ISSN : 2347-5447
British Biomedical Bulletin
Incl3-Catalyzed One-Pot Three-Component Aza-Friedel-Crafts Reactions (AFCR) Of 3-Substituted Indole Derivatives and Its In-Silico Mcl-1 Inhibition Studies
Rajasekar Mani*, Wilson Alphonse Carlton Ranjith, and Rajaretinam Rajesh Kannan
Centre for Molecular and Nanomedical Sciences, International Research Centre, Sathyabama Institute of Science and Technology (Deemed to be University), Chennai, INDIA.
*Corresponding author: Rajasekar Mani, Centre for Molecular and Nanomedical Sciences, International Research Centre, Sathyabama Institute of Science and Technology (Deemed to be University), Chennai - 600 119, INDIA, E-mail: drmrajasekar.irc@sathyabama.ac.in
Received date: April 29, 2021, Manuscript No. IPJOIC-22-13391; Editor assigned date: May 02, 2022, PreQC No. IPJOIC-22-13391 (PQ); Reviewed date: May 13, 2022, QC No IPJOIC-22-13391; Revised date: May 23, 2022, Manuscript No. IPJOIC-22-13391 (R); Published date: May 30, 2022, DOI: 10.36648/2472-1123.8.3.17
Citation: Mani R (2022) InCl3-Catalyzed One-Pot Three-Component Aza-Friedel-Crafts Reactions (Afcr) of 3-Substituted Indole Derivatives and Its In-Silico Mcl-1 Inhibition Studies. J Org Inorg Chem Vol.8 No.3:17
Abstract
InCl3-catalyzed three-component Aza-Friedel-Crafts reactions of Aldehydes, 4-Aminofluorescein, and indoles in methanol have been developed. The Aza-Friedel-Crafts products could be easily transformed into various 3-substituted indoles including biologically active compounds. This system offers a novel efficient method for the synthesis of 3-substituted indole derivatives in good yields. Further, the molecules are predicted to bind with Induced myeloid leukemia cell differentiation protein Mcl-1 using Extended Connectivity fingerprint ECfp4 NN (ECfp4)+NB (ECfp4). Molecular docking studies with Mcl-1 showed a docking score of -7.8 kcal/mol with two hydrogen bonds and nine hydrophobic contacts by interaction with the R263 hot-spots implying a potential inhibitor against Mcl-1.
Keywords: Aldehyde; Amine; Aza-Friedel-Crafts reactions; 3-Substituted indoles; Indoles; InCl3
Introduction
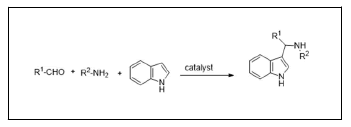
The Aza-Friedel-Crafts reactions (AFCR) have attracted much attention as a synthetically outstanding carbon-carbon bond-forming process that leads to functionalized amines in a green transformation and completely atom-economicF way. However, in spite of its significance, many potential problems related to arene nucleophile, electrophilic substrate, or functional group tolerance still remain for this transformation [1,3]. For example, the Aza-Friedel-Crafts reaction seems to be restricted to highly electrophilic substrates, such as trifluoromethyl imines, and highly nucleophilic substrates, such as indoles, electron-rich arenas due to the low reactivity of imines. In addition, the one-pot Multicomponent Coupling Reactions (MCRs), which introduce several elements of diversity into a molecule in a single step [4,6]. For a long time, the transition metal-catalyzed Multi-Component Reactions (MCRs) have attracted much interest because of their capability of offering many challenging transformations using one-pot method [7,8]. Particularly those metal catalysts, which are derived from the group VIII-X metals, display remarkable efficiency for the formation of carbon-carbon and carbon-heteroatom bonds are shown in (Figure 1) [9]. Furthermore, Piersanti and co-workers reported the reaction using enamines derived from α-ketoesters with indoles to give products with moderate enantioselectivities [10]. The recently reported the highly enantioselective Aza-Friedel-Crafts reaction of 2-substituted-3H-indol-3-one derivatives with pyrroles using novel chiral imidazoline-phosphoric acid catalysts [11].
In addition, Indoles are important structural units in many natural products and their derivatives are known to possess various biological properties (Figure 2), such as antibacterial, antioxidative, and insecticidal activities, and some indole derivatives have been used as antibiotics in pharmaceuticals [12]. Among indole derivatives, bis-indolylalkanes, 3-alkyl indoles, and 3-diarylmethyl indoles are an important class of bioactive metabolite, which can be synthesized by Lewis acid catalysis and much attention has been paid to the synthesis of them for a long while [13,14]. Although the synthesis of 3-alkyl indoles has been studied extensively, the synthesis of other unsymmetrical indole derivatives is still highly desirable in the synthetic community due of it needs more practical procedures and mild reaction conditions [15,16]. As part of our ongoing efforts devoted to InCl3-catalyzed organic reactions, [17] herein we wish to report the first genuinely and highly efficient InCl3 as Lewis acid-catalyzed one-pot three-component Aza-Friedel-Crafts reactions of indoles, Aldehyde, and Amine. The reactions generated the corresponding 3-substituted indole derivatives in good yields under mild reaction conditions.
Experimental Section
Aldehydes, 4-Aminofluorescein, Indole, Indium (III) Chloride, and other alcohols/solvents are obtained from SRL, Chennai, Tamil Nadu, and India. Column chromatography was performed on Silica Gel (100-200 mesh). NMR spectra were recorded on Bruker DRX 300 MHz in CDCl3 or DMSO-d6 at the University of Madras (Chennai, Tamil Nadu, and India). TMS was used as the internal standard (δ=0.00 ppm) and all the J values are given in hertz. Optical rotation was performed by using Rudolph-Autopol II digital polarimeter. Elemental analyses were performed using Perkin-Elmer 2400 elemental analyzer.
General procedure for the synthesis of 3-substituted indole (4a-e)
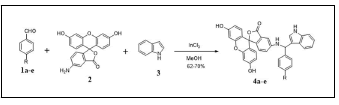
To a solution of the aldehydes 1a-e (1 mmol), 4-aminofluorescein 2 (1 mmol), Indole 3 (1 mmol), Indium trichloride (1 mmol, 0.22 g) as a catalyst, and dry MeOH (25 mL) were added. After stirring at 120 °C oil bath reflux temperature for a given period of time, the reaction mixture was evaporated under reduced pressure and extracted by chloroform-water. The chloroform layer was dried over anhyd. Na₂SO₄ and concentrated to dryness. The product was further purified by flash column chromatography.
Synthesis of 5-(((4-chlorophenyl)(1H-indol-3-yl)methyl)amino)-3',6'-dihydroxy-3H-spiro[isobenzofuran-1,9'-xanthen]-3-one (4a)
Compound, 4a was obtained by the reaction of 4-Chlorobenzaldehyde (1,1 mmol, 0.14 g), 4-Aminofluorescein (2,1 mmol, 0.34 mmol), Indole (3,1 mmol, 0.11 g) as brown solid: Yield: 0.41 g (70%); [α]D31+87.5 (c 0.1,CHCl₃); 1H NMR: (300 MHz,CDCl₃): δ 4.66 (s,1H,methine), 4.69, (s, 1H,-NH), 6.77 (d,2H,J=9.0 Hz,Ar-H), 6.93 (d,2H,J=8.5 Hz,Ar-H), 7.05 (t,2H,J=7.2 Hz, Ar-H), 7.28 (d,1H,J=7.5 Hz, Ar-H), 7.33 (t, 2H, J=7.2 Hz, Ar-H), 7.55 (t, 4H, J=7.5 Hz, Ar-H), 7.65-7.74 (m, 4H, Ar-H), 8.06 (s, 2H, Ar-OH), 8.25 (d, 1H, J=7.5 Hz, Ar-H), 10.90 (s, 1H,-NH). 13C NMR: (75 MHz, CDCl₃): δ 60.7, 82.1, 116.7, 117.2, 118.4, 119.1, 124.2, 125.3, 125.7, 126.1, 128.3, 128.7, 128.9, 129.1, 130.1, 130.3, 130.4, 130.6, 133.9, 134.1, 135.3, 149.8, 150.7, 151.2, 164.5, 169.1, 169.6. Elemental Anal. Found: C, 71.63; H, 3.93; N, 4.75. Calc. for C₃₅H₂₃ClN₂O₅: C, 71.61; H, 3.95; N, 4.77.
Synthesis of 5-(((4-bromophenyl)(1H-indol-3-yl)methyl)amino)-3',6'-dihydroxy-3H-spiro[isobenzofuran-1,9'-xanthen]-3-one (4b)
Compound, 4b was obtained by the reaction of 4-Bromobenzaldehyde (1b, 1 mmol, 0.18 g), 4-Aminofluorescein (2, 1 mmol, 0.34 mmol), Indole (3, 1 mmol, 0.11 g) as brown solid: Yield: 0.39 g (62%); [α]D31+84.5 (c 0.1,CHCl3); 1H NMR: (300 MHz, CDCl3): δ 4.67 (s, 1H, methine), 4.69, (s,1H,-NH), 6.76 (d,2H,J=7.2 Hz, Ar-H), 6.96 (d, 2H, J=7.2 Hz, Ar-H), 7.05 (t, 2H, J=7.2 Hz, Ar-H), 7.28 (d, 1H, J=7.5 Hz, Ar-H), 7.38 (t, 2H, J=7.2 Hz, Ar-H), 7.58 (t, 4H, J=7.5 Hz, Ar-H), 7.65-7.74 (m, 4H, Ar-H), 8.07 (s, 2H, Ar-OH), 8.25 (d, 1H, J=7.5 Hz, Ar-H), 10.90 (s, 1H,-NH). 13C NMR: (75 MHz, CDCl3): δ 60.8, 82.4, 116.8, 117.2, 118.6, 119.8, 124.6, 125.3, 125.7, 126.6, 128.3, 128.7, 128.9, 129.5, 130.1, 130.3, 130.4, 130.6, 133.9, 134.5, 135.3, 149.6, 150.6, 151.2, 164.5, 169.1, 169.5. Elemental Anal. Found: C, 66.55; H, 3.65; N, 4.46. Calc. for C35H23BrN2O5: C, 66.57; H, 3.67; N, 4.44.
Synthesis of 5-(((4-fluorophenyl)(1H-indol-3-yl)methyl)amino)-3',6'-dihydroxy-3H-spiro[isobenzofuran-1,9'-xanthen]-3-one (4c)
Compound, 4c was obtained by the reaction of 4-Fluorobenzaldehyde (1c, 1 mmol, 0.12 g), 4-Aminofluorescein (2, 1 mmol, 0.34 mmol), Indole (3, 1 mmol, 0.11 g) as brown solid: Yield: 0.39 g (68%); [α]D31+78.2 (c 0.1, CHCl3); 1H NMR: (300 MHz, CDCl3): δ 4.65 (s, 1H, methine), 4.68, (s, 1H,-NH), 6.79 (d, 2H, J=7.2 Hz, Ar-H), 6.95 (d, 2H, J=7.5 Hz, Ar-H), 7.07 (d, 2H, J=7.2 Hz, Ar-H), 7.28 (d, 1H, J=7.5 Hz, Ar-H), 7.33 (t, 2H, J=7.2 Hz, Ar-H), 7.58 (t, 3H, J=7.5 Hz, Ar-H), 7.65-7.75 (m, 5H, Ar-H), 8.06 (s, 2H, Ar-OH), 8.26 (d, 1H, J=7.5 Hz, Ar-H), 10.91 (s, 1H,-NH). 13C NMR: (75 MHz, CDCl3): δ 60.6, 82.2, 116.6, 117.2, 118.1, 119.2, 124.3, 125.3, 125.8, 126.7, 128.3, 128.7, 128.9, 129.4, 130.0, 130.3, 130.4, 130.6, 133.3, 134.4, 135.5, 149.8, 150.5, 151.2, 164.5, 169.1, 169.7. Elemental Anal. Found: C, 73.66; H, 4.08; N, 4.93. Calc. for C35H23FN2O5: C, 73.68; H, 4.06; N, 4.91.
Synthesis of 5-(((1H-indol-3-yl)(4-iodophenyl)methyl)amino)-3',6'-dihydroxy-3H-spiro[isobenzofuran-1,9'-xanthen]-3-one (4d)
Compound, 4d was obtained by the reaction of 4-Iodobenzaldehyde (1d, 1 mmol, 0.23 g), 4-Aminofluorescein (2, 1 mmol, 0.34 mmol), Indole (3, 1 mmol, 0.11 g) as brown solid: Yield: 0.44 g (65%); [α]D31+86.4 (c 0.1, CHCl3); 1H NMR: (300 MHz, CDCl3): δ 4.66 (s, 1H, methine), 4.68, (s, 1H,-NH), 6.79 (d, 2H, J=7.2 Hz, Ar-H), 6.95 (d, 2H, J=7.5 Hz, Ar-H), 7.06 (t, 2H, J=7.2 Hz, Ar-H), 7.29 (d, 1H, J=7.5 Hz, Ar-H), 7.33 (t, 3H, J=7.2 Hz, Ar-H), 7.56 (t, 3H, J=7.5 Hz, Ar- H), 7.62-7.75 (m, 4H, Ar-H), 8.06 (s, 2H, Ar-OH), 8.26 (d, 1H, J=7.5 Hz, Ar-H), 10.91 (s, 1H,-NH). 13C NMR: (75 MHz, CDCl3): δ 60.5, 82.1, 116.7, 117.5, 118.4, 119.1, 124.5, 125.3, 125.7, 126.5, 128.3, 128.7, 128.9, 129.4, 130.1, 130.3, 130.4, 130.6, 133.5, 134.3, 135.3, 149.8, 150.7, 151.5, 164.2, 169.1, 169.6. Elemental Anal. Found: C, 61.98; H, 3.44; N, 4.15. Calc. for C35H23IN2O5: C, 61.96; H, 3.42; N, 4.13.
Synthesis of 5-(((1H-indol-3-yl)(p-tolyl)methyl)amino)-3',6'-dihydroxy-3H-spiro[isobenzofuran-1,9'-xanthen]-3-one (4e)
Compound, 4e was obtained by the reaction of 4-Methylbenzaldehyde (1e, 1 mmol, 0.12 g), 4-Aminofluorescein (2, 1 mmol, 0.34 mmol), Indole (3, 1 mmol, 0.11 g) as brown solid: Yield: 0.38 g (68%); [α]D31+82.5 (c 0.1, CHCl3); 1H NMR: (300 MHz, CDCl3): δ 2.31 (s, 3H,-CH3), 4.65 (s, 1H, methine), 4.67, (s, 1H,-NH), 6.76 (d, 2H, J=7.5 Hz, Ar-H), 6.94 (d, 2H, J=7.5 Hz, Ar-H), 7.06 (t, 2H, J=7.2 Hz, Ar-H), 7.28 (d, 1H, J=7.2 Hz, Ar-H), 7.36 (t, 2H, J=7.2 Hz, Ar-H), 7.56 (t, 4H, J=7.5 Hz, Ar- H), 7.65-7.78 (m, 4H, Ar-H), 8.06 (s, 2H, Ar-OH), 8.26 (d, 1H, J=7.5 Hz, Ar-H), 10.91 (s, 1H,-NH). 13C NMR: (75 MHz, CDCl3): δ 21.2, 60.6, 82.1, 116.8, 117.2, 118.5, 119.5, 124.6, 125.3, 125.7, 126.1, 128.3, 128.7, 128.9, 129.1, 130.1, 130.3, 130.4, 130.6, 133.6, 134.4, 135.3, 149.8, 150.7, 151.2, 164.5, 169.1, 169.5. Elemental Anal. Found: C, 76.33; H, 4.65; N, 4.96. Calc. for C36H26N2O5: C, 76.31; H, 4.63; N, 4.94.
Ligand-based target prediction
Prediction of ligands based target protein was performed using Extended Connectivity fingerprint ECfp4 NN (ECfp4)+NB (ECfp4) of the PPB2 server [18]. Extended-Connectivity Fingerprints (ECFPs) are circular topological fingerprints designed for molecular characterization, similarity searching, and structure-activity modeling. It is among the most popular similarity search tools in drug discovery and they are effectively used in a wide variety of applications. NB (ECfp4) stands for a multinomial Naive Bayes model built on a reference database (~350K compounds and 1720 targets) extracted from ChEMBL using ECfp4 fingerprint. This model was built only once and uses to predict the targets for any query molecule. NN (ECfp4) is a similarity searching based target predictions method using ECfp4 fingerprint.
Initially the similarity between a query molecule and each of the compounds in the reference database and followed by sorting of the compounds in the reference database as per similarity score. The top 2000 compounds (nearest neighbor of a query) selected from the reference database and collect the targets associated with these top 2000 compounds. Scoring is performed as per the similarity of the most similar nearest neighbor associated with a target.
Molecular docking studies using autodock vina
The best target predicted from the PPB2 server was used to further studies. The protein structure of Estrogen receptor alpha, Mcl-1, and Progesterone receptor was modeled using the Swiss model server [19,20]. The 3D structure of the protein and the small molecules were obtained and converted to the PDBQT file. The active site amino acids were determined using PDBSum [21] from the PDB ids: 3ERT, 4WMR, and 2OVH respectively. Docking was performed using the grid for the whole protein with a default value of 0.375 Å with exhaustiveness of 24 using AutodockVina [22,23]. The interaction of the molecule and the protein was further analyzed using Discovery studio [24].
Result and Discussion
Synthesis of 3-substituted indole (4a-e)
Exhaustive studies of the Aza-Friedel-Crafts reaction (AFCR) conditions for the synthesis of 3-substituted indole 4a-e, aldehydes (1a-e) with 4-aminofluorescein (2) and indole (3) in the presence of InCl₃ were conducted (Scheme 1). The 1H NMR spectra of 3-substituted indole 4a-e, show a signal at around 6.0-8.0 ppm for aromatic proton and singlet resonance for the methine hydrogen (sp3 C-H) appears at 5.92 ppm. The expected high field chemical shift for 3-substituted indole N-H resonances is observed at 10.9 ppm. The presence of the unsaturated carbonyl group was confirmed from the 13C NMR spectrum [δ (C=O) ~169 ppm]. Structure of reaction time and product yields are given in Table 1.
| S. No | R | Time (h) | Yield (%) |
|---|---|---|---|
| 1 | Cl (4a) | 8 | 70 |
| 2 | Br (4b) | 8 | 62 |
| 3 | F (4c) | 8 | 68 |
| 4 | I (4d) | 8 | 65 |
| 5 | Me (4e) | 8 | 68 |
Table 1: Structure of reaction time and product yields
Target prediction using extended connectivity fingerprint ECfp4 NN (ECfp4)+NB (ECfp4) of 3-substituted indole
A fingerprint is the numerical representation of a molecule. The Fingerprint of a molecule contains a set of different numbers, which usually describe the properties of a molecule such as physicochemical properties, composition, topological features, substructure, etc. Once the fingerprints of the molecules are computed they can be used for a variety of different calculations such as similarity searching or model building.
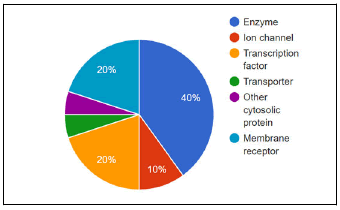
The possible interaction of 3-substituted indole remains mostly with the enzymes followed by transcription factors, membrane receptors ion channels, transporters, and other cytosolic protein are shown in figure 3. The molecule 5-(((4-chlorophenyl)(1H-indol-3-yl)methyl)amino)-3',6'-dihydroxy-3H-spiro[isobenzofuran-1,9'-xanthen]-3-one (4) is predicted to interact with proteins like Estrogen receptor alpha, Induced myeloid leukemia cell differentiation protein Mcl-1, Progesterone receptor, Estrogen receptor beta, Cannabinoid CB2 receptor, Serine/threonine-protein kinase PIM1, Serine/threonine-protein kinase AKT, Glycogen synthase kinase-3 beta, Cannabinoid CB1 receptor, Vascular endothelial growth factor receptor 2, Serotonin 2a (5-HT2a) receptor, Vanilloid receptor, Glucocorticoid receptor, Serotonin 2c (5-HT2c) receptor, PI3-kinase p110-alpha subunit, Serotonin transporter, HERG, Epidermal growth factor receptor erbB1, Cyclooxygenase-2 and Anandamide amidohydrolase based on ECfp4 NN (ECfp4)+NB (ECfp4) method.
Interaction of 3-substituted indole with Estrogen receptor alpha, Mcl-1, and Progesterone receptor
The protein targets (Estrogen receptor alpha, Mcl-1, and Progesterone receptor) assessed using ECfp4 NN (ECfp4)+NB (ECfp4) method was used for docking studies. The active site of Estrogen receptor alpha includes Met 343, Leu 346, Thr 347, Ala 350, Asp 351, Glu 353, Trp 383, Leu 387, Arg 394, Phe 404, Glu 419, Gly 420, Met 421 and Leu 428, for Mcl-1 the active site amino acid are His 224, Ala 227, Phe 228, Met 231, Leu 246, Val 249, Met 250, Val 253, Phe 254, Arg 263, Thr 266, Leu 267, Phe 270, Gly 271 and Ile 294. The active site of Progesterone receptor includes Leu 715, Leu 718, Asn 719, Leu 721, Gly 722, Glu 723, Gln 725, Leu 726, Trp 755, Met 756, Met 759, Arg 766, Phe 778, Phe 794, Leu 797, Met 801, Leu 887, Cys 891 and Thr 894. After performing Autodock Vina 5-(((4-chlorophenyl)(1H-indol-3-yl)methyl)amino)-3',6'-dihydroxy-3H-spiro[isobenzofuran-1,9'-xanthen]-3-one (4) showed a docking score of -5.8, -7.8 and -6.7 with Estrogen receptor alpha, Mcl-1 and Progesterone receptor respectively.
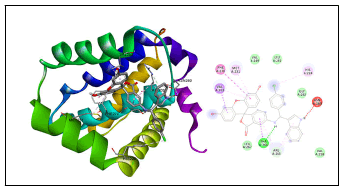
Indole derivatives are considered as potential Mcl-1 inhibitors [25,27]. Mcl-1 is involved in the regulation of apoptosis versus cell survival, and in the maintenance of viability but not of proliferation. It mediates its effects by interactions with a number of other regulators of apoptosis [28]. Mcl-1 is a major cancer target and its overexpression is observed commonly in human cancer. The overexpression has been reported in different cancer like breast cancer, lung cancer, prostate cancer, pancreatic cancer, cervical and ovarian cancers, and leukemia. Targeting Mcl-1 is a potential way the treatment cancer. The highest docking score is observed against Induced myeloid leukemia cell differentiation protein Mcl-1 showed 2 hydrogen bonds (Thr 266 and Arg 263) and 9 hydrophobic interactions with Val 253, Thr 266, His 224, Met 231, Val 253 and Arg 263 with the highest docking score are shown in (Figure 4) [29]. The binding hot spot of the Mcl-1 is at the amino acid Arg 26330 and this indole derivative has formed a hydrogen bond at this position making the compound a potential inhibitor.
Interaction with Estrogen receptor alpha showed 5 hydrophobic interactions with the amino acids Leu 327, Arg 352, Pro 325, Leu 327, and Val 355. The molecule showed 2 hydrogen bond with Val 698 and Trp 765 of the Progesterone receptor followed by 2 electrostatic interaction with Arg 766 and Glu 695, and 10 hydrophobic interactions were observed at Val 698, Trp 765, Pro 696, Lys 822, Val 698, Pro 780, Pro 696 and Lys 769.
Conclusion
In conclusion, we have demonstrated a very simple, efficient, and practical method for the one-pot Aza-Friedel-Crafts reactions of 3-substituted indole derivatives from Aldehyde, Indole, and aromatic amines in the presence of a catalytic amount of Indium trichloride. The existence of the methine hydrogen (sp3 C-H) was identified from 1H and 13C NMR studies. Preliminary In-silico studies on this molecule have shown that it interacts with Mcl-1 efficiently at the Arg 263 position, which is a hotspot of Mcl-1. The major advantage of this method is that it is truly a one-pot procedure that does not require a separate step to prepare an imine for subsequent use. The significant features of the protocol include operational simplicity, inexpensive reagents, mild condition, and high yields of the products. Further in vivo and in vitro studies on the molecule will help in understanding the efficiency of the molecule as an anticancer agent.
Acknowledgment
MR acknowledges financial support from the DBT, New Delhi. The authors thank Sathyabama Institute of Science and Technology, Chennai, Tamil Nadu, India’ for providing infrastructure and instrumentation support to execute the research work.
References
- Nakamura S, Furukawa T, Hatanaka T, Funahashi Y (2018) Enantioselective Aza-Friedel-Crafts reaction of cyclic ketimines with indoles using chiral imidazoline-phosphoric acid catalysts. Chem Commun 54: 3811-3814.
- Zhao ZB, Shi L, Li Y, Meng FJ, Zhou YG (2019) Facile synthesis of chiral õ-sultams: Via an organocatalytic Aza-Friedel-Crafts reaction. Org Biomol Chem 17: 6364-6368.
- You Y, Lu WY, Xie KX, Zhao JQ, Wang ZH, Yuan WC (2019) Enantioselective synthesis of isoquinoline-1,3(2: H,4 H)-dione derivatives via a chiral phosphoric acid catalyzed Aza-Friedel-Crafts aeaction. Chem Commun 55: 8478-8481.
- Xie E, Huang S, Lin X (2019) Design of planar chiral phosphoric acids with a [2.2]paracyclophanyl backbone as organocatalysts for the highly enantioselective Aza-Friedel-Crafts reaction. Org Lett 21: 3682-3686.
- Arai T, Araseki K, Kakino J (2019) Catalytic asymmetric Aza-Friedel-Crafts-type reaction of indoles with isatin-derived N-cbz-ketimines using a chiral bis(Imidazolidine)-containing NCN-pincer palladium catalyst. Org Lett 21: 8572-8576.
- Xie E, Rahman A, Lin X (2017) Asymmetric synthesis of cf3- and indole-containing tetrahydro-β-carbolines: Via chiral spirocyclic phosphoric acid-catalyzed Aza-Friedel-Crafts reaction. Org Chem Front 4: 1407-1410.
- Rahman A, Xie E, Lin X (2018) Organocatalytic asymmetric synthesis of benzazepinoindole derivatives with trifluoromethylated quaternary stereocenters by chiral phosphoric acid catalysts. Org Biomol Chem 16: 1367-1374.
- Jiang B, Huang ZG (2005) Synthesis of α-(3-Indolyl) Glycine derivatives via spontaneous Friedel-Crafts reaction between indoles and glyoxylate imines. Synthesis (Stuttg) 2005: 2198-2204.
- Kumar A, Sharma S, Maurya RA (2009) A novel multi-component reaction of indole, formaldehyde, and tertiary aromatic amines. Tetrahedron Lett 50: 5937-5940.
- Sherry BD, Furstner A (2008) The promise and challenge of iron-catalyzed cross coupling. Acc Chem Res 41: 1500-1511.
- Righi M, Bartoccini F, Lucarini S, Piersanti G (2011) Organocatalytic synthesis of α-quaternary amino acid derivatives via Aza-Friedel-Crafts alkylation of indoles with simple α-amidoacrylates. Tetrahedron (Oxford. Print) 67: 7923-7928.
- Nakamura S, Matsuda N, Ohara M (2016) Organocatalytic enantioselective Aza-Friedel-Crafts reaction of cyclic ketimines with pyrroles using imidazolinephosphoric. Acid Catalysts Chem Eur J 22: 9478.
- Mahato SK, Acharya C, Wellington KW, Bhattacharjee P, Jaisankar P (2020) Incl3: A versatile catalyst for synthesizing a broad spectrum of heterocycles. ACS Omega, 2020: 5.
- Neto JSS, Zeni G (2019) Recent advances in the synthesis of indoles from alkynes and nitrogen sources. Org Chem Front 7: 155-210.
- Festa AA, Voskressensky LG, Van Der Eycken E V (2019) Visible light-mediated chemistry of indoles and related heterocycles. Chem Soc Rev 48: 4401-4423.
- Chen L, Zou YX (2018) Recent progress in the synthesis of phosphorus-containing indole derivatives. Org Biomol Chem 16: 7544-7556.
- Mohammadi Z G, Moradi R, Ahmadi T, Lashgari N (2018) Recent advances in the application of indoles in multicomponent reactions. RSC Adv 8: 12069-12103O.
- Awale M, Reymond JL (2019) Polypharmacology browser PPB2: Target prediction combining nearest neighbors with machine learning. J Chem Inf Model 59: 10-17.
- Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G et al. (2014) SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res 42: 1.
- Hancock JM, Zvelebil MJ, Dunbrack R (2004) Swiss model in dictionary of bioinformatics and computational biology: Wiley-Liss (1stedn) Wiley-Liss Inc is located in Wilmington, DE, United States.
- Laskowski RA, Jablonska J, Pravda L, Varekova RS, Thornton JM (2018) PDB sum: Structural summaries of PDB entries. Protein Sci 27: 129-134.
- Trott O, Olson A J (2010) Auto Dock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31: 455-461.
- Brooks BR, III CLB, Mackerell J, Nilsson L, Petrella RJ (2010) CHARMM: The biomolecular simulation program. J Comput Chem 30: 1545-1614.
- Pelz NF, Bian Z, Zhao B, Shaw S, Tarr JC (2016) Discovery of 2-indole-acylsulfonamide myeloid cell leukemia 1 (mcl-1) inhibitors using fragment-based methods. J Med Chem 59: 2054-66.
- Burke JP, Bian Z, Shaw S, Zhao B, Goodwin CM (2015) Discovery of tricyclic indoles that potently inhibit mcl-1 using fragment-based methods and structure-based design. J Med Chem 58: 3794-805.
- Wan Y, Li Y, Yan C, Yan M, Tang Z (2019) Indole: A privileged scaffold for the design of anti-cancer agents. Eur J Med Chem 183: 111691.
- Quinn BA, Dash R, Azab B, Sarkar S, Das SK (2011) Targeting Mcl-1 for the therapy of cancer. Expert opin investig drugs 20: 1397-411.
- Young AIJ, Law AMK, Castillo L, Chong S, Cullen HD (2016) MCL-1 Inhibition provides a new way to suppress breast cancer metastasis and increase sensitivity to dasatinib. Breast Cancer Res 18: 125
- Denis C, Sopkova-De Oliveira Santos J, Bureau R, Voisin-Chiret AS (2020) Hot-spots of Mcl-1 protein. J Med Chem 63: 928-943.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences