Expression Profile of Hexosaminidases Genes in the Brain of Ob/Ob Mice Transplanted with Adipose Tissue
Maria de Nazareth Gamboa Ritto1,2, Marisa Terezinha Patriarca1, Ismael Dale Cotrim Guerreiro da Silva1, Carlos Fernandes Baptista1,2, Eduardo Henrique da Silva Freitas1,2, Silvana Aparecida Alves Correa3 and Samuel Marcos Ribeiro de Noronha3,4*
1Gynecology Department, Federal University of Sao Paulo/ Paulista School of Medicine (UNIFESP/EPM), Sao Paulo, Brazil
2General and Specific Surgery Department, Federal University of Rio de Janeiro State (UNIRIO), Rio de janeiro, Brazil
3Plastic Surgery Division, Surgery Department, Federal University of Sao Paulo/ Paulista School of Medicine (UNIFESP/EPM), Sao Paulo, Brazil
4University Center of United Metropolitan Schools (UniFMU), Sao Paulo, Brazil
- *Corresponding Author:
- Samuel Marcos Ribeiro de Noronha
Plastic Surgery Division, Surgery Department
Federal University of Sao Paulo/ Paulista School of Medicine (UNIFESP/EPM)
Rua Botucatu, 740 – Edifício Jairo Ramos – 1o andar
Vila Clementino - CEP 04023.062 - São Paulo, Brazil
Tel: 55 11 55764848
E-mail: labgineco@globo.com
Received Date: 24 February 2018; Accepted Date: 06 March 2018; Published Date: 15 March 2018
Citation: Ritto MNG, Patriarca MT, Silva IDCG, Freitas EHS, Correa SAA, et al. (2018) Expression Profile of Hexosaminidases Genes in the Brain of ob/ob Mice Transplanted with Adipose Tissue. Endocrinol Metab Vol. 2 No.1:8
Copyright: © 2018 Ritto MNG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Purpose: This study aims to assess the expression profile of hexosaminidase genes in the brain of ob/ob mice, to better understand the mechanisms underlying posttranslational protein modifications that leads to Polycystic Ovary Syndrome (PCOS). Glycoproteins assembled within the endoplasmic reticulum carry biological codes determining their fate. Reproduction depends on important glycoproteins and their circulating levels might be affected by leptin.
Methods: B6.V-Lepob/J mice were divided into three groups: Control normal weight; control obese and 7-daytreated obese. Their brains were processed for RNA extraction. PCR Array assays were performed in plates containing 11 mannosidases genes.
Results: Genetic profile of the 7-day group compared to the Control group showed that 82% of the 11 hexosaminidase genes were differentially expressed. These genes were downregulated: Edem1 and 3 can be highlighted. Only two out of six glucosidase-related genes were differentially expressed, Hexa and b, both downregulated.
Conclusion: Leptin alters glycosylation processes of brain molecules pivotal for the malfunctioning of reproductive system, Gene expression levels of hexosaminidases are altered after a seven-day leptin treatment, that causes a gene-expression profile similar to those of the normal weight. Leptin treatment might lead to lower levels of monoglucosylated glycoforms of LH, a hallmark of glycoprotein degradation.
Keywords
Polycystic ovary syndrome; Leptin; Glycosylation; Gonadotropins; Adipose tissue; Hexosaminidases and ovary
Introduction
The Polycystic Ovary Syndrome (PCOS) is a common reproductive and metabolic disorder that is characterized by the presence of polycystic ovaries, obesity, infertility and periods of amenorrhea [1-4]. These patients are bushy and have increased risk for developing endometrial cancer and cardiovascular disease, as well as changes in glucose metabolism and early diabetes risk [5,6]. The PCOS has an estimated prevalence of 4-12% among women in childbearing age [7].
Among the genetic factors involved in the pathogenesis of PCOS are Insulin-like and Growth Factor I (IGF-1), which act along with luteinizing hormone (LH) to increase the production of androgens by the ovaries [8,9]. Clinically, it was observed menstrual changes and hirsutism, usually accompanied by weight gain [10-12].
Weight loss of 5 to 10% may be sufficient to restore the reproductive function and to improve the ovarian response to ovulation induction [13,14].
Leptin is not the only hormone secreted by the adipose tissue [15] and the placenta [16,17]. However it is wellcharacterized as a regulator of energy balance and energy expenditure that exerts these two main actions by acting on neurons in the hypothalamic arcuate nucleus [18].
It has been demonstrated that administration of recombinant leptin in ob/ob mice can regulate body weight and glucose metabolism, and it can also restore fertility in these animals [19]. A leptin peak during the luteal phase of the sex cycle correlates with maximal level of progesterone. The role played by the absence of leptin action in obese mice infertility has been the focus of intense research [20].
Previous studies suggest that patients with leptin deficiency treated with recombinant leptin leads to successful results [21]. In addition to conventional treatment with leptin injection to correct obesity, there is also the possibility of using adipose tissue transplantation, which would cause the physiological release of this hormone. It is known that transplantation of adipose tissue from normal mice improves metabolic abnormalities in receptor mice with lipodystrophy [22,23]. Adipose tissue transplantation technique as an alternative to hormone treatment is effective for prevention and treatment of obese mice presenting some type of lipodystrophy and obesity. However, little is known about the early effects, namely in the short term, this procedure in obese and infertile mice. There is a need to carry out more intensive studies in order to assess the fertility restoration in ob/ob mice transplanted with adipose tissue regarding studies of molecular markers of glycosylation in PCOS involved with obesity. In the literature, there are studies describing experiments with models of obese diabetic mice presenting IR and micro polycystic ovaries. Considering the importance of leptin to the functioning of the hypothalamic-hypophysealgonadal axis, many other studies focused on obese mice fertility restoration as well [24].
The brain is rich in glycoproteins and different hexosaminidases are associated with neurological disorders of this nature, like Tay-Sachs disease and GM1-glangliosidosis. Both gonadotropins are glycoproteins displaying distinct glycosylation levels of their molecules, according to many different physiological conditions and these levels are key to determine affinity for membrane-bound receptors [25]. Male and female ob/ob mice present lower levels of circulating LH and FSH when compared to lean mice, indicating a persistent immaturity of the hypothalamic-pituitary function of the genetically obese animals [26].
Information in the literature regarding the behavior of transplantation of adipose tissue in obese mice with PCOS are lacking, and considering it to be an efficient animal model, we decided to conduct this study. Therefore, we aimed to assess the main changes in glycosylation-related genes expression and how they correlate to fertility restoration in ob/ob mice and to gonadotropins (LH/FSH) glycosylation after adipose tissue transplantation.
Materials and Methods
Experimental animals and surgical procedure
In this study, ten transgenic obese and anovulatory leptindeficient mice (B6.V-Lep ob/J, designated as ob/ob mice) and five isogenic lean ovulatory littermates (wild-type) were obtained from the Center for the Development of Experimental Models, Federal University of São Paulo, Brazil (CEDEME). These animals were maintained in a temperaturecontrolled environment at approximately 24°C under a 12/12- h light-dark cycle and were handled at least once a week. Five ob/ob mice received a white adipose tissue (WAT) transplant as described by Gavrilova and Marcus-Samuels et al. [27] and Pereira et al. [28]: the adipose gonad tissue samples that were obtained from the wild-type mice were placed in phosphate buffer solution (PBS) and fragmented into small pieces. Gonadal fat pads were collected from donor control mice and grafts of approximately 1 g were implanted in the subcutaneous tissue of receptor mice through small, shaved skin incisions on the dorsal region of the animal, which was anesthetized with halothane (Baxter Healthcare Corp, Round Lake, IL). Animals were also euthanized with halothane overdose. The brains of all of the animals were removed by the reported procedure and kept in liquid nitrogen until use.
Experimental groups
The animals that were described in the previous section were divided into three experimental groups as follows:
Control group (CG): Five (5) normal-weight B6.V-Lepob/J mice at two to three months of age and ovulatory cycles. Their mean weight in g was 24.0 ± 0.7.
Obese group (OG): Five (5) ob/ob mice at two to three months of age and anovulatory cycles. Their mean weight in g was 52.0 ± 1.3.
7-day Transplanted Mice group (7dTM): Five (5) ob/ob mice at two to three months of age and anovulatory cycles were implanted with adipose gonadal tissue from mice with ovulatory cycles. These animals were sacrificed seven (7) days after the surgical procedure. Their mean weight in g was 52.8 ± 1.8 before transplantation and 50.8 ± 2.5 seven days after the procedure.
RNA extraction
After using liquid nitrogen for cryogenic soaking, the tissues were homogenized in TrizolTM reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. After the complete dissociation of the nucleoprotein complexes, phase separation was achieved with chloroform and centrifugation. The precipitated RNA from the aqueous phase was washed with 75% ethanol. The RNA was dried and dissolved in RNasefree water. The total RNA was then purified with the Qiagen RNeasy Mini Kit (Qiagen, Valencia, CA) and submitted to DNase treatment. The amount and quality of the extracted RNA were assessed by spectrophotometry using NanoDrop v3.3.0 (NanoDrop Technologies Inc., Rockland, DE).
qPCR
The total RNA (1.0 μg) per plate/array from each experimental group pool was used to synthesize the cDNA. The samples were treated with buffers from the kit, and reverse transcription reactions were performed using the RT2 First Strand Kit from SA Biosciences (Qiagen Company) according to the manufacturer's protocol. The qPCR array was performed using the RT2 Profiler™ PCR array of SA Biosciences (https://www.sabiosciences.com/ArrayList.php). For each experimental group, 84 genes were examined in triplicate (Glycosylation RT² Profiler PCR Array - Cat. no. PAMM-046). The amplification, data acquisition and analysis curves were performed on an ABI Prism 7500 Fast Sequence Detection System (Applied Biosystems, Foster City, CA). In turn, each gene was checked for the efficiency and the minimum and maximum threshold curve pattern. To ensure accurate comparisons between the curves, the same threshold was established for every gene. Three genes were used for normalization (Gapdh and Actb), and the average qC values were used to standardize the gene expression (2-CT change table) and to determine the difference between the groups. To consider a gene differentially expressed, we used a differential cut-off of two-fold (up- or downregulated).
Statistical analysis
The p values were calculated based on Student’s t-test of the three replicate 2(-Δct) values for each gene in the control and treatment groups, and p values less than 0.05 were considered significant. The qPCR reactions were processed through the online software RT2 Profiler™ PCR Array Data Analysis (SA Biosciences).
Ethics
The procedures were performed in accordance with the ethical standards of the institution and national guidelines for the care and use of laboratory animals. The study protocol for the use of laboratory animals in research was approved by the local ethics committee (CEP/UNIFESP, number 0295/12).
Results
We investigated 17 genes related to protein glycosilation, which belong to two different groups of enzymes: 11 mannosidases-related genes (Edem1, Edem2, Edem3, Man1a, Man1a2, Man1b1, Man1c1, Man2a1, Man2a2, Man2b1, Manba) and six glucosidases-related genes (Ganab, Glb1, Hexa, Hexb, Mogs, Prkcsh). Next, we describe the expression pattern of each set of genes.
Mannosidases
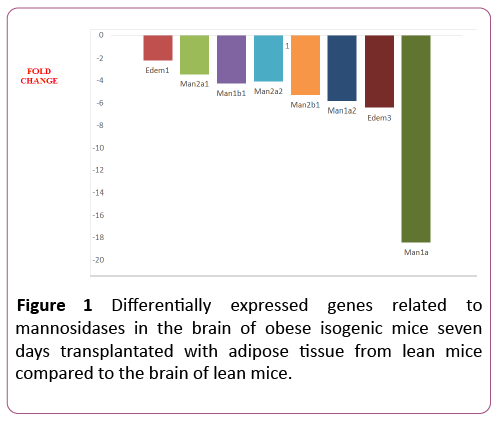
Nine out of the eleven (82%) glycosylation-related genes were differentially expressed in the 7dTM group, using the CG as calibrator. All of these nine genes were downregulated, and none were upregulated.The downregulated genes included Edem1, Edem3, Man1a, Man1a2, Man1b1, Man1c1, Man2a1, Man2a2, Man2b1. Among the non-differentially expressed genes, Edem2 and Manba were detected in both groups (7dTM and CG). The fold-change values of each mannosidaserelated gene can be observed in Table 1 and Figure 1.
Table 1 Fold-regulation values that were obtained for the glycosylation-related genes in the 7dTM group using the CG group as a calibrator. The data were processed with the online program Data AssistTM SA Biosciences (Qiagen Company). nde = nondifferentially expressed.
| Gene Symbol | GeneName | Refseq | 7dTM X CG | ||
|---|---|---|---|---|---|
| Fold Regulation | p value | ||||
| MANNOSIDASES | Edem1 | ER degradation enhancer, mannosidase alpha-like 1 | NM_138677 | -2.20 | 0.0018 |
| Edem2 | ER degradation enhancer, mannosidase alpha-like 2 | NM_145557 | nde | ||
| Edem3 | ER degradation enhancer, mannosidase alpha-like 3 | NM_001039644 | -6.43 | 0.0001 | |
| Man1a | Mannosidase 1,alpha | NM_008541 | -18.45 | 0.0045 | |
| Man1a2 | Mannosidase, alpha, class 1a, member 2 | NM_010763 | -5.82 | 0.0015 | |
| Man1b1 | Mannosidase, alpha, class 1b, member 1 | NM_001029983 | -4,21 | 0.0002 | |
| Man1c1 | Mannosidase, alpha, class 1c, member 1 | NM_207237 | -10.48 | 0.0014 | |
| Man2a1 | Mannosidase 2, alpha1 | NM_008549 | -3.45 | 0.0055 | |
| Man2a2 | Mannosidase 2, alpha1 | NM_172903 | 4.08 | 0.0007 | |
| Man2b1 | Mannosidase 2, alphab1 | NM_010764 | -5.30 | 0.0018 | |
| Manba | Mannosidase, beta a, lysosomal | NM_027288 | nde | ||
| GLUCOSIDASES | |||||
| Glb1 | Galactosidase, beta1 | NM_009752 | nde | ||
| Ganab | Alpha glucosidase2 alpha neutral subunit | NM_008060 | nde | ||
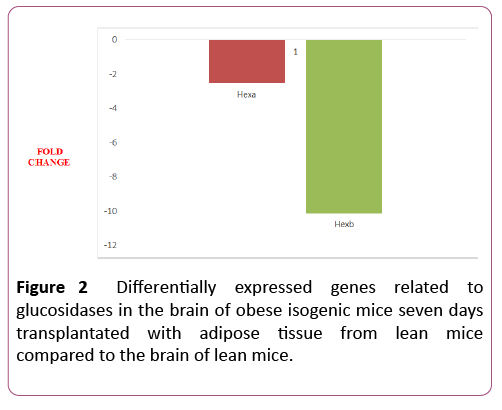
| Hexa | Hexosaminidase a | NM_010421 | -2.55 | 0.0009 | |
| Hexb | Hexosaminidase b | NM_010422 | -10.13 | 0.000 | |
| Mogs | Mannosyl-oligosaccharide glucosidase | NM_020619 | nde | ||
| Prkcsh | Protein kinase C substrate 80k-h | NM_008925 | nde | ||
Glucosidases
Two out of six genes (33%) were differentially expressed in the 7dTM group, using the CG as calibrator. These two genes were downregulated. The downregulated genes were Hexa and Hexb. Among the non-differentially expressed genes, Ganab, Glb1, Mogs and Prkcsh were detected in both groups (7dTM and CG). The fold-change values of each glucosidaserelated gene can also be observed in Table 1 and Figure 2.
Discussion
After treating ob/ob mice with adipose tissue, we observed fertility restoration and, therefore, regular levels of circulating hypothalamic-hypophyseal-gonadal axis-related hormones [28]. Pereira et al. also demonstrated that gonadal tissue transplantation increases the production of Leptin Receptors in the ovary [28]. However, what are the molecular changes underlying gonadal reactivation? More specifically, what are the changes in the expression of the enzymes that regulate glycosylation of gonadotropic hormones? The glycosylationrelated gene expression data shown in this study make us believe that, in order to restore fertility, intracellular interferences of leptin over the action of mannosidases and of glucosidases during early glycoprotein synthesis take place in the endoplasmic reticulum, reducing circulating levels of monoglucosylated glycoforms of LH. This reduction will explain the alteration of the ob/ob mice’s hormonal profile, that is, ob/ob mice present low levels of circulating LH and leptin treatment might early reduce intracellular levels of monoglucosylated glycoforms of LH, which is a hallmark of glycoprotein degradation.
The O-linked oligosaccharides are not important for in vitro bioactivity or receptor binding, but they play an important role in the in vivo bioactivity and half-life of the glycoprotein hormones. Addition of the O-linked oligosaccharide chains to the backbone of glycoprotein hormones could be an interesting strategy for designing long acting agonists of glycoprotein hormones. On the other hand, the N-linked oligosaccharides are not important for receptor binding, but they are critical for bioactivity of glycoprotein hormones. Deletion of the N-linked oligosaccharides resulted in the development of glycoprotein hormone antagonists [29-31].
Expression of glycosyltransferase genes is essential for glycosylation. However, the detailed mechanisms of how glycosyltransferase gene expression is regulated in a specific tissue or during disease progression are poorly understood. In particular, epigenetic studies of glycosyltransferase genes are limited, although epigenetic mechanisms, such as histone and DNA modifications, are central to establish tissue-specific gene expression. New epigenetic mechanism of brain-specific GnTIX expression regulated by defined chromatin modifiers have been described, providing new insights into the tissue-specific expression of glycosyltransferases [32]. The absence of leptin action alters key glycosylation processes of brain molecules in obese mice, which are pivotal for the malfunctioning of the hypothalamic-hypophyseal-gonadal axis, including N-Glycan processing in the Endoplasmic Reticulum. Gene expression levels of hexosaminidases are markedly altered after a sevenday leptin treatment.
Differentially expressed genes detected in the 7-day group like Manba, Edem2 Mogs, Edem3 Man1c1 e Hexb are associated with the neural cell adhesion molecule (NCAM), but also influences key neural functions, including synaptic plasticity, neurite growth, and cell migration [33].
Conclusion
Leptin treatment seems to trigger a gene-expression profile similar to those of the normal weight mice. Leptin treatment might lead to lower levels of monoglucosylated glycoforms of LH, which is a hallmark of glycoprotein degradation. These mechanisms are markedly suppressed after a seven day treatment of leptin, which induces the treated mice brain to display a generic profile similar to those of the normal weight.
In summary, the data presented here indicate an early decrease in the central glucose metabolism after leptin treatment. These results confirm the ability of the adipose tissue-derived hormone leptin to regulate crucial genes that are related to glycolysis mechanisms and to the TCA cycle. Leptin seems to early revert central physiological conditions associated with PCOS in the central nervous system, however, the morphological alterations that are associated with fertility in the peripheral tissues can only be observed within a 45-day treatment. The extrapolation of these results to patients with metabolic syndrome must await further investigation.
Acknowledgements
Thanks to Sao Paulo Research Foundation (FAPESP) for Research Grants numbers 2008/50776-7 and 2008/54383-0, that allowed us to make this project become reality.
Declaration
The procedures reported in this article were conducted at the Molecular Biology Lab, of the Gynecology Department, Federal University of Sao Paulo/ Paulista School of Medicine (UNIFESP/EPM), Sao Paulo, Brazil. The authors declare that they have no conflicts of interest.
References
- Berger JJ, Bates GW Jr (2014) Optimal management of subfertility in polycystic ovary syndrome. Int J Womens Health 13: 613-621.
- Caldwell AS, Middleton LJ, Jimenez M, Desai R, McMahon AC, et al. (2011) Characterization of reproductive, metabolic and endocrine features of polycystic ovary syndrome in female hyperandrogenic mouse models. Endocrinology 30: en20141196.
- Aquino CI, Nori SL (2014) Complementary therapy in polycystic ovary syndrome. Transl Med UniSa 9: 56-65.
- Madnani N, Khan K, Chauhan P, Parmar G (2013) Polycystic ovarian syndrome. Indian J Dermatol Venereol Leprol 79: 310-321.
- Baldwin CY, Witchel SF (2006) Polycystic ovary syndrome. Pediatr Ann 35: 888-896.
- Costello MF, Shrestha B, Eden J, Sjoblom P, Johnson N (2007) Insulin sensitizing drugs versus the combined oral contraceptive pill for hirsutism, acne and risk of diabetes, cardiovascular disease, and endometrial cancer in polycystic ovary syndrome. Cochrane Database Syst Rev 24: CD005552.
- Kansra AR, Menon S (2013) PCOS: perspectives from a pediatric endocrinologist and a pediatricgynecologist. Curr Probl Pediatr Adolesc Health Care 43: 104-113.
- Kelly CJ, Stenton SR, Lashen H (2011) Insulin-like growth factor binding protein-1 in PCOS: a systematic reviewand meta-analysis. Hum Reprod Update 17: 4-16.
- Franks S, Stark J, Hardy K (2008) Follicle dynamics and anovulation in polycystic ovary syndrome. Hum Reprod Update 14: 367-378.
- Sirmans SM, Pate KA (2013) Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol Dec 18: 1-13.
- Dantas WS, Gualano B, Rocha MP, Barcellos CR, dos Reis Vieira Yance V, et al. (2013) Metabolic disturbance in PCOS: clinical and molecular effects on skeletal muscle tissue. ScientificWorld Journal 5:178364.
- Vera L, Accornero M, Mora M, Valenzano-Menada M, Minuto F, et al. (2013) Increasing hirsutism due to a granulosa-cell tumor in a woman with polycystic ovary syndrome: case report and review of the literature. Gynecol Endocrinol 29:273-277.
- Ben-Shlomo I, Younis JS (2014) Basic research in PCOS: are we reaching new frontiers? Reprod Biomed Online 28:669-683.
- Perales-Puchalt A, Legro RS (2013) Ovulation induction in women with polycystic ovary syndrome. Steroids 78:767-772.
- Al-Suhaimi EA, Shehzad A (2013) Leptin, resistin and visfatin: the missing link between endocrine metabolic disorders and immunity. Eur J Med Res 1:12.
- Maymó JL, Pérez Pérez A, Gambino Y, Calvo JC, Sánchez-Margalet V, et al. (2011) Review: Leptingene expression in the placenta-regulation of a key hormone in trophoblast proliferation and survival. Placenta 32:S146-153.
- Hauguel-de Mouzon S, Lepercq J, Catalano P (2006) The known and unknown of leptin in pregnancy. Am J Obstet Gynecol 194:1537-1545.
- Bi S, Kim YJ, Zheng F (2012) Dorsomedial hypothalamic NPY and energy balance control. Neuropeptides 46:309-314.
- Klebanov S, Astle CM, DeSimone O, Ablamunits V, Harrison DE (2005) Adipose tissue transplantation protecsob/ob mice from obesity, normalizes insulin sensitivity and restores fertility. J Endocrinol 186: 203-211.
- Walters KA, Allan CM, Handelsman DJ (2012) Rodent models for human polycystic ovary syndrome. Biol Reprod 86: 1-12.
- Costello MF, Chapman M, Conway U (2006) A systematic review and meta-analysis of randomized controlled trials on metformin co-administration during gonadotrophin ovulation induction or IVF in women with polycystic ovary syndrome. Hum Reprod 21:1387-1399.
- Recabarren SE, Smith R, Rios R, Maliqueo M, Echiburú B, et al. (2008) Metabolic profile in sons of women with polycystic ovary syndrome. J Clin Endocrinol Metab 93:1820-1826.
- Sam S, Coviello AD, Sung YA, Legro RS, Dunaif A (2008) Metabolic phenotype in the brothers of women with polycystic ovary syndrome. Diabetes Care 31: 1237-1241.
- Arlt W, Neogi P, Gross C, Miller WL (2004) Cinnamic acid based thiazolidinediones inhibit human P450c17 and 3beta-hydroxysteroid dehydrogenase and improve insulin sensitivity independent of PPAR gamma agonist activity. J Mol Endocrinol 32: 425-436.
- Bayleran J, Hechtman P, Kolodny E, Kaback M (1987) Tay-Sachs disease with hexosaminidaseA: carachterization of the defective enzyme in two patients. Am J Hum Genet 41: 532-548.
- Swerdloff RS, Batt RA, Bray GA (1976) Reproductive hormonal function in the genetically obese (ob/ob) mouse. Endocrinology. 98: 1359-1364.
- Gavrilova O, Marcus-Samuels B, Graham D, Kim JK, Schulman GI, et al. (2000) Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. Journal of Clinical Investigation 105: 271-278.
- Pereira Jr M, Vidotti DB, Borra RC, SimoesMdeJ, Da Silva ID, et al. (2011) Involvment of GDF-9, leptinand IGF-1 receptors associated with adipose tissue transplantation on fertility restoration in obese anovulatory mice. Gynecological Endocrinology 27: 759-766.
- Fares F (2006) The role of O-linked and N-linked oligosaccharides on the structure-function of glycoprotein hormones: Development of agonists and antagonists. Biochim Biophys Acta-Gen Sub 1760: 560-667.
- Helenius A, Aebi M (2004) Roles of N-linked Glicans in the endoplasmic reticulum. Ann Rev Biochem 73: 1009-1049
- Van den Steen P, Rudd PM, Dwek RA, Opdenakker G (1998) Concepts and principles of O-linked Glycosylation. Crit Rev Biochem Mol Biol 33: 151-208.
- Kizuka Y, Kitazume S, Okahara K, Villagra A, Sotomayor EM, et al. (2014) Epigenetic regulation of a brain-specific glycosyltransferase N-acetylglucosaminyltransferase-IX (GnT-IX) by specific chromatin modifiers. J Biol Chem 289:11253-61.
- Kreuzmann D, Horstkorte R, Kohla G, Kannicht C, Bennmann D (2017) Increased polysialylation of the neural cell adhesion molecule in a transgenic mouse model of sialuria.Chembiochem 18:1188-1193

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences