Lipodystrophy, NAFLD and Pancreatic Neuroendocrine Tumor in a Patient with LIPE Mutation
Jeremy Wang1-3, Jihane N Benhammou1-3, Wayne Grody4 and Joseph R Pisegna1-4*
1Division of Digestive Diseases, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA
2Department of Medicine, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA
3Division of Gastroenterology, Hepatology and Parenteral Nutrition, VA Greater Los Angeles Healthcare System, Los Angeles, CA, USA
4Departments of Pathology & Laboratory Medicine and Human Genetics, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA
- *Corresponding Author:
- Joseph R. Pisegna
Professor of Medicine and Human
David Geffen School of Medicine at UCLA and Division of Gastroenterology
Hepatology and Parenteral Nutrition
VA Greater Los Angeles Healthcare System (691/111C)
11301 Wilshire Blvd., Los Angeles, CA 90073, USA
Tel: 310-268-3578
Fax: 310-268-4096
E-mail: jpisegna@ucla.edu
Received Date: September 18, 2017; Accepted Date: September 26, 2017; Published Date: October 03, 2017
Citation: Wang J, Benhammou JN, Grody W, Pisegna JR (2017) Lipodystrophy, NAFLD and Pancreatic Neuroendocrine Tumor in a Patient with LIPE Mutation. J Genet Disord. 1:5.
Abstract
The LIPE gene encodes hormone-sensitive lipase, also known as cholesteryl ester hydrolase that converts chlesteryl esters to free cholesterol to generate fatty acid and monglyceride. The enzyme is expressed in adipose tissues and functions to mobilize fats for use as energy. Mutation of LIPE has been shown to be involved in the development of lipodystrophies. Herein, we report a novel mutation of LIPE resulting in a lipodystrophy phenotype and the first report of an association with, non-alcoholic fatty liver disease (NAFLD) and a pancreatic neuroendocrine tumor (PNET). An understanding of this mutation may shed light on the role of this gene in the development of NAFLD and PNET and therefore deserves further study.
Keywords
Lipodystrophy; Non-Alcoholic Fatty Liver Disease (NAFLD); Pancreatic Neuroendocrine Tumor (PNET); Gene mutation
Case Report
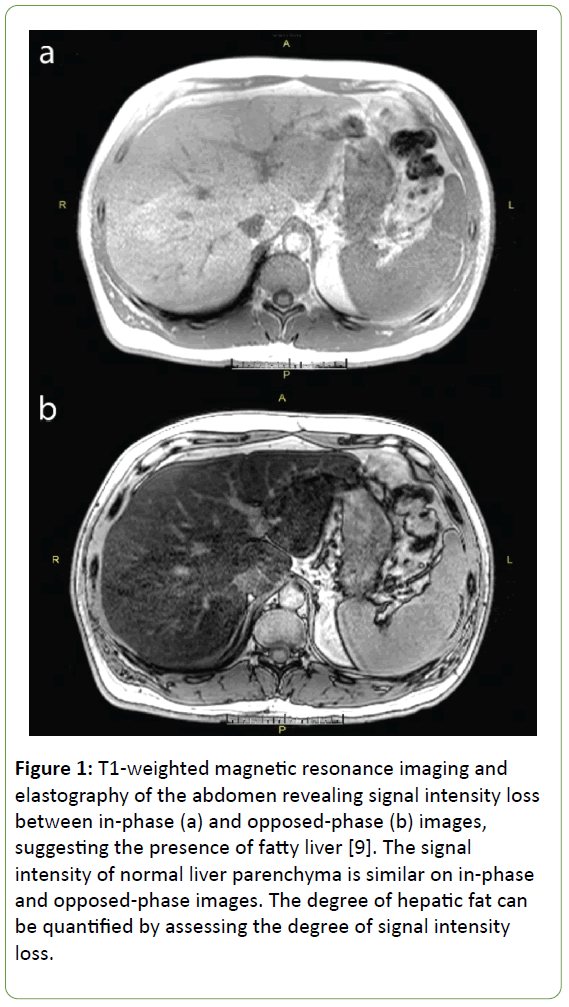
A 54 year old man with a history of type 2 diabetes mellitus and hypertriglyceridemia initially presented for evaluation of a pancreatic neuroendocrine tumor (PNET) that was diagnosed after recurrent episodes of pancreatitis. On exam, he was noted to have lipodystrophy with excess fat distribution in the neck, upper back, and upper thorax. These findings in conjunction with his recently diagnosed PNET raised concern for a possible underlying genetic syndrome. Whole exome sequencing was performed, which was negative for multiple endocrine neoplasia type 1 (MEN-1) but notable for two variants in the LIPE gene on chromosome 19 (RefSeq NM_005357.2: c.1491A>T and c.436C>T) responsible for encoding hormone-sensitive lipase (HSL). Analysis of these mutations revealed an association with autosomal recessive familial partial lipodystrophy type 6. The patient subsequently underwent ultrasound elastography (Fibroscan®), which was notable for non-alcoholic fatty liver disease (NAFLD) and mild to moderate liver fibrosis with a MetaVir score of F2-F3. Follow-up magnetic resonance imaging and elastography of the abdomen revealed severe fatty liver with a fat fraction of 35%, but no evidence of fibrosis (Figure 1a and b). This case is notable for a newly described association between a mutation in the LIPE gene and the presence of both lipodystrophy and a pancreatic neuroendocrine tumor.
Figure 1: T1-weighted magnetic resonance imaging and elastography of the abdomen revealing signal intensity loss between in-phase (a) and opposed-phase (b) images, suggesting the presence of fatty liver [9]. The signal intensity of normal liver parenchyma is similar on in-phase and opposed-phase images. The degree of hepatic fat can be quantified by assessing the degree of signal intensity loss.
Discussion
Adipose tissue plays a critical role in regulating fatty acid storage and metabolism. Disorders in this process are a prominent feature of lipodystrophies, rare syndromes characterized by abnormal fat tissue distribution that are also associated with obesity, dyslipidemia, insulin resistance, and an increased risk of type 2 diabetes mellitus [1]. Lipodystrophy can be congenital or acquired, with one subset of congenital lipodystrophies being the familial partial lipodystrophies (Table 1). These lipodystrophies are phenotypically characterized by the above described metabolic derangements, as well as reduced subcutaneous fat in the arms and legs, and in some individuals, excess subcutaneous fat deposition in the neck, face, and intra-abdominal regions.
| Type | Inheritance | Chromosome Location | Gene/Locus |
|---|---|---|---|
| 1 (Kobberling type) | Likely autosomal dominant | N/A | N/A |
| 2 (Dunnigan type) | Autosomal dominant | 1q22 | LMNA |
| 3 | Autosomal dominant | 3p25.2 | PPARG |
| 4 | Autosomal dominant | 15q26.1 | PLN1 |
| 5 | Autosomal recessive | 3p25.3 | CIDEC |
| 6 | Autosomal recessive | 19q13.2 | LIPE |
Table 1: Subdivisions of familial partial lipodystrophy.
Type 6 familial partial lipodystrophy has previously been described by Carboni et al. in a sister and brother pair born of consanguineous Italian parents [2]. The siblings developed accumulation of fat in the abdomen and axillae with reduced subcutaneous fat in the legs. The sister also had biopsy-proven muscular dystrophy, hepatic steatosis, and elevated levels of triglycerides and cholesterol. Farhan et. al performed whole genome exome sequencing in this sibling pair and identified a frameshift mutation in the LIPE gene encoding hormone-sensitive lipase (HSL) on chromosome 19 that segregated with disease in the family [3].
HSL is an intracellular lipase with many lipid substrates, and is thought to be the primary enzyme responsible for the hydrolysis of stored triglycerides and the subsequent release of free fatty acids in adipose tissue [4]. Albert et al. previously described the effects of a deletion in the LIPE gene encoding HSL in a cohort of Amish participants [5]. They sequenced 12 lipolytic-pathway genes in 24 Old Order Amish individuals whose fasting serum triglyceride levels were at the extremes of the distribution and detected a 19-bp frameshift deletion in the LIPE gene in one study participant. Genotyping this deletion in 2,738 participants in the Amish Complex Disease Research Program identified 1 individual homozygous for the deletion (DD genotype), 140 heterozygotes (ID genotype), and 2597 non-carriers (II genotype). Recruitment of family members of the proband with the homozygous genotype identified three additional DD homozygous siblings. All four had been diagnosed with type 2 diabetes before age 50 and had higher liver fat percentage when compared to their heterozygous or wild type siblings. Analysis of the remainder of the study participants revealed that heterozygous carriers of the deletion (ID) had higher hepatic fat content compared to non-carriers (II).
Our patient’s lipodystrophic phenotype and hepatic steatosis is consistent with these previously described findings and further illustrates the clinical significance of HSL and the metabolic sequlae of impaired lipolysis. HSL’s association with fatty liver is clinically significant given the potential for NAFLD to lead to cirrhosis. HSL likely maintains hepatic lipid homeostasis by mobilizing triglycerides from the liver and releasing free fatty acids into the circulation. This association between HSL and fatty liver allow us to hypothesize that patient’s diagnosed with NAFLD at a young age or normal body mass index may possess genetic polymorphisms in HSL or other lipid-storage regulatory genes that predisposes them to the development of hepatic steatosis. There are also therapeutic implications to HSL as current therapy for NAFLD focuses on addressing its associated metabolic conditions of insulin resistance and dyslipidemia. For example, the thiazolidinedione class of medications improve insulin sensitivity by activating peroxisome proliferator-activated receptor gamma (PPAR-y), another regulator of fatty acid metabolism that is affected in type 3 familial partial lipodystrophy [6,7]. Furthermore, Reid et al. previously demonstrated that adenoviral overexpression of HSL in mice reduced liver triglycerides by 40-60% and had the potential to ameliorate hepatic steatosis [8,9]. Thus, activation of HSL may have a role in the treatment and reversal of NAFLD in future patients. Overall, our patient’s case demonstrates the physiologic significance of HSL and provides additional insight into the pathophysiology of hepatic steatosis. Furthermore, our case represents the first case of an associated PNET occurring in a patient with a lipodystrophy.
Acknowledgement
This work received grant support from: NIH Training Grant NIDDK T32 DK07180-30 (JNB). Department of Veterans Affairs RR&D Merit Review (JRP) I01 RX000194; Human Studies CORE through CURE: Digestive Diseases Research Center supported by NIH grant P30DK41301.
References
- Frayn KN (2002) Adipose tissue as a buffer for daily lipid flux. Diabetologia 45: 1201-1210.
- Carboni N, Brancati F, Cocco E, Solla E, D'Apice MR, et al. (2014) Partial lipodystrophy associated with muscular dystrophy of unknown genetic origin. Muscle Nerve 49: 928-930.
- Farhan SM, Robinson JF, McIntyre AD, Marrosu MG, Ticca AF, et al. (2014) A novel LIPE nonsense mutation found using exome sequencing in siblings with late-onset familial partial lipodystrophy. Can J Cardiol 30: 1649-1654.
- Haemmerle G, Zimmermann R, Zechner R (2003) Letting lipids go: hormone-sensitive lipase. Curr Opin Lipidol 14: 289-297.
- Garg A, Misra A (2004) Lipodystrophies: rare disorders causing metabolic syndrome. Endocrinol Metab Clin North Am 33: 305-331.
- Agarwal AK, Garg A (2002) A novel heterozygous mutation in peroxisome proliferator-activated receptor-gamma gene in a patient with familial partial lipodystrophy. J Clin Endocrinol Metab 87: 408-411.
- Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, et al. (1999) Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature 402: 880-883.
- Reid BN, Ables GP, Otlivanchik OA, Schoiswohl G, Zechner R, et al. (2008) Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis. J Biol Chem 283: 13087-13099.
- Hamer OW, Aguirre DA, Casola G, Lavine JE, Woenckhaus M, et al. (2006) Fatty liver: imaging patterns and pitfalls. Radiographics : a review publication of the Radiological Society of North America, Inc 26: 1637-1653.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences