ISSN : ISSN: 2576-1412
Journal of Applied Microbiology and Biochemistry
Multiple Antibiotic Resistance, Antibiogram and Phenotypic Detection of Metallo-Beta-Lactamase (MBL) from Escherichia coli of Poultry Origin
Ejikeugwu Chika1*, Iroha Ifeanyichukwu1, Amaechi Clement O1, Ugwu Malachy2, Eze Peter2, Iroha Chidinma S3, Ogene Lilian1 and Orinya Chinedu1
1Department of Applied Microbiology, Ebonyi State University, Ebonyi State, Nigeria
2Department of Pharmaceutical Microbiology and Biotechnology, Faculty of Pharmaceutical Sciences, Nnamdi Azikiwe University, Anambra State, Nigeria
3Department of Pharmacy, Federal Teaching Hospital Abakaliki (FETHA I), Ebonyi State, Nigeria
- *Corresponding Author:
- Ejikeugwu Chika
Department of Applied Microbiology
Faculty of Science, Ebonyi State University
Abakaliki, P.M.B 053, Ebonyi State, Nigeria
Tel: +2348097684562
E-mail: ejikeugwu_chika@yahoo.com
Received date: August 08, 2017; Accepyed date: August 21, 2017; Published date: August 28, 2017
Citation: Chika E, Ifeanyichukwu I, Clement OA, Malachy U, Peter E, et al. (2017) Multiple Antibiotic Resistance, Antibiogram and Phenotypic Detection of Metallo-Beta- Lactamase (MBL) from Escherichia coli of Poultry Origin. J Appl Microbiol Biochem. Vol. 1 No. 4:15
Abstract
Background: Bacteria produce antibiotic-degrading enzymes such as carbapenemases. Carbapenemases are a consortium of carbapenem-hydrolyzing enzymes such as metallo-β-lactamase (MBL) that gives Gram-negative bacteria the exceptional ability to degrade and render the carbapenems inefficacious.
Aim: This study evaluated the antibiogram, multiple antibiotic resistance and occurrence of MBL-producing E. coli from cloacal swabs of poultry birds in a local poultry farm in Abakaliki, Nigeria.
Materials and methods: A total of 40 cloacal swab samples from the cloacal region of poultry birds were bacteriologically analyzed for the isolation of E. coli. E. coli isolates were identified using standard microbiology techniques and the antibiogram of the isolates was determined using the disk diffusion technique. The multidrug resistance nature of the E. coli isolates was determined using multiple antibiotics resistance index (MARI) protocol while MBL production was phenotypically confirmed using the inhibition based assay.
Results: A total of 29 (72.5%) E. coli isolates was recovered from the 40 cloacal swab samples. The E. coli isolates were highly resistant to imipenem (31%), meropenem (58.6%), ertapenem (75.9%), cefotaxime (55.2%), ciprofloxacin (89.7%), cefoxitin (93.1%). and ceftazidime (69.0%). MBL production was phenotypically detected in 3 (10.3%) E. coli isolates out of the 29 isolates of E. coli recovered in this study. The resistant E. coli isolates were multiply resistant to antibiotics in the class of fluoroquinolones, cephalosporins, aminoglycosides and carbapenems; and they had a multiple antibiotic resistance of 0.4 on average.
Conclusion: This presumptive study has shown that E. coli isolates of poultry origin produce MBL. The emergence and spread of drug resistant bacteria in the community can be contained if we use antibiotics rationally and find alternative measures for promoting animal growth without the use of antimicrobial agents.
Keywords
Metallo-β-lactamase; Escherichia coli; Multidrug resistance; Pathogens
Introduction
In Nigeria, the detection rate of multidrug resistant Gramnegative bacteria in both the community and hospital environment is still at a pitiable state. More worrisome is the fact that the detection of multidrug resistant bacteria such as metallo-β-lactamase (MBL)-producing bacteria has not yet been institutionalized in our healthcare system. And this could contribute to poor prognosis of the patient as well as lead to inappropriate use or application of antimicrobial therapy. The emergence of new beta-lactamases such as metallo-β-lactamases (MBLs), extended spectrum β-lactamases (ESBLs) and AmpC enzymes to mention but a few is an important mechanism by which bacteria develop resistance to some available antibiotics [1-3]. Microbial infections caused by antibiotic resistant bacteria lead to increase in the length of hospitalization, severity of illness and the overall cost of treatment. Gram-negative bacteria that produce MBL are widely reported as an emerging threat to the efficacy of the carbapenems including imipenem and meropenem. The increasing frequency of these multidrug resistant organisms from several countries have become of major clinical significance owing to their antibiotic degrading potentials [2,4-11]. MBLs are beta-lactamases that hydrolyze and confer on Gram negative bacteria the singular ability to be resistant to the carbapenems – which are stable against the antibiotic degrading ability of ESBL-producing bacteria [1,2,4,11]. They are encoded by genes that have been procured by bacteria either by mutation or horizontally from other organisms, and they can be chromosomally or plasmid-mediated. MBLs were first formally described from serine beta-lactamases in the 1980s; and since then, MBL-producing Gram negative bacteria is now an increasing problem in many parts of the world [1,2,4-7,12]. Pathogens that produces MBLs can hydrolyze all types of β-lactams including penicillins, cephalosporins, and most especially the carbapenems. However, the monobactams such as aztreonam and serine β-lactamase inhibitors still have antimicrobial activity against the MBLs [1,3,13,14]. The MBLs belong to the class of carbapenemases that depends on zinc ions as cofactors for enzyme activity. Their presence in Gram-negative bacteria can be phenotypically detected in the laboratory using ethylene diamine tetraacetic acid (EDTA) – to which MBLs are sensitive to [1,9,10,11]. The genes that encode MBL production in Gramnegative bacteria are either chromosomally encoded or plasmid encoded as earlier stated; and they can easily be transmitted via mobile genetic elements such as plasmids and transposons in a bacterial population [1,13,14]. The use of antibiotics in livestock production and poultry practices is a good breeding ground for the emergence and spread of antibiotic resistant bacteria in the community [15-17]. According to Wegener [18], the use of antibiotics in animal feeds place a crucial role in the development and spread of antibiotic resistant bacteria in the community. This avenue enables the selection of resistance strains of bacteria including E. coli exposed to the antibiotics in the intestinal flora of the birds. In this study, the antibiogram, multiple antibiotic resistance and occurrence of MBL-producing E. coli from cloacal swabs of poultry birds was determined.
Materials and Methods
Sample collection and processing
A total of 40 consecutive and non-duplicated cloacal swab samples were collected from the cloacal region of poultry birds from a local poultry farm in Abakaliki metropolis, Ebonyi State, Nigeria using sterile swab sticks. The samples were labeled and transported to the Microbiology Laboratory unit of Ebonyi State University, Abakaliki, Nigeria for bacteriological analysis. Each sample was inserted into sterile test tubes of 5 ml freshly prepared nutrient broth (Oxoid, UK) and incubated at 30oC overnight. Following overnight incubation at 30oC, the test tubes containing the samples were examined for visible bacterial growth as evidenced by the presence of turbidity.
Bacterial isolation and identification
Test tubes positive for bacterial growth as evidenced by turbidity were aseptically sub-cultured onto freshly prepared MacConkey agar (MAC) and eosin methylene blue (EMB) agar plates for the isolation of E. coli. The inoculated agar plates were incubated at 30oC overnight. Suspected colonies of E. coli were sub-cultured onto freshly prepared MAC and EMB agar plates for the isolation of pure colonies of E. coli. E. coli was identified using standard microbiology identification technique including: colonial/ morphological appearance on culture media, Gram staining, indole test and methyl red test [19].
Maintenance of bacteria stock culture
Each of the isolated E. coli isolates were maintained as stock cultures for further studies on nutrient agar (Oxoid, UK) slants in Bijou bottles. This was done by streaking the isolates on the agar slants, and incubating at 30oC for 18-24 hrs. After incubation, the inoculated slants were stored in the refrigerator at ambient temperature and these stock cultures served as source of E. coli isolates for further bacteriological studies.
Antimicrobial susceptibility testing (AST)
AST was performed according to a previously described methodology using the Kirby-Bauer disk diffusion technique on Mueller-Hinton agar plates as per the Clinical and Laboratory Standard Institute (CLSI) guidelines [4,20]. Imipenem (10 μg), meropenem (10 μg), ertapenem (10 μg), amikacin (30 μg), ofloxacin (5 μg), ceftazidime (30 μg), ceftriaxone (30 μg), cefotaxime (30 μg), ciprofloxacin (10 μg) and cefoxitin (30 μg) (Oxoid, UK) were used for antimicrobial susceptibility testing. Susceptibility test plates were incubated at 30oC for 18-24 hrs; and the ensuing inhibition zone diameters (IZDs) were measured to the nearest millimeter using a meter rule. All the IZDs were recorded and interpreted as percentage susceptible, intermediate and resistant using the CLSI standard antibiotic breakpoints [20].
Screening test for MBL
MBL enzyme-producing E. coli isolates was suspected when the test organism(s) was resistant to any of the carbapenems including imipenem and meropenem as was described previously [4,5,9]. Bacterial isolates showing inhibition zone diameter (IZD) of ≤ 23 mm to any of the carbapenems were suspected to produce MBL enzyme and these isolates were subjected to phenotypic confirmation test.
Phenotypic detection of MBL-positive E. coli
The test bacteria isolates (adjusted to 0.5 MacFarland turbidity standard) were aseptically swabbed on Muller-Hinton (MH) agar plates, and standard antibiotics disks of imipenem (10 μg) and meropenem (10 μg) disks impregnated with EDTA (1 μg) and imipenem and meropenem disks without EDTA was aseptically placed on the MH agar plates. A distance of 25 mm was maintained between the antibiotic disks. All test plates were incubated at 30oC for 18-24 hours and zone of inhibition were recorded and interpreted as per the CLSI criteria [20]. A difference of ≥ 7 mm between the zones of inhibition of any of the carbapenems with EDTA and disks without EDTA infers MBL production phenotypically [4,9].
Determination of multiple antibiotic resistance index (MARI)
Multiple antibiotic resistance index (MARI) was determined by the method of [21] with little modification. MARI was evaluated to determine the multidrug resistant nature/profile of resistant isolates in our study. To determine resistant profile of these resistant isolates, MARI was evaluated using the formular: MARI = a/b, where “a” is the number of antibiotics to which the resistant bacteria was resistant to and “b” is the total number of antibiotics to which the resistant bacteria has been evaluated for.
Results
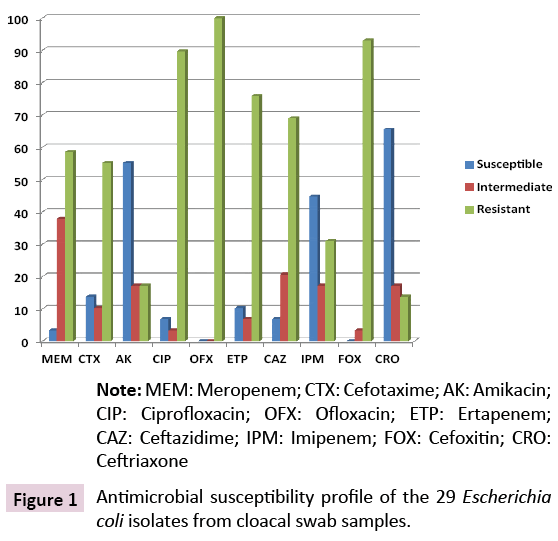
This study detected the production of metallo-β-lactamase (MBL) from Escherichia coli isolates that emanated from the cloacal region of poultry birds in a local poultry farm in Abakaliki metropolis, Ebonyi State, Nigeria. The antibiogram and multiple antibiotic resistance index of the isolated Escherichia coli isolates was also evaluated. After the culture of the samples on MacConkey agar and eosin methylene blue agar plates, a total of 29 (72.5%) isolates of Escherichia coli was bacteriologically isolated from the cloacal swab samples analyzed in this study. The isolated E. coli isolates produced pink-red colonies on MacConkey agar plates as well as green metallic sheen colonies on eosin methylene blue (EMB) agar plates. The result of the identification studies showed that the E. coli isolates were positive for methyl red test, indole production and Gram-negative (Table 1). The percentage susceptibility of the isolated Escherichia coli isolates to some selected antibiotics is shown in (Figure 1).
| Organism | Total samples analyzed | Positive samples | Prevalence (%) | Morphological appearance on culture media |
Gram reaction | Indole test |
MR test |
|---|---|---|---|---|---|---|---|
| Escherichia coli | 40 | 29 | 72.5 | Lactose-fermenting colonies on MAC; and green metallic sheen colonies on EMB | Gram-negative | Positive | Positive |
Note: MAC-MacConkey agar, EMB-Eosin methylene blue agar, MR-Methyl red
Table 1 Prevalence of Escherichia coli isolates.
The Escherichia coli isolates recovered in this study from the cloacal swab samples were highly resistant to the 3rd-generation cephalosporins including cefotaxime (55.2%), ceftriaxone (13.8%) and ceftazidime (69.0%). Ofloxacin had no inhibitory activity against the E. coli isolates which were found to be completely resistant to this fluoroquinolone (100%). Similar high level of resistance was also observed amongst the E. coli isolates to ciprofloxacin (89.7%), which is also a fluoroquinolone (Figure 1). The E. coli isolates were also found to be highly resistant to cefoxitin (93.1%), which is a 2nd-generation cephalosporin. A handful of the E. coli isolates also showed reduced susceptibility to the carbapenems including meropenem, ertapenem and imipenem at the rate of 58.6%, 75.9% and 31.0% respectively (Figure 1). Table 2 shows the frequency of MBL-producing E. coli isolates in this study.
| Organism | Sample source | Number (%) | MBL positive n (%) |
MBL negative n (%) |
|---|---|---|---|---|
| Escherichia coli | Cloacal swabs of poultry birds | 29 (72.5) | 3 (10.3) | 26 (89.7) |
Key: n - number of samples, % - percentage
Table 2 Detection of MBL in 29 E. coli isolates.
Out of the 29 E. coli isolates that were phenotypically screened for MBL production, only 3 (10.3%) isolates of E. coli were phenotypically confirmed to produce MBL (Table 2). The other 26 isolates of E. coli did not produce MBL by the inhibition based assay technique used in this study. On average, the resistant E. coli isolates had a multiple antibiotic resistance index of 0.4. They were multiply resistant to antibiotics in the class: aminoglycosides, fluoroquinolones, carbapenems, and cephalosporins (Table 3).
| Isolate No. | MARI | Antibiotics |
|---|---|---|
| E1 | 0.3 | AK, FOX, CIP, OFX, CAZ, CTX, IPM, MEM, ETP |
| E9 | 0.3 | AK, FOX, CIP, OFX, CAZ, CTX, IPM, MEM, ETP |
| E3 | 0.4 | AK, FOX, CIP, OFX, CAZ, CTX, IPM, MEM, ETP |
| E5 | 0.4 | AK, FOX, CIP, OFX, CAZ, CTX, IPM, MEM, ETP |
| E6 | 0.3 | AK, FOX, CIP, OFX, CAZ, CTX, IPM, MEM, ETP |
| E8 | 0.4 | AK, FOX, CIP, OFX, CAZ, CTX, IPM, MEM, ETP |
| E10 | 0.4 | AK, FOX, CIP, OFX, CAZ, CTX, IPM, MEM, ETP |
| E12 | 0.3 | AK, FOX, CIP, OFX, CAZ, CTX, IPM, MEM, ETP |
Note: AK: Amikacin; FOX: Cefoxitin; CIP: Ciprofloxacin; OFX: Ofloxacin; CAZ: Ceftazidime; CTX: Cefotaxime; IPM: Imipenem; MEM: Meropenem; ETP: Ertapenem; MARI: Multiple antibiotics resistance index
Table 3 Multiple antibiotics resistance index (MARI) of selected E. coli isolates.
Discussion
The production of metallo-β-lactamase (MBL) by Gram-negative bacteria especially in members of the Enterobacteriaceae family is an important resistance mechanism that allows these organisms to resist the antimicrobial onslaught of some potent antibiotics such as the carbapenems. This study was aimed at determining the antibiogram, multiple antibiotic resistance and production of MBL from Escherichia coli isolates recovered from cloacal swab samples from poultry birds in a local poultry farm in Abakaliki, Nigeria. A total of 40 consecutive, non-duplicated cloacal swab samples were used for this study. The result of the bacteriological investigation shows that a total of 29 (72.5%) E. coli isolates were isolated from the 40 cloacal swab samples. Feacaloral transmission is the major route through which pathogenic strains of the bacterium cause disease; and most E. coli serotypes or strains are occasionally responsible for product recalls due to food contamination [22]. All the E. coli isolates in this study were completely resistant to ofloxacin (100%), a fluoroquinolone. This resistance rate was followed by ciprofloxacin (89.7%), another fluoroquinolone to which the test E. coli isolates also showed reduced susceptibility to. This report of E. coli resistance to the fluoroquinolones in this study was reported by [23]. In Iran, [24] also reported multiple antimicrobial resistance in E. coli isolates recovered from chickens in Iran as was obtainable in this study. The percentage of E. coli isolates that was found to be resistant to ciprofloxacin in our study (89.7%) was lesser than that reported by [25] in Egypt – in which 40% of the tested E. coli isolates in their study was found to be resistant to ciprofloxacin. Members of the Enterobacteriaceae family including E. coli often contain resistance determinants for other classes of antibiotics such as the aminoglycosides, sulfonamides, and fluoroquinolones which are readily transmissible from one strain of organism to another and between different species of Gram-negative bacteria [26,27]. The E. coli strains in this study were found to be highly resistant to the cephalosporins and carbapenems used in this study especially cefoxitin (93.1%), ceftazidime (69.0%), cefotaxime (55.2%), imipenem (31.0%), meropenem (58.6%) and ertapenem (75.9%). The high resistance of E. coli isolates of poultry origin as reported in this study is in line with a similar study carried out in Ibadan, Nigeria, [28] who reported that E. coli from poultry frozen foods were resistant to beta-lactam antibiotics and non-beta-lactams alike. In terms of their multiple antibiotic resistance indexes, the E. coli isolates were found to be multiply resistant to antibiotics in the class: cephalosporins, carbapenems, aminoglycosides, and fluoroquinolones. The average multiple antibiotic resistance index of the isolated E. coli in this study was pegged at 0.4. This indicate that the E. coli isolates bacteriologically recovered in this study was found to be multiply resistant to antibiotics from four different classes as was aforementioned. This result of ours is in agreement with the report by Tawab AA et al. [25] and Talebiyan R et al. [24] who reported the multiple antibiotic resistance nature of E. coli isolates from poultry origin in Egypt and Iran respectively. In China, [29] reported in a similar study that E. coli isolates of poultry origin are multidrug resistant, and that they express enzymes that allow them to be multidrug resistant in nature. Out of the 29 isolates of E. coli that was bacteriologically recovered from the 40 cloacal swab samples, the production of metallo-β- lactamase (MBL) was phenotypically detected in only 3 isolates of E. coli. MBL production was not detected in the other 26 E. coli isolates by the inhibition-based assay technique that was used in this study. The prevalence rate of E. coli isolates that produced MBL phenotypically in this study was 10.3%. This result is similar to a previous study that we conducted in Abakaliki metropolis in 2016, in which we phenotypically screened Enterobacteriaceae isolates of poultry origin for the production of MBL [4]. However, the prevalence rate of MBL-positive E. coli isolates in this current study (10.3%) is lesser than what we obtained in the previous year in which MBL production was detected phenotypically in 9 (23.1%) E. coli isolates. Our report of MBL production in E. coli isolates of poultry origin is in agreement with the study done by Wadekar et al. [30] who showed that Enterobacteriaceae isolates including E. coli produce multidrug resistance enzymes such as MBL that allows them to be multidrug resistant in nature. The results of this study contribute to the growing reports of antibiotic resistance in the community. This goes to show that antibiotics are irrationally used in the community. The occurrence of an MBL-positive isolate in a poultry farm portends serious public health implication because this isolate could serve as route via which resistance traits could spread undetected.
Conclusion
Conclusively, this current study has presumptively shown that E. coli isolates of poultry origin are multidrug resistant in nature. Our report also shows that these isolates produce metallo-β- lactamase (MBL) phenotypically, and this allows them to be resistant to the carbapenems. The rapid spread of resistance among bacteria may be due to widespread and inappropriate use of antibiotics in the community. Absolute care should be taken in the treatment and handling of poultry birds. The use of antibiotics in animal husbandry and poultry production is a major driving force that contributes to the development of antibiotic resistant bacteria amongst food-producing animals. Aggressive action is therefore needed now to nip in the bud, the emergence and spread of drug resistant bacteria in the community.
Acknowledgement
We appreciate Professor Charles O. Esimone (Nnamdi Azikiwe University, Awka, Nigeria) and Professor Michael U. Adikwu (University of Abuja, Nigeria) for their tireless academic advice and scientific assistance over the years that lead to the formulation and execution of this research project.
References
- Walsh TR, Toleman MA, Poirel L, Nordmann P (2005) Metallo β – Lactamases: The quiet before the Storm? Clin Microbiol Rev 18: 306-325.
- Toleman MA, Biedenbach D, Bennett DMC, Jones RN, Walsh TR (2005) Italian metallo – β – lactamases: A national problem? Report from the SENTRY Antimicrobial Surveillance Programme. J Antimicrob Chemother 55: 61-70.
- Bush K, Jacoby GA (2010) Updated functional classification of β – Lactamases. Antimicrob Agents Chemother 54: 969-976.
- Ejikeugwu C, Iroha I, Duru C, Ayogu T, Orji O, et al. (2016) Occurrence of metallo-beta-lactamase-producing Enterobacteriaceae in abakaliki nigeria. Int J Appl Pharm Sci Res 1: 70-75.
- Aibinu I, Nwanneka T, Odugbemi T (2007) Occurrence of ESBL and MBL in clinical isolates of Pseudomonas aeruginosa from Lagos Nigeria. J Am Sci 3: 81-85.
- Libisch B, Muzslay M, Gacs M, Minarovits J, Knausz M, et al. (2006) Molecular epidemiology of VIM-4 metallo – β – lactamase – producing pseudomonas spp isolates in Hungary. Antimicrob Agents Chemother 50: 4220-4223.
- Saderi H, Karimi Z, Owlia P, Bahar MA, Rad SM (2008) Phenotypic detection of metallo – beta – lactamase producing pseudomonas aeruginosa strains isolated from burned patients. Iran J Pathol 3: 20-24.
- Tortola MT, Lavilla S, Miro E, Gonzalez JJ, Larrosa N, et al. (2005) First detection of a carbapenem – hydrolyzing metalloenzyme in two Enterobacteriaceae isolates in Spain. Antimicrob Agents Chemother 49: 3492-3494.
- Ejikeugwu C, Ugwu M, Iroha I, Eze P, Gugu T, et al. (2014) Phenotypic detection of metallo-beta-lactamase (MBL) enzyme in Enugu, Southeast Nigeria. Am J Biol Chem Pharmaceut Sci 2: 2328-2814.
- Kalantar D, Mansouri S (2010) Emergence of multiple β – lactamases produced by Escherichia coli clinical isolates from hospitalized patient in Kerman Iran. Jundishapur J Microbiol 3: 137-145.
- Pitout JDD, Chow BL, Gregson DB, Laupland KB, Elsayad S, et al. (2007) Molecular epidemiology of metallo-β-lactamase – producing pseudomonas aeruginosa in the calgary health region: Emergence of VIM-2- Producing Isolates. J Clin Microbiol 45: 294-298.
- Lye DC, Wijaya L, Chan J, Teng CP, Leo YS (2008) Ertapenem for treatment of extended – spectrum beta – lactamase – producing and multidrug – resistant gram – negative bacteraemia. Ann Acad Med Singapore 37: 831-834.
- Franco MRG, Caiaffa-Filho HH, Burattini MN, Rossi F (2010) Metallo – beta – lactamases among imipenem –resistant Pseudomonas aeruginosa in a Brazilian university hospital. Clinics (Sao Paulo) 65: 825-829.
- Carfi A, Pares S, Duéé E, Galleni M, Duez C, et al. (1995) The 3 – D structure of a zinc metallo – β – lactamase from Bacillus cereus reveals a new type of protein fold. EMBO J 14: 4914-4921.
- Shiraki Y, Shibata N, Doi Y, Arakawa Y (2004) Escherichia coli producing CTX-M-2 β – lactamase in cattle, Japan. Emerg Infect Dis 10: 69-75.
- Oyinloye JMA, Ezekiel CN (2011) Extended spectrum beta – lactamase (ESBL) – producing multidrug resistant Enterobacteriaceae from commercial poultry feeds in Nigeria. Ann Biol Res 2: 250-254.
- Kader AA, Kamath KA (2009) Faecal carriage of extended-spectrum β-lactamase – producing bacteria in the community. East Mediterr Health J 15: 1365-1370.
- Wegener HC (2003) Antibiotics in animal feed and their role in resistance development. Curr Opin Microbiol 6: 439-445.
- Cheesbrough M (2010) Biochemical tests to identify bacteria. In: District laboratory practice in tropical countries (part 2). Cambridge University Press, Cambridge, UK. pp: 71-76.
- Clinical Laboratory Standard Institute (CLSI) (2015) Performance standards for antimicrobial disk susceptibility test. Fifteenth informational supplement, CLSI document M100-S15. Wayne PA, USA.
- Akinjogunla OJ, Enabulele IO (2010) Virulence factors, plasmid profiling and curing analysis of multidrug resistant Staphylococcus aureus and coagulase negative Staphylococcus spp. isolated from patients with acute otitis media. J Am Sci 6: 1022-1033.
- Russell JB, Jarvis GN (2001) Practical mechanisms for interrupting the oral-fecal lifecycle of Escherichia coli. J Mol Microbiol Biotechnol 3: 265-272.
- Sharada R, Ruban S, Thiyageeswaran M (2008) Antibiotic resistance pattern of Escherichia coli isolated from poultry in Bangalore. Internet J Microbiol 7: 1-5.
- Talebiyan R, Kheradmand M, Khamesipour F, Rabiee-Faradonbeh M (2014) Multiple antimicrobial resistance of Escherichia coli isolated from chickens in Iran. Vet Med Int 2014: 491418.
- Tawab AA, Ammar AM, Soad AN, Reda RM (2015) Prevalence of E. coli in diseased chickens with its antibiogram pattern. BENHA Veterinary Medical Journal 28: 224-230.
- Mehrgan H, Rahbar M, Arab-Halvali Z (2010) High prevalence of extended spectrum beta – lactamase – producing Klebsiella pneumoniae in a tertiary care hospital in Tehran Iran. J Infect Dev Ctries 4: 132-138.
- Madigan MT, Martinko JM, Dunlap PV, Clark DP (2009) Brock biology of microorganisms (12th edn.). Pearson Benjamin Cummings Publishers, USA. pp: 795-796.
- Adetunji VO, Odetokun IA (2012) Antibiogram profiles of Escherichia coli, Salmonella and Listeria species isolated along the processing line of sale of frozen poultry foods. Research Journal of Microbiology 7: 235-241.
- Zhang CH, Liu YL, Wang JH (2010) Detection of ESBLs and antimicrobial susceptibility of Escherichia coli isolated in Henan, China. J Anim Vet Adv 9: 2030-2034.
- Wadekar MD, Anuradha K, Venkatesha D (2013) Phenotypic detection of ESBL and MBL in clinical isolates of Enterobacteriaceae. Int J Curr Res Aca Rev 1: 89-95.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences