ISSN : 0976-8505
Der Chemica Sinica
Lemon Juice: An Environmentally Benign Catalyst for Synthesis of Benzothiazoles and Benzoxazole Derivatives in Aqueous Medium
Monika A Patil, Panchsheela A Ubale, Shrikrishna S Karhale and Vasant B Helavi*
Department of Chemistry, Rajaram College, Kolhapur, 416004, Maharashtra, India
Abstract
Lemon juice as a natural and eco-friendly catalyst has been utilized for the synthesis of benzothiazole and benzoxazole derivatives by the reaction of 2-aminothiophenol or 2-aminophenol with a variety of aryl aldehydes in good-excellent yields. The beneficial features of this new synthetic approach include short reaction time, a clean reaction profile with mild reaction condition and an easy work up procedure.
Keywords
Natural catalyst, Aryl aldehydes, Benzothiazole, Benzoxazole
Introduction
The traditional reaction conditions increases the environmental pollution posed by utility of toxic, volatile organic solvents. Thus these organic processes generate lot of waste and have rigorous impact on living systems. Hence cleaner green reaction conditions with environmentally acceptable and renewable raw materials which offer environmental and economic advantages over traditional synthetic processes are necessary.
Uses of natural catalyst, environmentally benign solvent under mild reaction condition are the fundamental aspects of green chemistry. Natural catalysts represent a unique class of biocatalysts which are eco-friendly, inexpensive, nonhazardous; biodegradable has large number of application in the organic transformations [1-5].
Benzazoles such as benzathiazole, benzoxazole are privileged structural motifs having potential applications in the pharmacological and biological activities in medicinal chemistry [6-10]. Benzothiazoles are biologically active compounds which is used as valuable intermediates in various organic syntheses [11] and found in variety of natural products showing diverse medicinal applications [12-17]. Further, benzoxazoles exhibits photophysical and photochemical properties and shows chromophoric effect by enhancing the emission quantum yield through decrease in the non-radioactive decay rate constant on the fluorescence of conjugated compound [18]. Some of the typical reported methods for the synthesis of benzothiazoles and benzoxazoles include the reaction of 2-aminothiophenol or 2-aminophenol with variety of aryl aldehydes [19-30]. However, present methods exhibits certain drawbacks such as tedious catalyst preparation, expensive reagents, prolonged reaction times, use of toxic solvent, lower yields, and complicated workup procedures. Therefore, synthesis of benzazole derivatives using natural catalyst, eco-benign solvent under mild reaction condition is highly desirable. Herein, we chose water as a reaction media for condensation of 2-aminothiophenol or 2-aminophenol with variety of aryl aldehydes using natural catalyst, because water is considerably safe, eco-friendly, non-toxic, and inexpensive compared to other organic solvents.
Citrus limonium, Citrus aurantium, and Citrus indica are important species of the citrus family, locally known as Limbu or Nimbu in India. The juice obtained from lemon is sour in taste used to control the high blood pressure, arthritis and rheumatism, asthma and to prevent kidney stone it is also well known plant species for antioxidant activity as well as for anti-carcinogenic activity. Acidity caused due to presence of citric and ascorbic acids (vitamin C) to lemon juice are responsible to works as an acid catalyst in organic transformation.
In continuation our research work for the development of sustainable methodologies for the synthesis of bio-active heterocyclic scaffolds [31-32], herein we report the synthesis of benzothiazoles and benzoxazoles by employing lemon juice as a natural, bio-degradable catalyst under mild reaction condition.
Materials and Methods
All chemicals were purchased from local sources (Spectrochem and Thomas Bakers) and were of used without further purification. All reactions were carried out under air atmosphere in dried glassware. Melting points were measured by an open capillary method under open atmosphere. The products were in good agreements with those of known compounds by their spectral data. The FT-IR spectra were measured on Bruker ALPHA FT-IR spectrometer. The NMR spectra were recorded on a Bruker AC (400 MHz for 1H NMR and 100 MHz for 13C NMR) spectrometer using CDCl3 as the solvent and TMS as an internal standard. The δ -values are expressed in parts per million (ppm).
Preparation of lemon juice
Fresh lemon was purchased from the local market. The pieces were made using a knife and pressed in a fruit juicer to obtain the juice. Then the juice was filtered through filter paper to remove solid material and clear portion of juice was used as a catalyst. The pH of the extracted lemon juice was found to be 2.3.
General procedure for the synthesis of benzazoles
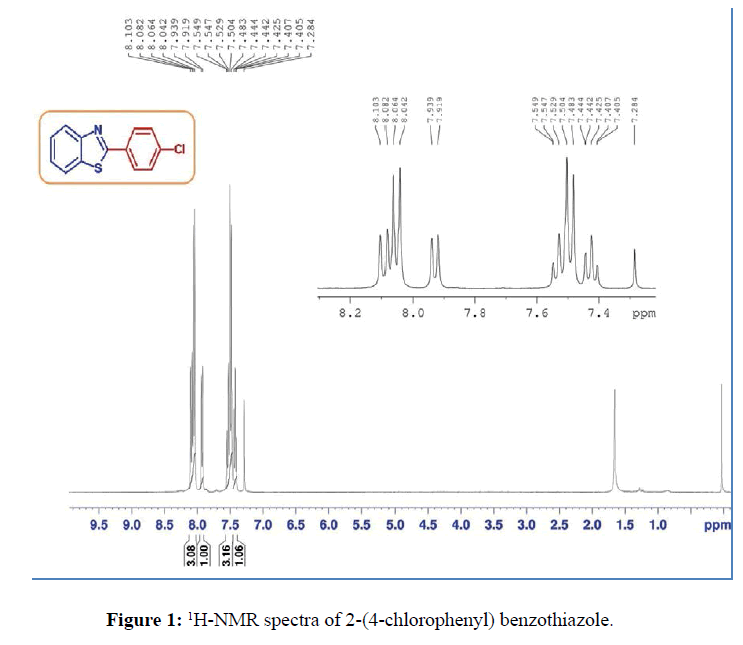
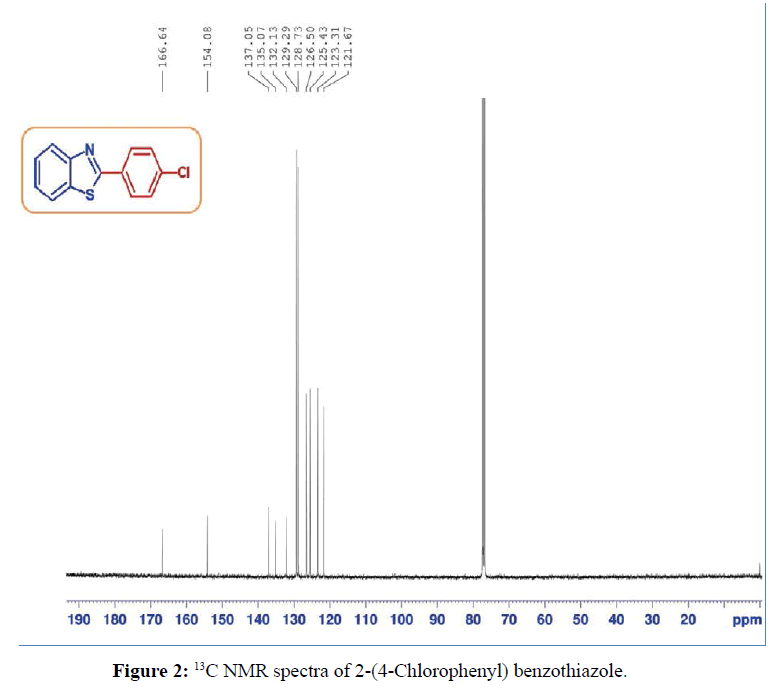
In typical procedure, 2-aminothiophenol or 2-aminophenol (1 mmol), aryl aldehyde (1 mmol), and lemon juice: water mixture (1:1 v/v) (4 mL) was added in the reaction vessel. The reaction mixture was stirred at 80°C temperature for a specified time (Table 1). After reaction completion as analyzed by TLC, the reaction mixture was quenched with cold water and stirred continuously until free flowing solid was obtained. The resulting solid was filtered, air dried and recrystallization from ethanol to get pure product. The identity of the compound [2-(4-chlorophenyl) benzothiazole] was ascertained on the basis of 1H NMR and 13C NMR spectroscopy as shown in Figures 1 and 2. The physical and spectral data are in agreement with the literature data.
| Entry | Amine | Aldehyde | Product (3) |
Lemon juice | Pineapple juice | Orange juice | |||
|---|---|---|---|---|---|---|---|---|---|
| Time (min) |
Yieldb (%) |
Time (min) |
Yieldb (%) |
Time (min) |
Yieldb (%) |
||||
| 1 | 1a | H | 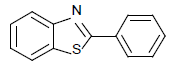 |
45 | 91 | 50 | 90 | 50 | 88 |
| 2 | 1a | 4-NO2 | 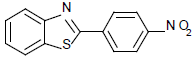 |
50 | 90 | 55 | 88 | 55 | 87 |
| 3 | 1a | 3-NO2 |  |
51 | 88 | 56 | 87 | 58 | 86 |
| 4 | 1a | 4-Cl | 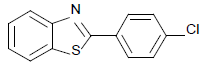 |
52 | 88 | 55 | 86 | 60 | 84 |
| 5 | 1a | 4-Br |  |
60 | 87 | 64 | 85 | 65 | 83 |
| 6 | 1a | 4-CN |  |
50 | 60 | 55 | 88 | 55 | 86 |
| 7 | 1a | 4-CH3 |  |
65 | 88 | 70 | 87 | 75 | 86 |
| 8 | 1a | 4-OCH3 |  |
70 | 87 | 74 | 80 | 80 | 83 |
| 9 | 1a | 4-OH | 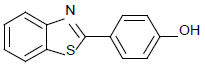 |
72 | 88 | 75 | 84 | 85 | 82 |
| 10 | 1a | 3,4,5-OCH3 | 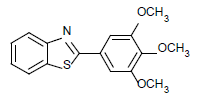 |
90 | 84 | 95 | 85 | 99 | 80 |
| 11 | 1b | H | 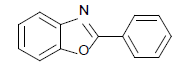 |
120 | 82 | 125 | 81 | 130 | 80 |
| 12 | 1b | 4-NO2 | 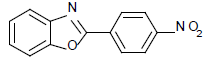 |
75 | 90 | 90 | 86 | 90 | 84 |
| 13 | 1b | 4-Cl |  |
80 | 89 | 85 | 85 | 90 | 81 |
| 14 | 1b | 4-OCH3 |  |
100 | 88 | 105 | 83 | 105 | 80 |
aReaction conditions: Aryl aldehydes (1 mmol), 2-aminothiophenol/2-aminophenol (1 mmol), lemon juice: water (4 mL, 1:1, v/v) at 80°C.
bIsolated yields after purification
Table 1: Lemon juice catalyzed synthesis of benzothiazole and benzoxazolea.
Selected spectral data
2-(phenyl) benzothiazole (Table 1, entry 1)
Off-white solid, MP: 111-113°C (34); 1H NMR (400 MHz, CDCl3): δ (ppm) 8.10-8.13 (m, 3H), 7.92-7.94 (d, 1H, J=8 Hz), 7.50-7.54 (m, 4H), 7.39-7.43 (m, 1H) ); 13C NMR (100 MHz, CDCl3): δ (ppm) 121.6, 123.2, 125.2, 126.2, 127.5, 129, 130.9, 133.6, 135, 154.1, 168; IR (KBr) (cm-1): 3056, 1622,1448, 755, 623.
2-(4-nitorphenyl) benzothiazole (Table 1, entry 2)
Yellow Solid, MP: 229-331°C (34); 1H NMR (400 MHz, CDCl3): δ (ppm) 8.09-8.14 (m, 2H), 7.93-7.95 (d, 2H, J=7.6 Hz), 7.66-7.93 (m, 1H), 7.18-7.28 (m, 2H), 7.03-7.15 (m, 1H); 13C NMR (100 MHz, CDCl3): δ (ppm) 121.6, 123.8, 124.3, 125.8, 126.2, 127.1, 128, 129.7, 132.8, 141.2, 147.7, 149.5, 157; IR (KBr) (cm-1): 3044, 1627, 1557, 1360, 822, 756.
2-(4-chlorophenyl) benzothiazole (Table 1, entry 4)
White Solid, MP: 107-109°C (34); 1H NMR (400 MHz, CDCl3): δ (ppm) 8.04-8.10 (m, 3H), 7.92-7.93 (d, 1H, J=8 Hz) 7.48-7.54 (m, 3H), 7.40-7.44 (m, 1H); 13C NMR (100 MHz, CDCl3): δ (ppm) 121.6, 123.3, 125.4, 126.5, 128.7, 129.2, 132.1, 135, 137, 154, 166.6; IR (KBr) (cm-1): 3056, 1630,1445, 850, 733.
2-(4-nitrophenyl) benzoxazole (Table 1, entry 12)
Yellow Solid, MP: 263-265°C (34); 1H NMR (400 MHz, CDCl3): δ (ppm) 8.23-8.28 (m, 2H), 7.45-7.52 (m, 2H), 7.33-7.42 (d, 2H, J=8.4 Hz), 7.22-7.31 (d, 2H, J=7.4 Hz), 7.33-7. 84 (m, 1H); 13C NMR (100 MHz, CDCl3): δ (ppm) 122.6, 124.8, 124.5, 126.8, 128.4, 130.7, 132.4, 142.2, 148.7, 158.4; IR (KBr) (cm-1): 3048, 1621, 1556, 1533, 1352.
Results and Discussion
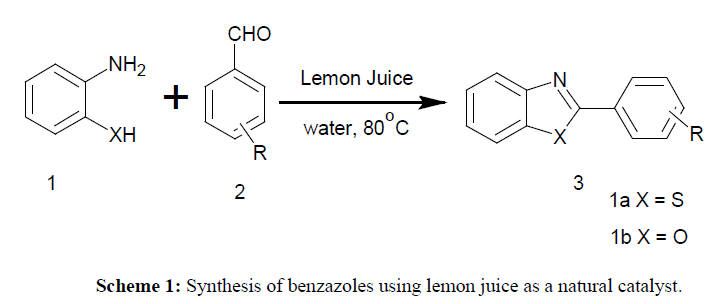
Initially, in order to optimize various reaction conditions for the synthesis of benzothiazole we conducted the model reaction between 2-aminothiophenol (1 mmol), 4-nitrobenzaldehyde (1 mmol) and lemon juice:water (4 mL, 1:1, v/v) Scheme 1.
Initially, to study the effect of catalyst loading the model reaction was carried out at ambient temperature by increasing amount of lemon juice from 1 to 3 mL, but moderate product was obtained with prolonged reaction time (Table 2, entries 1-5). Continuing our effort by paying attention on yield, surprisingly the considerable influence on the yield (81%) obtained when the model reaction was carried out at 80°C in presence of 2 ml lemon juice (Table 2, entry 6). Further in order to check the effect of the solvent on the yield of the product, the above reaction was performed in different solvents like methanol, ethanol, and water at temperature 80°C. In all cases 81-90% yield of desired product was obtained (Table 2, entry 7-9). We also optimized the catalyst-solvent proportion on conducting the model reaction by changing lemon juice: water ratio (Table 2, entry 9-11). The result showed that 2:2 (lemon juice: water) proportions suitable for smooth conversion of reactant to corresponding product with respect to time and yield.
| Entry | Amount of lemon juice (mL) | Solvent | Temperature (°C) | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| 1 | 1.0 | - | RT | 120 | Trace |
| 2 | 1.5 | - | RT | 120 | 20 |
| 3 | 2.0 | - | RT | 120 | 35 |
| 4 | 2.5 | - | RT | 120 | 35 |
| 5 | 3.0 | - | RT | 120 | 35 |
| 6 | 2.0 | - | 80 | 50 | 80 |
| 7 | 2.0 | Methanol | 80 | 70 | 81 |
| 8 | 2.0 | Ethanol | 80 | 65 | 83 |
| 9 | 2.0 | Water | 80 | 50 | 90 |
| 10 | 2.5 | Water | 80 | 50 | 90 |
| 11 | 3.0 | Water | 80 | 50 | 89 |
aReaction conditions: 4-nitrobenzaldehydes (1 mmol), 2-aminothiophenol (1 mmol), lemon juice:water (4 mL, 1:1, v/v) at 80°C.
bIsolated yield after purification
Table 2: Effect of catalytic amount of catalyst and temperature on time and yield of the model reactiona.
With these optimized condition in hand, we extended this protocol for the synthesis of benzothiazoles and benzoxazole derivatives by using various natural catalyst such as pineapple juice, and orange juice. It was observed that, both pineapple and orange juice efficiently undergo this transformation giving satisfactory yield of anticipated product. The pH analysis of all above natural catalyst is summarized in Table 3. As juices are acidic in nature it was used as acid catalyst for this protocol. Among all, the pH of lemon juice was found to be lowest as 2.3. Therefore, the yield of benzothiazoles and benzoxazole derivatives by using lemon juice was quite high as compared to pineapple juice and orange juice (Table 1).
Next, we turned our efforts towards the scope and generality of protocol for the synthesis of benzothiazole and benzoxazole derivatives by reacting 2-aminothiophenol or 2-aminophenol with diverse array of aryl aldehydes and the results are depicted in Table 1. It was observed that, aromatic aldehydes bearing electron donating as well electron withdrawing groups reacted efficiently with 2-aminothiophenol and 2-aminophenol giving corresponding product in good to excellent yields. It was noticed that the aldehydes with electron donating groups required longer reaction time than the aldehydes with electron withdrawing groups.
We then compared results obtained by above protocol with the reported catalysts for synthesis of benzothiazoles and benzoxazole derivatives (Table 4). It was observed that the present protocol is best fitted with the previous results in terms of reaction time and product yields.
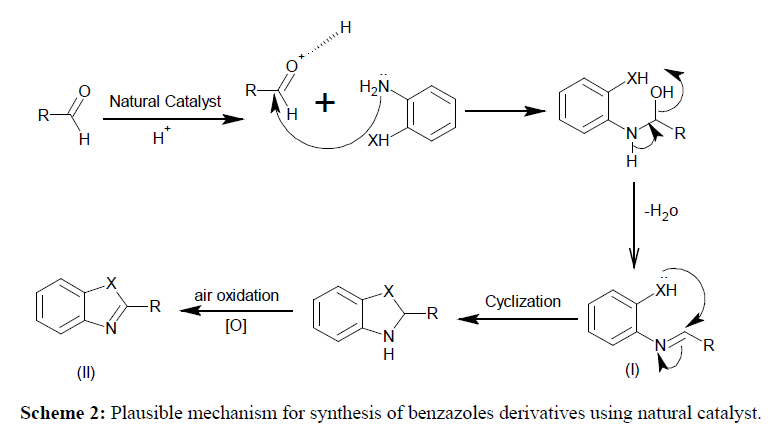
The possible mechanism for the synthesis of benzazole is depicted in Scheme 2. It is believed that carbonyl group is activated by acidity of natural catalyst for nucleophilic attack that led to the formation of intermediate (I). The intermediate I undergo intra-molecular cyclization, followed by air oxidation, leading to the formation of the desired product (II).
Conclusion
In conclusion, we have developed simple and green protocol for synthesis of benzothiazoles and benzoxazole derivatives comprising the reaction of various aryl aldehydes with aminobenzenes in good to excellent yield using natural catalyst. This method offers several advantages such as mild reaction condition high yield, short reaction time, and simple experimental and workup procedure.
References
- Morbale ST, Jadhav SD, Deshmukh MB, Patil SS (2015) Bronsted acid-type biosurfactant for heterocyclization: a green protocol for benzopyran synthesis. RSC Adv 5: 84610-84620.
- Deshmukh MB, Patil SS, Jadhav SD, Pawar PB (2012) Green Approach for Knoevenagel Condensation of Aromatic Aldehydes with Active Methylene Group. Synth Commun 42: 1177-1183.
- Mote K, Pore S, Rashinkar G, Kambale S, Kumbhar A, et al. (2010) Acacia concinna pods: as a green catalyst for highly efficient synthesis of Acylation of amines. Arch Apll Sci Res 2: 74-80.
- Vekariya RH, Patel KD, Patel HD (2016) Fruit juice of Citrus limon as a biodegradable and reusable catalyst for facile, eco-friendly and green synthesis of 3,4-disubstituted isoxazol-5(4H)-ones and dihydropyrano[2,3-c]-pyrazole derivatives. Res Chem Intermed 42: 7559.
- Pal R (2014) Visible light induced Knoevenagel condensation: A clean and efficient protocol using aqueous fruit extract of tamarindus indica as catalyst. Int J Adv Chem 2: 27-33.
- Radi M, Saletti S, Botta M (2008) A one-pot, two-step microwave-assisted synthesis of highly functionalized benzoxazoles using solid-supported reagents (SSRs). Tetrahedron Lett 49: 4464.
- Etna T, Yildiz I, Tekiner-Gulbas B, Bolelli K, Temiz-Arpaci O, et al. (2009) Synthesis, biological evaluation and 2D-QSAR analysis of benzoxazoles as antimicrobial agents. Eur J Med Chem 44: 501.
- Alper-Hayta S, Arisoy M, Temiz-Arpaci O, Yildiz IE, Semiha OA, et al. (2008) Synthesis, antimicrobial activity, pharmacophore analysis of some new 2-(substitutedphenyl/benzyl)-5-[(2-benzofuryl)carboxamido]benzoxazoles. J Med Chem 43: 2568.
- Kumar D, Jacob MR, Reynolds MB, Kerwin SM (2002) Synthesis and evaluation of anticancer benzoxazoles and benzimidazoles related to UK-1. Bioorg Med Chem 10: 3997-4004.
- Song X, Vig BS, Lorenzi PL, Drach JC, Townsend LB, et al. (2005) Amino Acid Ester Prodrugs of the Antiviral Agent 2-Bromo-5,6-dichloro-1-(β-d-ribofuranosyl)benzimidazole as Potential Substrates of hPEPT1 Transporter. J Med Chem 48: 1274-1277.
- Lu J, Fu H (2011) Copper-Catalyzed Cascade Synthesis of Alkyl 6-Aminobenzimidazo[2,1-a]isoquinoline-5-carboxylates. J Org Chem 76: 4600-4605.
- Kashiyama E, Hutchinson I, Chua MS, Stinson SF, Phillipes LR, et al. (1992) Antitumor Benzothiazoles Synthesis, Metabolic Formation, and Biological Properties of the C- and N-Oxidation Products of Antitumor 2-(4-Aminophenyl)benzothiazoles. J Med Chem 42: 4172.
- CMathis CA, Bacskai BJ, Kajdasz ST, McLellan ME, Frosch MP, et al. (2002) A lipophilic thioflavin-T derivative for positron emission tomography (PET) imaging of amyloid in brain. Bioorg Med Chem Lett 12: 295-298.
- Yamamoto K, Fujitta M, Tabashi K, Kawashima Y, Kato E, et al. (1988) Novel calcium antagonists. Synthesis and structure-activity relationship studies of benzothiazoline derivatives. J Med Chem 31: 919-930.
- Yoshida H, Nakao R, Nohta H, Yamaguchi M (2000) Chemiluminescent properties of some luminol-related compounds-Part 3. Dyes Pigm 47: 239.
- Lee BC, Jung JH, Jeong JM, Kim SE (2011) Aromatic radiofluorination and biological evaluation of 2-aryl-6-[18F]fluorobenzothiazoles as a potential positron emission tomography imaging probe for β-amyloid plaques. Bioorg Med Chem 19: 2980.
- Xie Y, Deng S, Chen Z, Yan S, Landry DW (2006) Identification of small-molecule inhibitors of the Abeta-ABAD interaction. Bioorg Med Chem Lett 16: 4657-4660.
- Reiser A, Leyshon LJ, Saunders D, Mijovic MV, Bright A, et al. (1972) Fluorescence of aromatic benzoxazole derivatives. J Am Chem Soc 94: 2414-2421.
- Chaudhari C, Siddiki MAH, Ken-ichi S (2015) Acceptorless dehydrogenative synthesis of benzothiazoles and benzimidazoles from alcohols or aldehydes by heterogeneous Pt catalysts under neutral conditions. Tetrahedron Lett 56: 4885.
- Kardanpour R, Tangestaninejad S, Mirkhani V, Moghadam M, Mohammadpoor-Baltork I, et al. (2016) Anchoring of Cu(II) onto surface of porous metal-organic framework through post-synthesis modification for the synthesis of benzimidazoles and benzothiazoles. J Solid State Chem 235: 145.
- Pardeshi SD, Sonar JP, Pawar SS, Dekhane D, Gupta S, et al. (2014) Sonicated assisted synthesis of benzimidazoles, benzoxazoles and benzothiazoles in aqueous media. J Chil Chem Soc 59: 2335-2340.
- Chakraborti AK, Rudrawar S, Jadhav KB, Kaur G, Chankeshwara SV (2007) “On water” organic synthesis: a highly efficient and clean synthesis of 2-aryl/heteroaryl/styryl benzothiazoles and 2-alkyl/aryl alkyl benzothiazolines. Green Chem 9: 1335-1340.
- Naveen KT, Sai KB, Chandana K (2016) Micropropagation, Micromorphological Studies, and In Vitro Flowering in Rungia pectinata L. J Basic Appl Res 2: 226.
- So YH, Zaleski JM, Murlick C, Ellaboudy A (1996) Synthesis and Photophysical Properties of Some Benzoxazole and Benzothiazole Compounds. Macromolecules 29: 2783-2795.
- Banerjeea S, Payraa S, Sahaa A, Sereda G (2014) ZnO nanoparticles: a green efficient catalyst for the room temperature synthesis of biologically active 2-aryl-1, 3-benzothiazole and 1, 3-benzoxazole derivatives. Tetrahedron Lett 55: 5515-5520.
- Chikhale RV, Pant AM, Menghani SS, Wadibhasme PG, Khedekar PB (2014) Facile and efficient synthesis of benzoxazole derivatives using novel catalytic activity of PEG-SO3H. Arabian J Chem pp. 1878-5352.
- Sharma P, Gupta M, Kant R, Gupta VK (2015) Formation of a nanorod shaped ionogel and its high catalytic activity for one-pot synthesis of benzothiazoles. New J Chem 39: 5116-5120.
- Mohammadi M, Bardajee GR, Pesyan NN (2014) A novel method for the synthesis of benzothiazole heterocycles catalyzed by a copper–DiAmSar complex loaded on SBA-15 in aqueous media. RSC Adv 4: 62888-62894.
- Sharma H, Singh N, Jang DO (2014) A ball-milling strategy for the synthesis of benzothiazole, benzimidazole and benzoxazole derivatives under solvent-free conditions. Green Chem 16: 49224930.
- Mortimer CG, Wells G, Crochard JP, Stone EL, Bradshaw TD, et al. (2006) Antitumor Benzothiazoles. 26.1 2-(3,4-Dimethoxyphenyl)-5-fluorobenzothiazole (GW 610, NSC 721648), a Simple Fluorinated 2-Arylbenzothiazole, Shows Potent and Selective Inhibitory Activity against Lung, Colon, and Breast Cancer Cell Lines. J Med Chem 49: 179-185.
- Karhale S, Patil K, Bhenki C, Rashinkar, Helavi GV (2016) Zirconocene catalyzed synthesis of 2-substituted benzimidazole derivatives. Res Chem Intermed 42: 7257.
- Survase DN, Chavan HV, Dongre SB, Helavi VB (2016) Polyethylene glycol in water: Simple, efficient, and catalyst-free synthesis of 4H-pyran derivatives. Synth Commun 46: 1665-1670.
- Das S, Samanta S, Maji SK, Samanta PK, Dutta AK, et al. (2013) visible light driven synthesis of 2-substitutedbenzothiazoles using CdS nanosphere as heterogenous recyclable catalyst. Tetrahedron Lett 54: 1090.
- Teimouria, Chermahini AN, Salavati H, Ghorbanian L (2013) An efficient and one-pot synthesis of benzimidazoles, benzoxazoles, benzothiazoles and quinoxalines catalyzed via nano-solid acid catalysts. J Mol Catal A: Chem 373: 38.
- Araujo DP, Morais VSS, de Fatima A, Modolo LV (2015) Efficient sodium bisulfite-catalyzed synthesis of benzothiazoles and their potential as ureases inhibitors. RSC Adv 5: 28814-28821.
- Bardajee GR, Mohammadi M, Yari H, Ghaedi A (2016) Simple and efficient protocol for the synthesis of benzoxazole, benzoimidazole and benzothiazole heterocycles using Fe(III)-Schiff base/SBA-15 as a nanocatalyst. Chin Chem Lett 27: 265.
- Nalage SV, Bhosale SV, Bhosale DS, Jadhav WN (2010) P2O5 mediated rapid condensation of 2-aminothiophenol with aromatic aldehydes at ambient temperature. Chin Chem Lett 21: 790.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences