ISSN : ISSN: 2576-1455
Journal of Heart and Cardiovascular Research
Hypochlorite Raises Intracellular Free Ca2+ in Primary Cultured Smooth Muscle Cells of Rat Aorta: Participation of Cellular Signaling Pathways
Jian-Ping Liu1, Weimin Liu2, Wenyan Li1, Tao Zheng1, Bella T Altura1,3-5 and Burton M Altura1,3-5*
1Department of Physiology and Pharmacology, State University of New York, Downstate Medical Center, Brooklyn, NY, USA
2Department of Anatomy and Cell Biology, State University of New York, Downstate Medical Center, Brooklyn, NY, USA
3The Center for Cardiovascular and Muscle Research, State University of New York, Downstate Medical Center, Brooklyn, NY, USA
4School of Graduate Studies in Molecular and Cellular Science, State University of New York, Downstate Medical Center, Brooklyn, USA
5Department of Medicine, State University of New York, Downstate Medical Center, Brooklyn, NY, USA
- *Corresponding Author:
- Professor BM Altura
Suny Downstate Medical Center
Brooklyn, New York, USA
Tel: +1 -718 -270 -2194
Fax: +1 -718- 270 3103
E-mail: baltura@downstate.edu
Received date: 06 June 2017; Accepted date: 01 August 2017; Published date: 7 August 2017
Citation: Liu JP, Liu W, Li W, Zheng T, Altura BT, et al. (2017) Hypochlorite Raises Intracellular Free Ca2+ in Primary Cultured Smooth Muscle Cells of Rat Aorta: Participation of Cellular Signaling Pathways. J Heart Cardiovasc Res. Vol. 1 No. 2: 108.
Copyright: © 2017 Liu JP, et al. This is an open-access article distributed under the terms of the creative Commons attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
It has been reported that the local concentration of hypochlorite potentially exceeds 100 µM/l under pathological conditions in vivo and could cause vasoconstriction and damage of blood vessels in the microcirculation. Hypochlorite is found in atherosclerotic lesions and has been implicated in a number of cardiovascular disease processes. During moderate inflammatory conditions, the concentration of hypochlorite can reach as high as 340 µM. Thus, hypochlorite might be implicated in some pathological conditions such as inflammatory diseases, hypertension, heart diseases and stroke. The present study was designed to investigate the effects of three different protein kinase C inhibitors (bisindolylmaleimide I, staurosporine and Gö6979), PD-098059 (an inhibitor of extracellular signal-regulated kinases), genistein (an inhibitor of protein tyrosine kinase) and wortmannin {an antagonist of phosphatidylinositol 3-kinases (PI3Ks)} on intracellular free Ca2+ ({Ca2+}i) in primary, cultured rat aortic smooth muscle cells exposed to hypochlorite. The present investigation represents the first demonstration, to our knowledge, that hypochlorite increases intracellular Ca2+ concentration (from approximately 78 ± 11.4 nM to 206.3 ± 23.8 nM) in rat aortic smooth muscle cells. All the protein kinase C inhibitors (PD-098059, genistein and wortmann) significantly inhibited the elevation in {Ca2+}i induced by hypochlorite at different concentrations of 200, 400 and 800 µM from control levels. Our data suggest that protein kinase C as well as extracellular signal-regulated kinases, protein tyrosine kinases and PI3Ks, may play essential roles in hypochlorite-triggered signal transduction pathways in vascular smooth muscle cells in both normal and pathological conditions.
Keywords
Hypochlorite; Rat aorta; PKC inhibitor; ERK inhibitor; Protein tyrosine kinases inhibitor; PI3Ks antagonist; Ca2+, Intracellular free; Smooth muscle cells; Signal transduction
Introduction
Hypochlorite is one of the most aggressive oxidants of reactive oxygen species which can be produced by the myeloperoxidase (MPO)-hydrogen peroxide (H2O2)-chloride system [1-4]. MPO, a member of the hem-peroxidasecyclooxygenase superfamily, is abundantly expressed in neutrophils, and to a lesser extent in monocytes, and certain types of macrophages. MPO participates in innate immune defense mechanisms through formation of microbicidal reactive oxidants and diffusible radical species. A unique activity of MPO is its ability to use chloride as a co-substrate with hydrogen peroxide to generate chlorinating oxidants such as hypochlorous acid, a potent antimicrobial agent [4]. Hypochlorite can be released to the outside of cells, where it may attack normal tissues and cells, and thus contribute to the pathogenesis of disease processes [3-5]. It is known that hypochlorite plays important roles in both normal and pathological conditions. Reactive oxygen species are continuously generated in vivo, and an excessive level of these species are considered to be responsible for several pathophysiological processes, partly by affecting vascular tone [6]. Hydrogen peroxide serves as a substrate for myeloperoxidase, an enzyme that is released by activated neutrophils during inflammatory processes, as seen, for instance, in reperfusion injury and atherosclerosis [7]. Myeloperoxidase catalyzes the oxidation of chloride by hydrogen peroxide, yielding hypochlorite, an extremely potent oxidant [4]. Under pathological conditions, hypochlorite may be formed in relatively high concentrations. It has been documented that myeloperoxidase is a component of human atherosclerotic lesions [2] and certain inflammatory conditions [5]. Up to 40% of the H2O2 generated by activated leukocytes is used to form hypochlorite, and the local concentrations of hypochlorite can potentially exceed 100 μM/l under pathological conditions in vivo [1,8-10]. During moderate inflammatory conditions, the concentration of hypochlorite can reach as high as 340 μM [5]. The hypochlorite concentrations in our studies could be considered as biologically/pathologically-relevant to some diseases such as atherosclerosis and inflammation in vivo.
Previous studies from our labs have shown that hypochlorite can modulate vascular tone directly or, indirectly, leading to vasodilation or vasoconstriction [11,12]. Protein kinase C, mitogen-activated protein kinase (MAPK), and nonreceptor tyrosine kinase have been demonstrated to play essential roles in H2O2-tiggered signal transduction pathways in vascular smooth muscle cells [13,14]. It is, however, unclear whether hypochlorite acts on vascular smooth muscle cells via similar or different mechanisms/pathways. The current study was undertaken to investigate the effects of hypochlorite on vascular smooth muscle cells of rat aorta and to gain insight into the underlying mechanisms of its action, with special attention given to potential signal transduction mediators generated by hypochlorite.
Materials and Methods
Primary cell culture and hypochlorite treatment
The experiments were performed on single vascular smooth muscle cells of aorta, which were obtained from male Wistar rats (250–350 g) after pentobarbital sodium anesthesia and sacrifice. The procedures employed to isolate and culture rat aortic SMCs have been reported [15]. Briefly, the SMCs were cultured in Dullbecco’s modified Eagle’s medium, mixed 1:1 with Ham’s nutrient mixture F-12 at 37˚C in a humidified atmosphere composed of 95% air and 5% CO2 [15]. The culture medium contained 10% fetal calf serum, 100 U/ml penicillin, and 100μg streptomycin. More than 97% of the primary cultured cells were SMCs as confirmed by immunostaining with a monoclonal α-smooth muscle actin antibody [15]. Cells were regularly subcultured with 0.05% trypsin and seeded onto 12 mm circular coverslips. Cells at the second to sixth passages, at 80% confluence, were used for our experiments. Twenty-four hours before hypochlorite treatment, the medium was replaced with 1% serum medium. Hypochlorite was diluted in phosphate-buffered saline (PBS; pH 7.3) and was directly added to the culture medium at final concentrations ranging from 100 to 800 μM. Control samples were treated with PBS (pH 8.3) only. All of the experiments described above were performed under normal laboratory light, and approved by the Animal Care and Use Committee of our institution.
Intracellular Ca2+ measurements
Intracellular Ca2+ ({Ca2+}i) was measured in isolated primary SMCs in the presence or absence of hypochlorite, using the Ca2+-sensitive membrane-permeant fluorescent dye fura 2-AM (acetoxymethylester of 1-2-{5-carboxyoxazol-2-yl}- 6- aminobenzofuran-5-oxy-2-{2'-amino-5'methylphenoxy} ethane-N,N,N',N'-tetra-acetic acid [15], according to previously established methods [16,17]. Monolayers of the aortic SMCs, grown on the coverslips, were loaded with 2.0 μM fura 2-AM and 0.12% pluronic acid F-127 (60 mins, 37˚C). The monolayers were washed two to three times with PBS and 20 mM HEPES (pH 7.4) and incubated with this buffer at room temperature until ready to use. The monolayers were inserted in a leakproof coverslip holder. Buffer was added to the monolayer on the coverslip. The coverslip holder was mounted onto the stage of a temperature-controlled Nikon TMS inverted microscope with a long working distance Nikon Fluor objective (n.a. 0.5), attached to a 300-W xenon light source and the CCD camera for image acquisition. Buffer (control) and hypochlorite were added to the monolayers. The primary cultured aortic SMC monolayers, preloaded with fura 2-AM, were excited alternatively, at 340 and 380 nm, and the emission intensity was recorded at 510 nm using a silicon-intensified target camera [17]. Background autofluorescence for both excitation wavelengths was acquired from blanks for each experiment and subtracted from each pair of images separately before ratioing. Fluorescence ratios (R) were obtained by dividing the 340 nm image by the 380 nm image. No image misalignments occurred when those two ratiometric images were superimposed. The resulting images were then used to calculate [Ca2+]i in smooth muscle cells using external standards containing 2.54 mM Ca2+ and 0 mM Ca2+ plus 10 mM EGTA for maximum (Rmax) and minimum (Rmin) fluorescence ratios of the 340-nm and 380-nm images. [Ca2+]i was calculated according to the following equation [18]:
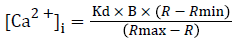
A Kd of 224 nM was used for the fura-2/Ca2+ complex [16]. B is the ratio of fluorescence intensity of fura-2 to Ca2+ : fura-2 complex excited at 380 nm.
Drugs and chemicals
The following pharmacological agents were purchased from Sigma Chemical (St. Louis, Mo., USA): sodium hypochlorite, bisindolylmaleimide I HCl, staurosporine and genistein. The fura 2-AM was purchased from Molecular Probes (Eugene, Ore., USA). Dimethyl sulfoxide (DMSO), Gö6976, PD-98059 and wortmannin were purchased from CALBIOCHEM (La Jolla, Calif., USA). All other organic and inorganic chemicals were obtained from Fisher Scientific (Fair Lawn, N.J., USA) and were of the highest purity.
Calculations and statistical analyses
Where appropriate, results are expressed as mean ± S.E.M of at least 35-40 cells each. Statistical evaluation of the results was carried out by analysis of Student t tests and ANOVA. The results were considered significant at a P value of <0.05.
Results
Effects of hypochlorite on [Ca2+]i in isolated smooth muscle cells
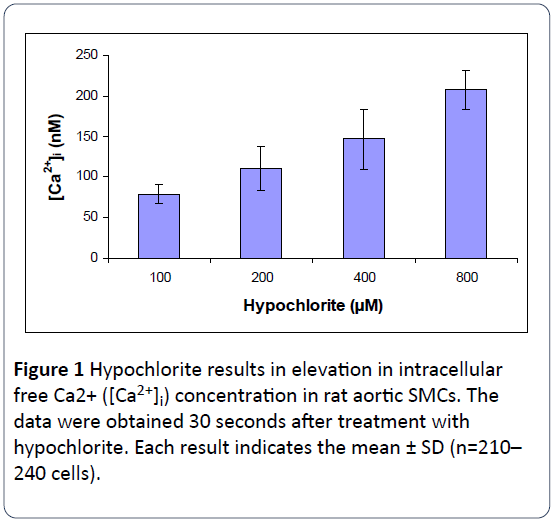
The quantitative effects of hypochlorite on [Ca2+]i in SMCs isolated from rat aorta were determined by using the direct technique of Ca2+ visualization in single cells as revealed by the digital imaging microscope using fura 2-AM [16]. The control basal level of [Ca2+]i was 78.57 ± 11.43 nM. As shown in Figure 1, hypochlorite elicited a concentration-dependent increase in [Ca2+]i within 30 seconds, the threshold being ~100 μM; 800 μM hypochlorite resulted in an almost a 2.6-fold rise in [Ca2+]i.
Effects of PKC antagonists on hypochlorite induced elevations in [Ca2+]i
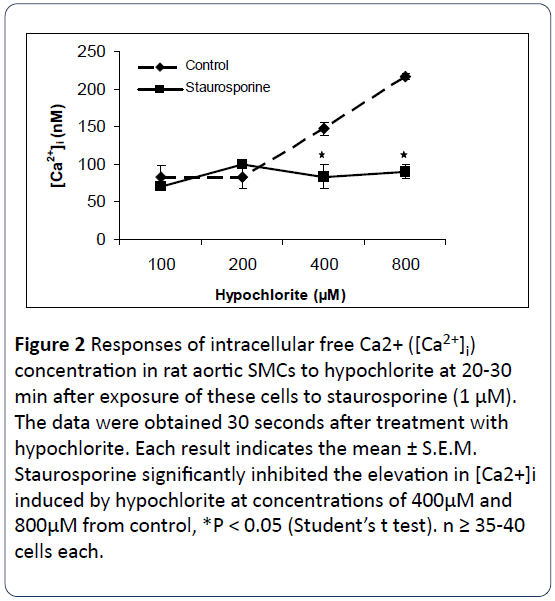
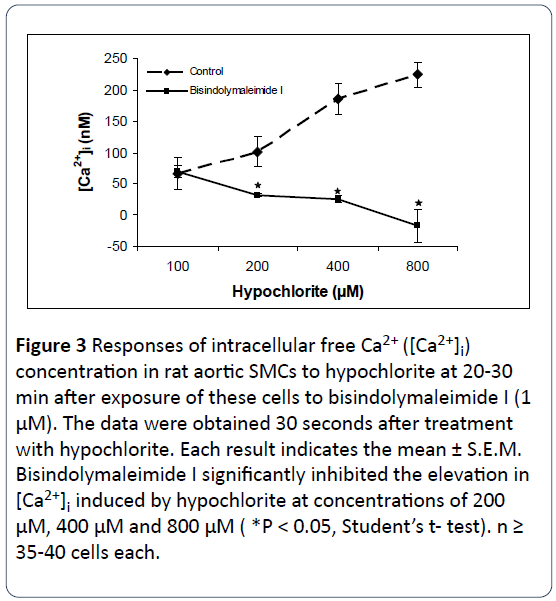
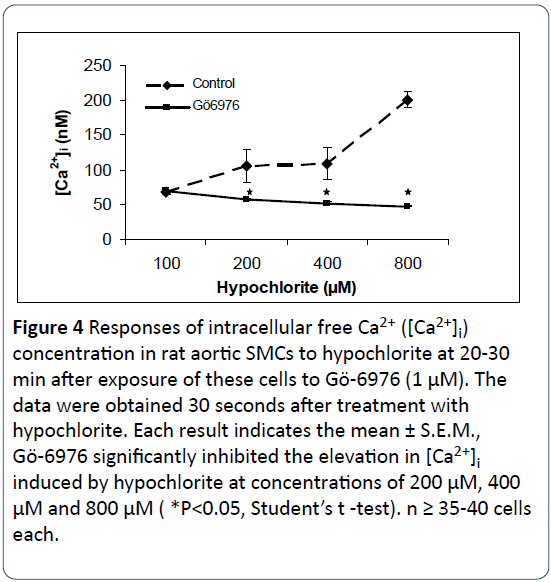
The response of three protein kinase C antagonists on hypochlorite- induced [Ca2+]i changes in SMCs isolated from rat aorta were determined by using the direct technique of Ca2+ visualization in single cells as revealed by the digital imaging microscope using fura 2-AM [16]. Figures 2-4 illustrate that preincubation of primary cultured smooth muscle cells from rat aorta with staurosporine (1 μM), bisindolylmaleimide I (1 μM) and Gö6979 (1 μM) effectively prevents hypochlorite - induced elevation in [Ca2+]i. The control basal level of [Ca2+]i in Figures 1-3 were 82.75 ± 16.26 nM, 66.22 ± 25.03 nM, 67.24 ± 2.89 nM, respectively. Somewhat to our surprise, the inhibitory effects of bisindolymaleimide I on hypochlorite induced elevations in [Ca2+]i displayed concentrationdependent effects (Figure 2).
Figure 2: Responses of intracellular free Ca2+ ([Ca2+]i) concentration in rat aortic SMCs to hypochlorite at 20-30 min after exposure of these cells to staurosporine (1 μM). The data were obtained 30 seconds after treatment with hypochlorite. Each result indicates the mean ± S.E.M. Staurosporine significantly inhibited the elevation in [Ca2+]i induced by hypochlorite at concentrations of 400μM and 800μM from control, *P < 0.05 (Student’s t test). n ≥ 35-40 cells each.
Figure 3: Responses of intracellular free Ca2+ ([Ca2+]i) concentration in rat aortic SMCs to hypochlorite at 20-30 min after exposure of these cells to bisindolymaleimide I (1 μM). The data were obtained 30 seconds after treatment with hypochlorite. Each result indicates the mean ± S.E.M. Bisindolymaleimide I significantly inhibited the elevation in [Ca2+]i induced by hypochlorite at concentrations of 200 μM, 400 μM and 800 μM ( *P < 0.05, Student’s t- test). n ≥ 35-40 cells each.
Figure 4: Responses of intracellular free Ca2+ ([Ca2+]i) concentration in rat aortic SMCs to hypochlorite at 20-30 min after exposure of these cells to Gö-6976 (1 μM). The data were obtained 30 seconds after treatment with hypochlorite. Each result indicates the mean ± S.E.M., Gö-6976 significantly inhibited the elevation in [Ca2+]i induced by hypochlorite at concentrations of 200 μM, 400 μM and 800 μM ( *P<0.05, Student’s t -test). n ≥ 35-40 cells each.
Effects of PD-098059, genistein and wortmannin on hypochlorite induced elevations in [Ca2+]i
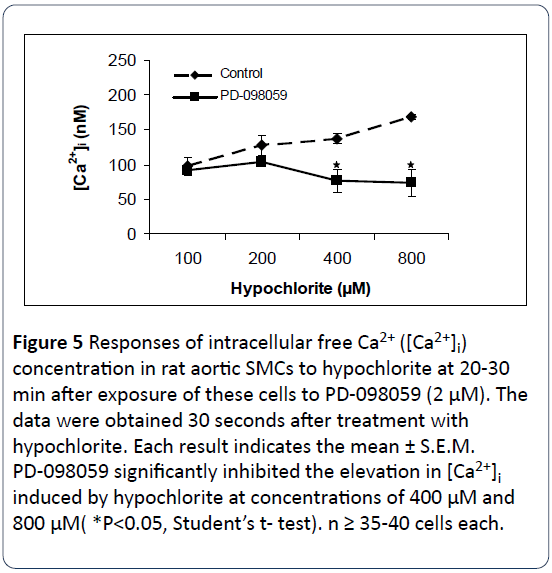
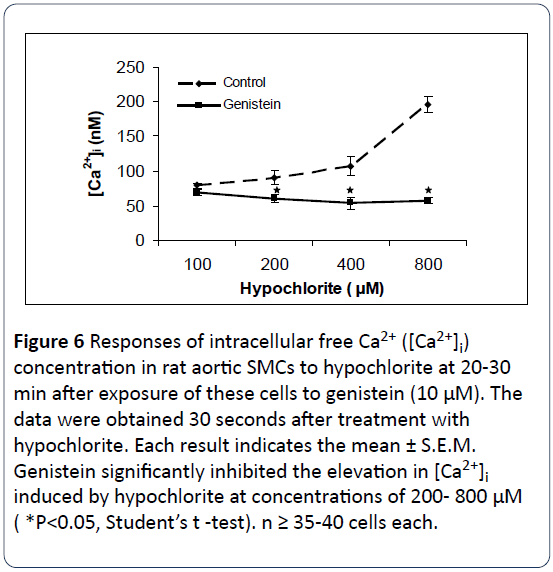
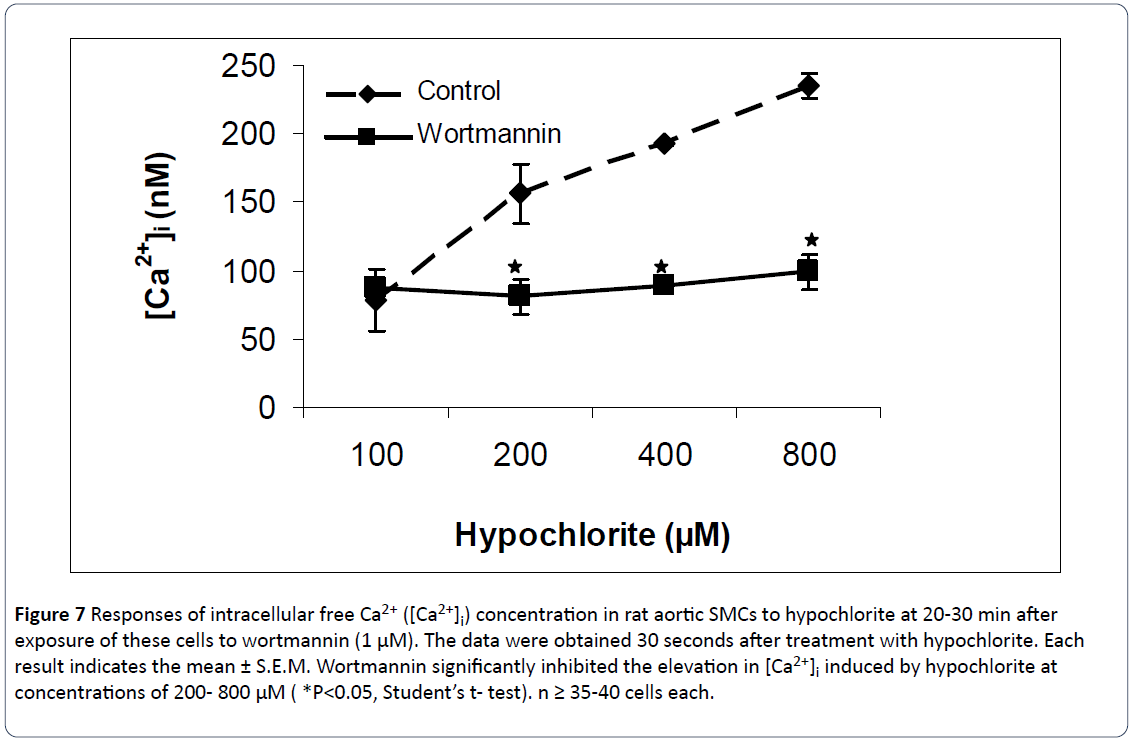
Figures 5-7 summarize the results of our experiments with hypochlorite and PD-098059, genistein and wortmannin on [Ca2+]i in primary, cultured rat aortic smooth muscle cells. Hypochlorite, in the concentration range of 100–800 μM, significantly increased [Ca2+]i in a concentration-dependent manner. The control basal level of [Ca2+]i in Figures 4-6 were 97.4 ± 12.6 nM, 79.42 ± 3.39 nM, and 78.41 ± 22.92 nM, respectively. Preincubation of primary cultured smooth muscle cells from rat aorta with PD-098059 (2 μM), genistein (10 μM) and wortmannin (1 μM) significantly prevents hypochloriteinduced elevation in [Ca2+]i. These three inhibitors inhibited almost completely the rises of [Ca2+]i induced by hypochlorite at the high concentrations (400 μM and 800 μM). Using 100 μM hypochlorite, on the preincubated cells, no changes in [Ca2+]i were observed from control levels.
Figure 5: Responses of intracellular free Ca2+ ([Ca2+]i) concentration in rat aortic SMCs to hypochlorite at 20-30 min after exposure of these cells to PD-098059 (2 μM). The data were obtained 30 seconds after treatment with hypochlorite. Each result indicates the mean ± S.E.M. PD-098059 significantly inhibited the elevation in [Ca2+]i induced by hypochlorite at concentrations of 400 μM and 800 μM( *P<0.05, Student’s t- test). n ≥ 35-40 cells each.
Figure 6: Responses of intracellular free Ca2+ ([Ca2+]i) concentration in rat aortic SMCs to hypochlorite at 20-30 min after exposure of these cells to genistein (10 μM). The data were obtained 30 seconds after treatment with hypochlorite. Each result indicates the mean ± S.E.M. Genistein significantly inhibited the elevation in [Ca2+]i induced by hypochlorite at concentrations of 200- 800 μM ( *P<0.05, Student’s t -test). n ≥ 35-40 cells each.
Figure 7: Responses of intracellular free Ca2+ ([Ca2+]i) concentration in rat aortic SMCs to hypochlorite at 20-30 min after exposure of these cells to wortmannin (1 μM). The data were obtained 30 seconds after treatment with hypochlorite. Each result indicates the mean ± S.E.M. Wortmannin significantly inhibited the elevation in [Ca2+]i induced by hypochlorite at concentrations of 200- 800 μM ( *P<0.05, Student’s t- test). n ≥ 35-40 cells each.
Discussion
The present investigation represents the first demonstration, to our knowledge, that:
• Hypochlorite increases free intracellular Ca2+ concentration (i.e., from approximately 80 nM to 210 nM in rat aortic smooth muscle cells).
• Antagonists of three different protein kinase C inhibitors (bisindolylmaleimide I, staurosporine and Gö6979), PD-098059, genistein and wortmannin produced striking inhibitory effects on the elevation in [Ca2+]i induced by hypochlorite when the SMCs were exposed to different concentrations( 200, 400 and 800 μM ), thereby giving us some insight into the potential contribution of four diverse cellular-signaling pathways to hypochlorite-induced Ca2+ mobilization in rat aortic smooth muscle cells.
Previous studies from our lab have shown that hypochloriteinduced contractions are mainly mediated via the elevation of intracellular free Ca2+ levels [11,12]. Multiple cellular signal pathways are known to participate in mechanisms of vasoconstriction, such as activation of protein kinase C (PKC), protein tyrosine kinase, MAPK, and PI3Ks [11-13,19-21].
PKC, a family of Ca2+-sensitive and Ca2+-insensitive phospholipid-dependent protein kinases, that are present in high concentrations in vascular smooth muscle, have been shown to play important roles in cellular signal transduction [11,13,14,19,20,22]. However, up until the present study, no experimental evidence has appeared on the possible role of PKC in hypochlorite-induced Ca2+ mobilization in rat aortic smooth muscle cells. In the present work, the elevation in [Ca2+]i induced by hypochlorite were found to be completely inhibited in the presence of two potent PKC antagonists (bisindolylmaleimide I and staurosporine) and one selective PKCα and PKCâ1 antagonist (Gö6976). This suggests that the inhibitory effect of bisindolylmaleimide I, staurosporine and Gö6976 is mediated through the activation of PKC. PKC isoforms, probably PKCα (possibly PKCβ1 as well) may be a pivotal signaling mechanism by which hypochlorite acts on the aortic smooth muscle cells to raise [Ca2+]i. This is, thus, similar to that found for H2O2 in rat aortic rings and cerebral arteries [13,14] and hydroxyl radicals in rat aortic rings [20].
It has been reported that PI3Ks are a group of enzymes that act directly to provide biochemical links between a novel phosphatidylinositol pathway and nonreceptor tyrosine kinases that appear to modulate Ca2+ channels through activity of these PI3Ks [23]. Up to now, there has been no information regarding PI3Ks on hypochlorite-induced Ca2+ mobilization in rat aortic smooth muscle cells. In this paper, we report that one potent antagonist of PI3Ks (wortmannin) completely attenuated the elevation in [Ca2+]i induced by hypochlorite, suggesting the possible involvement of products of PI3Ks in such responses. Approximately 20 years ago, we demonstrated that H2O2-induced contractions [14,22,24] and hydroxyl radical-induced contractions [20] were found to be somewhat dependent on PI3Ks as well.
Previous studies have shown that protein tyrosine phosphorylation is an essential component in signal transduction pathways in smooth muscle cells [7,25]. Our present data also demonstrate that genistein, an inhibitor of protein tyrosine kinase [25], which is an enzyme for catalyzing protein tyrosine phosphorylation, significantly inhibited the elevation in [Ca2+]i induced by hypochlorite in rat aortic SMCs, which indicates the likely involvement of protein tyrosine phosphorylation in hypochlorite-triggered signal transduction pathways in vascular smooth muscle cells. The conclusion is supported by other investigators who have demonstrated that oxidative stress can promote protein tyrosine phosphorylation in smooth muscle cells [13,14,20,22,24,26].
Mitogen-activated protein kinases [MAPKs], also called extracellular signal-regulated kinases (ERKs)] are constituents of numerous signal transduction pathways, and are activated by protein kinase cascades [27]. Several studies have been reported which indicate MAPK responds to diverse stimuli, including reactive oxygen species, and can transduce signals from the cell membrane to the nucleus [11,13,14,21,22,26,28,29]. Activation of MAPK plays an important role in modulating vascular tone [14,19,21,22]. In this investigation, the specific MAPK antagonist, PD-098059 [12], significantly inhibited the elevation in [Ca2+]i induced by hypochlorite in rat aortic SMCs. This suggests that activation of MAPK is probably an important pathway in hypochloritemediated action on rat aortic smooth muscle cells [30-37].
Conclusion
In conclusion, three different protein kinase C inhibitors (bisindolylmaleimide I, staurosporine and Gö6979), PD-098059, genistein and wortmannin significantly inhibited the elevation in [Ca2+]i induced by hypochlorite at different concentrations, i.e., from 200 to 800 μM , which would suggest that protein kinase C, extracellular signal-regulated kinases, protein tyrosine kinases and PI3Ks probably play essential roles in hypochlorite-triggered signal transduction pathways in vascular smooth muscle cells in both normal and pathological conditions.
Acknowledgment
The work described herein was supported in part by NIH Research Grants (National Heart, Lung and Blood Institute, National Institute on Alcohol and Alcohol Abuse, and National Institute on Mental Health) to B. M. A. and B.T.A.
References
- Baker MS, Rice Evans CA, Burdon RH (1992). Free Radical Damage and Its Control. Elsevier, New York. pp: 301-317.
- Daugherty A, Dunn JL, Rateri DL, Heinecke JW (1994) Myeloperoxidase, a Catalyst for Lipoprotein Oxidation, is Expressed in Human Atherosclerotic Lesions. J Clin Invest 94: 437-444.
- Klebanoff SJ (2005) Myeloperoxidase: Friend and Foe. J Leukoc Biol 77: 598-625.
- Malle E, Furtmüller PG, Sattler W, Obinger C. (2007) Myeloperoxidase: A Target for New Drug Development? Br J Pharmacol 152: 836-854.
- Katrantzis M, Baker MS, Handley CJ, Lowther DA (1991). The Oxidant Hypochlorite, a Product of the Myeloperoxidase System, Degrades Articular Cartilage Proteoglycan Aggregate. Free Radic Biol Med 10: 101-109.
- Fattman CL, Schaefer LM, Oury TD (2003). Extracellular Superoxide Dismutase in Biology and Medicine. Free Radic Biol Med 35: 236-256.
- Malarkey K, Belham CM, Paul A, Graham A, McLees A, et al. (1995) The Regulation of Tyrosine Kinase Signalling Pathways by Growth Factor and G-Protein-Coupled Receptors. Biochem J 309: 361-375.
- Fukai K, Kaneda M, Takahashi E, Washio M, Doi K (1994). Protective Effects of Sulfhydryl Compounds on HHypochloriteinduced Intracellular Calcium Increase in Single Rat Ventricular Myocytes. J Mol Cell Cardiol 26: 455-461.
- Jenner AM, Ruiz JE, Dunster C, Halliwell B, Mann GE, et al. (2002) Vitamin C Protects Against Hypochlorous Acid-induced Glutathione Depletion and DNA Base and Protein Damage in Human Vascular Smooth Muscle Cells. Arterioscler Thromb Vasc Biol 22: 574-580.
- Medina P, Segarra G, Torondel B, Chuan P, Domenech C, et al. (2000) Inhibition of Neuroeffector Transmission in Human Vas Deferens by sildenafil. Br J Pharmacol 131: 871-874.
- Liu JP, Li W, Altura BT, Altura BM (2017) Mechanisms of Sodium Hypochlorite-Induced Contraction of Rat Aorta.
- Liu JP, Altur BT, Altura BM (2017) Hypochlorite-induced relaxation in isolated rat aortic rings and mechanisms of action.
- Reeves EP, Nagl M, Godovac-Zimmermann J, Segal AW (2003) Reassessment of the Microbicidal Activity of Reactive Oxygen Species and Hypochlorous Acid with Reference to the Phagocytic Vacuole of the Neutrophil Granulocyte. J Med Microbiol 52: 643-651.
- Sand C, Peters SL, Pfaffendorf M, van Zwieten PA (2003) Effects of Hypochlorite and Hydrogen Peroxide on Cardiac Autonomic Receptors and Vascular Endothelial Function. Clin Exp Pharmacol Physiol 30:249-253.
- Post GR, Brown JH (1996) G Protein-coupled Receptors and Signaling Pathways Regulating Growth Responses. FASEB J 10: 741-749.
- Yang ZW, Zheng T, Zhang A, Altura BT, Altura BM (1998) Mechanisms of Hydrogen Peroxide-Induced Contraction of Rat Aorta. Eur J Pharmacol 344: 169-181.
- Yang ZW, Zheng T, Wang J, Zhang A, Altura BT, et al. (1999) Hydrogen Peroxide Induces Contraction and Raises [Ca2+]i in Canine Cerebral Arterial Smooth Muscle: Participation of Cellular Signaling Pathways. Naunyn-Schmiedeberg's Arch Pharmacol 360: 646-653.
- Grynkiewicz G, Poenie M, Tsien RY (1985) A New Generation of Ca2+ Indicators with Greatly Improved Fluorescence Properties. J Biol Chem 260: 3440-3450.
- Horowitz A, Menice CB, Laporte R, Morgan KG (1996) Mechanisms of Smooth Muscle Contraction. Physiol Rev 76: 967-1003.
- Li J, Li W, Liu W, Altura BT, Altura BM (2004) Mechanisms of Hydroxyl Radical-Induced Contraction of Rat Aorta. Eur J Pharmacol 499: 171-178.
- Touyz RM, Yao G (2003) Modulation of Vascular Smooth Muscle Cell Growth by Magnesium-Role of Mitogen-Activated Protein Kinases. J Cell Physiol 197: 326-335.
- Tsien RY (1980) New Calcium Indicators and Buffers with High Selectivity Against Magnesium and Protons: Design, Synthesis, and Properties of Prototype Structures. Biochemistry 19: 2396-2404.
- Blair LA, Marshall J (1997) IGF-1 Modulates N and L Calcium Channels in a PI 3-Kinase-Dependent Manner. Neuron 19: 421-429.
- Schraufstatter IU, Browne K, Harrisa A, Hyslop PA, Jackson JH et al. (1990) Mechanisms of Hypochlorite Injury of Target Cells. J Clin Invest 85: 554-562.
- Albrecht DE, Tidball JG (1997) Platelet-Derived Growth Factor-Stimulated Secretion of Basement Membrane Proteins by Skeletal Muscle Occurs by Tyrosine Kinase-Dependent and -Independent Pathways. J Biol Chem 272:2236-2244.
- Baas AS, Berk BC (1995) Differential Activation of Mitogen-Activated Protein Kinases by H2O2 and O2− in Vascular Smooth Muscle Cells. Circ Res 77: 29-36.
- English JM, Cobb MH (2002) Pharmacological Inhibitors of MAPK Pathways. Trends Pharmacol Sci 23: 40-45.
- Li J, Su J, Li W, Liu W, Altura BT et al. (2003) Peroxynitrite Induces Apoptosis in Canine Cerebral Vascular Muscle Cells: Possible Relation to Neurodegenerative Diseases and Strokes. Neurosci Lett 350: 173-177.
- Rao GN (1997) Protein Tyrosine Kinase Activity is Required for Oxidant-Induced Extracellular Signal-Regulated Protein Kinase Activation and c-fos and c-jun Expression. Cell Signal 9:181-187.
- Blumer KJ, Johnson GL, Lange-Carter CA (1994) Mammalian Mitogen-Activated Protein Kinase Kinase Kinase (MEKK) can Function in a Yeast Mitogen-Activated Protein Kinase Pathway Downstream of Protein Kinase C. Proc Natl Acad Sci USA 91: 4925-4929.
- Kariya K, Takai Y (1987) Distinct Functions of Down-Regulation-Sensitive and -Resistant Types of Protein Kinase C in Rabbit Aortic Smooth Muscle Cells. FEBS Lett. 219: 119-124.
- Li W, Altura BT, Altura BM (1998). Differential Effects of Methanol on Rat Aortic Smooth Muscle. Alcohol 16: 221-229.
- Yang ZW, Wang J, Zheng T, Altura BT, Altura BM (2000) Low [Mg2+]o Induces Contraction and [Ca2+]i Rises in Cerebral Arteries: Roles of Tyrosine and Mitogen-Activated Protein Kinases. Am J Physiol Heart Circ Physiol 279: H185-H194.
- Yang ZW, Wang J, Zheng T, Altura BT, Altura BM (2001a) Importance of PKC and PI3Ks in Ethanol-Induced Contraction of Cerebral Arterial Smooth Muscle. Am J Physiol Heart Circ Physiol 280: H2144-H2152.
- Yang ZW, Wang J, Zheng T, Altura BT, Altura BM (2001b) Ethanol-Induced Contractions in Cerebral Arteries: Role of Tyrosine and Mitogen-Activated Protein Kinases. Stroke 32: 249-257.
- Zhang A, Cheng TP, Altura BM (1992) Magnesium Regulates Intracellular Free Ionized Calcium Concentration and Cell Geometry in Vascular Smooth Muscle Cells. Biochim Biophys Acta 1134: 25-29.
- Zhang A, Cheng TP, Altura BT, Altura BM (1997). Chronic Treatment of Cultured Cerebral Vascular Smooth Muscle Cells With Low Concentration of Ethanol Elevates Intracellular Calcium and Potentiates Prostanoid-induced rises in [Ca2+]i: Relation to Etiology of Alcohol-Induced Stroke. Alcohol 14: 367-371.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences