case Report. J Ren Med Vol:8 No:1
Abstract
Background: Isolated arterial thrombosis is rare, with incidence of less than 1.8% to 5% in adults. Here, we present a case of an adult with isolated multiple arterial thrombosis, which preceded the diagnosis of nephrotic syndrome (due to C3 glomerulonephritis)–A rare presentation.
Case summary: 29-year-old male presented with nephrotic syndrome with renal failure. He had history of multiple partial arterial thrombosis involving the le t renal artery, superior and inferior mesenteric arteries, and femoral arteries without venous involvement, two months prior to his current presentation. The pro-cogulant work up, including APLA, was negative, but he was not screened for nephrotic syndrome at that time. He improved with heparin infusion and did not require thrombolysis or any interventional procedures. Renal biopsy done at current admission revealed C3 glomerulonephritis con irmed by electron microscopy. Genetic study revealed a deletion in the CFHR3 gene on Chromosome 1. Multiplex Ligation-dependent Probe Ampli ication (MLPA) revealed a heterozygous deletion of CFHR1/CFHR3 genes, with a dosage coe icient of 0.4. Despite treatment, the patient progressed to end-stage renal disease.
Conclusion: Without evidence of concomitant TMA in renal biopsy, the association between C3GN and isolated arterial thrombosis hasn’t been established in literature. This case has been presented in view of rarity in presentation.
Keywords
Nephrotic syndrome; Arterial thrombosis; Venous thrombosis; C3 glomerulopathy; Thrombotic microangiopathy; Hypercoagulability; CFH gene
Introduction
Venous thrombosis is a well-known complication in nephrotic syndrome, while arterial thrombosis, which is more severe than venous thrombosis has been reported less frequently. Isolated arterial thrombosis is rare with incidence of less than 1.8% to 5% in adults and are less common when compared with nephrotic syndrome in children. The cause of hypercogulability in nephrotic syndrome are complex
Case Presentation
A 29-year-old male, employed as a driver, was admitted to the nephrology department with complaints of frothy urine and pedal edema persisting for 2 weeks. He is a non-smoker and has no history of drug abuse. There is no reported history of hematuria, oliguria, or skin rashes. On examination, the patient was afebrile, pale, and had bilateral pitting pedal edema. His BP was 130/90 mmHg in the supine posture in both upper limbs. All peripheral pulses were palpable. Abdominal examination revealed a soft abdomen, with no free fluid/bruit, and fundus examination was normal.
Two months prior to his current presentation, he was admitted elsewhere in the surgical department for complaints of abdominal pain, vomiting, and leg pain. Upon evaluation, multiple thrombi were discovered with 70% occlusion in the inferior mesenteric, superior mesenteric, and femoral arteries, and partial occlusion of the left renal artery. There was no evidence of deep vein thrombosis.
He denied any history of trauma/surgical procedures/ COVID-19 infection. Additionally, there was no family history of venous and arterial thrombosis in first-degree relatives and no history suggestive of malignancies, connective tissue diseases, or native medications. The cause of arterial thrombosis could not be determined from his investigations mentioned below (Table 1).
| CBC | Hb: 12.8 mg/dl, platelet count: 1.98 lakhs |
| RFT | Creatinine: 1.5 |
| Peripheral smear | No evidence of schistocytes |
| Pro-coagulant workup | Protein C and S-slightly reduced; factor-V-Leiden -neg |
| ECHO | No vegetation’s, no patent foramen ovale |
| ANA | Negative |
| PT, INR | Normal |
| APLA workup | Negative |
Table 1: Investigations from previous records 2 months back.
The patient was initially treated with heparin and bridged with warfarin. His INR was well maintained in the therapeutic range. His symptoms improved with anticoagulants, and he did not require thrombolysis or thrombectomy. He was discharged with anticoagulants and antiplatelet drugs.
Now, his current investigations revealed nephrotic syndrome with a 24-hour urine protein of 7 gm and serum albumin of 2.3 mg/dl. His creatinine level was 2.4 mg/dl with urea of 58 mg/dl. Complete blood count was normal. His total cholesterol was elevated at 380 mg/dl. On ultrasound of the abdomen, the right kidney measured 9.3 × 4 cm, while the left kidney measured 8.5 × 4 cm with increased echoes and few scars in left kidney (probably due to left renal artery partial occlusion).
His C3 was low (24.6 mg/dl) with normal C4 (28 mg/dl) levels. Serum ANA, anti-cardiolipin antibodies, and lupus anticoagulant were negative. Peripheral smear didn't reveal any schistocytes.
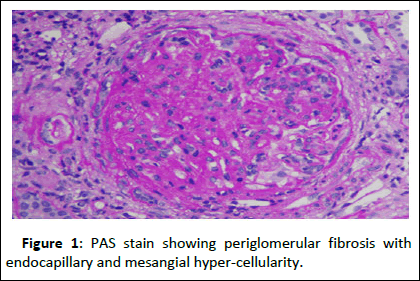
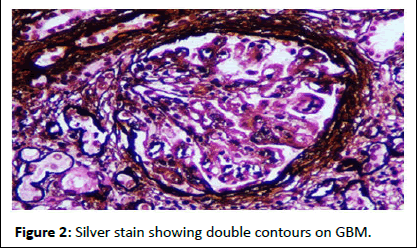
Since the patient was already on warfarin, he was bridged to heparin and biopsy was done in right kidney, as he had scars in left kidney. Renal biopsy revealed peri-glomerular fibrosis with ischemic retraction of the capillary tuft in four out of eleven glomeruli. All glomeruli showed endo-capillary and mesangial hypercellularity. None of the glomeruli were sclerotic. Double contours were seen on glomerular basement membranes. Interstitial fibrosis and tubular atrophy involved 20-25% of the biopsied core. Focal foam cell clusters are seen in the interstitium. No evidence of concomitant thrombotic-micro angiopathy was seen in biopsy. Immunofluorescence showed isolated C3 (+3) granular positivity in capillary loops and mesangium. Hence the diagnosis of complement-mediated membrano proliferative glomerulonephritis was made (Figures 1-3).
Figure 1: PAS stain showing periglomerular fibrosis with endocapillary and mesangial hyper-cellularity.
Figure 2: Silver stain showing double contours on GBM.
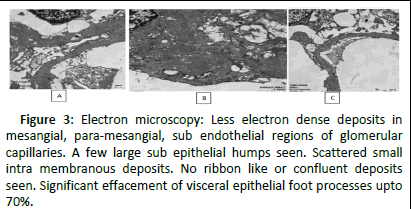
Figure 3: Electron microscopy: Less electron dense deposits in mesangial, para-mesangial, sub endothelial regions of glomerular capillaries. A few large sub epithelial humps seen. Scattered small intra membranous deposits. No ribbon like or confluent deposits seen. Significant effacement of visceral epithelial foot processes upto 70%.
Electron microscopy, which was done to differentiate C3GN and dense deposit disease, revealed prominent mesangial and sub-endothelial deposits with scattered small intramembranous deposits and observed, leading to the diagnosis of C3 glomerulonephritis (Figure 4).
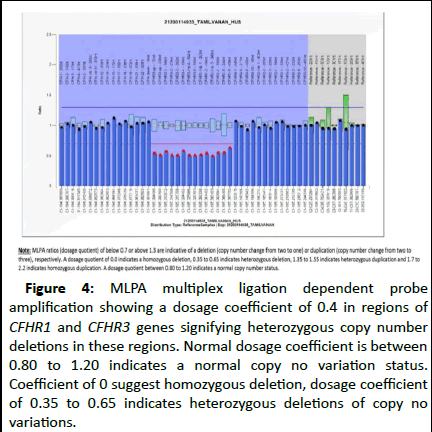
Figure 4: MLPA multiplex ligation dependent probe amplification showing a dosage coefficient of 0.4 in regions of CFHR1 and CFHR3 genes signifying heterozygous copy number deletions in these regions. Normal dosage coefficient is between 0.80 to 1.20 indicates a normal copy no variation status. Coefficient of 0 suggest homozygous deletion, dosage coefficient of 0.35 to 0.65 indicates heterozygous deletions of copy no variations.
Genetic study revealed a deletion in the CFHR3 gene on chromosome 1. Multiplex Ligation-dependent Probe Amplification (MLPA) revealed a heterozygous deletion of CFHR1/CFHR3 genes, with a dosage coefficient of 0.4. This deletion was reported as a variant of unknown significance since it was found in less than 10% of the healthy Asian population.
Serum protein electrophoresis was normal. ANA and ENA profile were negative. However, the anti-CFH antibody was weakly positive. As the patient had deranged renal function, with no definitive evidence regarding immunosuppression, management was focused on anti proteinuric measures and anticoagulation. As other causes of pro-thrombotic states have been ruled out, multiple arterial thrombosis is attributed to nephrotic syndrome.
During follow-up, the patient progressed to end-stage renal disease over the next 8 months and currently is on maintenance hemodialysis. The patient and his family were counseled about the possibility of a 50% post-transplant recurrence, and they were not willing to proceed with transplantation.
Discussion
Adults with nephrotic syndrome are at a higher risk of arterial thromboembolism, venous thromboembolism, and bleeding compared to the general population [1]. The exact cause of the hypercoagulable state in nephrotic syndrome is not fully understood, but it is attributed to alterations in various components of the coagulation cascade [2].
Hemostatic factors in nephrotic syndrome
Various hemostatic factors in nephrotic syndrome shows in Table 2.
| Coagulation components | Abnormality | Thrombosis |
| Zymogens | Loss of factor II, IX, X, XII increased factor VII, X | |
| Cofactors | Increased factor V and VIII | Compensation for hypoalbuminemia. |
| Fibrinogen | Increased | Compensation for hypo albuminemia |
| Coagulation inhibitors | Decreased anti-thrombin III increased alpha2 microglobulin | Urinary loss |
| Fibrinolytics | Decreased plasminogen | Urinary loss |
| Platelets | Thrombocytosis increased platelet aggregation and adhesivenes |
Table 2: Various hemostatic factors in nephrotic syndrome.
Pathogenic factors leading to hypercoagulability
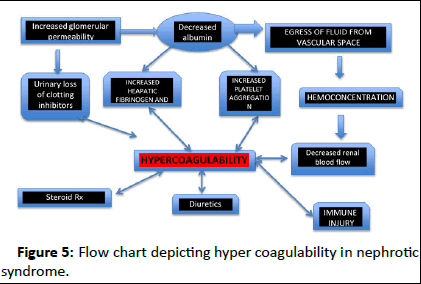
The hypercoagulability in nephrotic syndrome can be either disease-related or treatment-related. The risk of thrombosis is associated with the severity of hypoalbuminemia and tends to occur early in the course of the disease (Figure 5).
Figure 5: Flow chart depicting hyper coagulability in nephrotic syndrome.
Venous thrombosis is more frequently reported in nephrotic syndrome, presenting as deep vein thrombosis, renal vein thrombosis, pulmonary embolism, and cerebral vein thrombosis. Arterial thrombosis, on the other hand, is extremely rare and has mainly been reported in children. The most common site of arterial thrombosis is the femoral artery, but cases have also been reported in the aortic, renal, cerebral, mesenteric, and coronary arteries.
There are some case reports where isolated arterial thrombosis was the initial clinical manifestation, leading to the subsequent discovery of nephrotic syndrome in adults [3-5]. In a study by Xinqiang Hang, et al. nine adult nephrotic syndrome patients with acute isolated arterial thrombosis were reported. In this case series, seven out of nine patients had arterial thrombosis preceding the diagnosis of nephrotic syndrome.
In a case report by Ibrahim Fahal, et al. two cases of adult nephrotic syndrome (27 years and 47 years old) with mesangial proliferative GN on renal histology with isolated arterial thrombus were reported [6]. One of the patients had thrombosis of bilateral profunda femoris artery and distal popliteal artery. His symptoms settled with anticoagulation, while the other patient had severe right iliac arterial thrombus requiring thrombectomy. In our case, the patient's symptoms preceded the diagnosis of nephrotic syndrome and settled with anticoagulation, which may be attributed to partial thrombosis of the femoral arteries.
Arterial thrombosis in nephrotic syndrome–Indian case reports
Depicting Indian case reports of arterial thrombosis in nephrotic syndrome seen in Table 3.
| Case report author | Age | Site of arterial thrombosis | Renal histology | Serum albumin | Treatment | Comments |
| Vamsi Krishna Nagalla, et al. | 12 yrs/F | Right popliteal artery | Diffuse proliferative GN | 2.3 g/dl | Amputation of right foot | Genetic study shows heterozygous mutation for factor V Leiden |
| BK Brahmbhatt, et al. | 44 yrs/M | Right brachiocephalic trunk with embolism in right MCA and right axillary artery | Minimal change disease | 1.4 g/dl | Embolectomy followed by anti-coagulation | Cerebral thrombosis |
| Nikhil John and Elenjickal, et al. | 29 yrs/M | Coronary artery LAD | FSGS tip lesion | 1.15 g/dl | Thrombolysis and anti-coagulation | |
| Pandian JD, et al. | 42 yrs/M | Right anterior and middle cerebral artery infarction | Minimal change disease | 1.5 g/gl | Conservative |
Table 3: Depicting Indian case reports of arterial thrombosis in nephrotic syndrome.
C3 glomerulopathy is a rare disease resulting from dysregulation of the alternate complement pathway, comprising two major subgroups: Dense deposit disease and C3 glomerulonephritis [7]. Our patient had C3 glomerulonephritis with electron-dense deposits prominently in mesangial and subendothelial regions. No confluent deposits were seen in the membrane, with significant (70%) effacement [8-11].
The CFH gene family includes CFHR1, CFHR2, CFHR3, CFHR4, and CFHR5 on chromosome 1q31.3. Our patient's genetic analysis report revealed a deletion in the CFHR3 gene associated with diseases like atypical HUS and C3GN. The genomic region of the CFH family has large segmental duplications and repetitive sequences between them that predispose them to genomic rearrangements like duplication, deletion, and inversions [12]. When larger than 1 kb, they are called structural variants. The most common structural variant described in the CFH gene family is the deletion of the CFHR1 and CFHR3 genes. In our case, MLPA showed a heterozygous deletion in the CFHR1 and CFHR3 genes, which was a variant of unknown significance.
Transplants in CFHR3 gene-associated C3GN showed a 50% disease recurrence [13]. The association of C3GN and CFHR3 gene mutations with thrombosis without concomitant evidence of TMA hasn't been described in the literature [14].
Conclusion
Arterial thrombosis/embolization is characteristic of antiphospholipid antibody syndrome and myelo-proliferative disorders, but can occur in nephrotic syndrome and malignancies. In our case, arterial thrombosis could be attributed to underlying nephrotic syndrome, which was missed at the initial presentation. This case, presenting as isolated arterial thrombosis, is rarer presentation in adult nephrotic syndrome. Genetic analysis in C3 glomerulonephritis is mandatory, as it is required for predicting post-transplant recurrence. This case is presented as it underscores the importance of a simple screen for proteinuria in thrombotic episodes, which would have avoided unnecessary costly investigations and prevented delays in diagnosis and therapy.
Declaration of Patient Consent
The authors certify that they have obtained all appropriate patient consent forms. In the forms, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understand that her name and initials will not be published and due efforts will be made to conceal her identity, but anonymity cannot be guaranteed.
Financial Support and Sponsorship
Nil
Conflict of Interest
There are no conflicts of interest.
References
- Vestergaard SV, Birn H, Darvalics B, Nitsch D, Sørensen HT, et al. (2022) Risk of arterial thromboembolism, venous thromboembolism, and bleeding in patients with nephrotic syndrome: A population-based cohort study. Am J Med 135:615-625
[Crossref] [Google Scholar] [PubMed]
- Al-Azzawi HF, Obi OC, Safi J, Song M (2016) Nephrotic syndrome-induced thromboembolism in adults. Int J Crit Illn Inj Sci 6:85-88
[Crossref] [Google Scholar] [PubMed]
- Han X, Zhao P, Wang Z, Ji X, Zhao M (2023) Acute lower extremity arterial thrombosis associated with nephrotic syndrome in adults: Case series and literature review. BMC Nephrol 24:318
[Crossref] [Google Scholar] [PubMed]
- Almehmi A, Bates MC (2006) Extensive upper extremity arterial thrombosis as the initial manifestation of nephrotic syndrome. W V Med J 102:16-17
[Google Scholar] [PubMed]
- Kimura A, Nishimura K, Miyasaka S, Maeta H, Morimoto K, et al. (2010) A case of acute arterial thrombosis caused by nephrotic syndrome. Ann Vasc Dis 3:68-70
[Crossref] [Google Scholar] [PubMed]
- Fahal IH, McClelland P, Hay CC, Bell GM (1994) Arterial thrombosis in the nephrotic syndrome. Postgrad Med J 70:905-909
[Crossref] [Google Scholar] [PubMed]
- Hou J, Ren KY, Haas M (2022) C3 glomerulopathy: A review with emphasis on ultrastructural features. Glomerular Dis 2:107-120
[Crossref] [Google Scholar] [PubMed]
- Nagalla VK, Raju SB, Bura NR (2021) Arterial thrombosis associated with factor V leiden mutation in a child with nephrotic syndrome. Indian J Nephrol 31:187-189
[Crossref] [Google Scholar] [PubMed]
- Brahmbhatt BK, Mathew A, Rajesh R, Kurian G, Unni VN (2011) Brachiocephalic artery thrombosis in adult nephrotic syndrome. Indian J Nephrol 21:204-207
[Crossref] [Google Scholar] [PubMed]
- John EN, Chellappan A, Ghodeshwar G, Sharma A, Chaudhari SR (2023) Acute myocardial infarction in a patient with focal segmental glomerulosclerosis tip lesion: Hit with a double whammy. Cureus 15:e48860
[Crossref] [Google Scholar] [PubMed]
- Pandian JD, Sarada C, Elizabeth J, Visweswaran RK (2000) Fulminant cerebral infarction in a patient with nephrotic syndrome. Neurol India 48:180-181
[Google Scholar] [PubMed]
- Lupski JR, Stankiewicz P (2005) Genomic disorders: Molecular mechanisms for rearrangements and conveyed phenotypes. PLoS Genet 1:e49
[Crossref] [Google Scholar] [PubMed]
- Wong L, Moran S, Lavin PJ, Dorman AM, Conlon PJ (2016) Kidney transplant outcomes in familial C3 glomerulopathy. Clin Kidney J 9:403-407
[Crossref] [Google Scholar] [PubMed]
- Ravindran A, Palma LM, Fervenza FC, Sethi S (2023) Overlap of C3 glomerulopathy and thrombotic microangiopathy: A case series. Kidney Int Rep 8:619-627
[Crossref] [Google Scholar] [PubMed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences