ISSN : 2393-8862
American Journal of Pharmacology and Pharmacotherapeutics
Enhancing Gastric Cancer Therapy: Nanoparticle Insights from In vitro Studies
Mehrnaz Mostafavi1* and Mahtab Shaabani2
1Department of Allied Medical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran
2Department of Medical Sciences, Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- *Corresponding Author:
- Mehrnaz Mostafavi
Department of Allied Medical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran
E-mail:mzmostafavi@gmail.com
Received date: January 06, 2024, Manuscript No. IPAPP-24-18539; Editor assigned date: January 09, 2024, PreQC No. IPAPP-24-18539 (PQ); Reviewed date: January 23, 2024, QC No. IPAPP-24-18539; Revised date: March 20, 2025, Manuscript No. IPAPP-24-18539 (R); Published date: March 27, 2025, DOI: 10.36648/2393-8862.12.1.203
Citation: Mostafavi M, Shaabani M (2025) Enhancing Gastric Cancer Therapy: Nanoparticle Insights from In vitro Studies. Am J Pharmacol Pharmacother Vol:12 No:1
Abstract
Cancer, particularly gastric cancer, poses a significant global health challenge, ranking among the leading causes of death worldwide. Current therapies, including surgery, chemotherapy, and radiotherapy, exhibit varied effectiveness across different cancer stages and often lead to chemo resistance and adverse effects. Nanomedicine, owing to its unique nanoscale properties, presents promising opportunities in cancer detection and treatment.
This study investigates the potential therapeutic implications of nanoparticles, specifically Gold Nanoparticles (GNPs), in gastric cancer therapy. The research elucidates the cytotoxic effects of chemotherapy drug 5-Fluorouracil (5FU) and GNPs on human gastric cancer cell line MLN-45 cells. Initial experiments revealed optimal incubation times of 72 hours for 5FU and 24 hours for GNPs, achieving significant reductions in cell viability. A critical finding was the enhanced cytotoxicity observed when combining chemotherapy with GNPs and laser irradiation. Notably, lower concentrations of chemotherapeutic agents yielded higher cell destruction when combined with GNPs, potentially reducing adverse drug effects. Furthermore, the study sheds light on the challenges and opportunities associated with nanomedicine. While GNPs exhibit promise in cancer therapy, there remain uncertainties regarding their clinical applicability and biological interactions. Understanding the equilibrium between exposure duration and toxicity is crucial in optimizing therapeutic strategies involving nanoparticles.
In essence, this study emphasizes the capacity of nanotechnology to enhance conventional cancer treatments and emphasizes the necessity for continued exploration to maximize therapeutic benefits while minimizing undesired outcomes. These results set the stage for future inquiries into the practical application of nanoparticles in treating gastric cancer
Keywords
Gastric cancer; Gold nanoparticle; MKN-45 gastric cancer cell line; 5-fluorouracil
Introduction
Gastric Cancer (GC), one of the common cancers, has ascended to become the fifth most commonly diagnosed cancer and the fourth main contributor to cancer-related fatalities [1]. As GC is often detected in its advanced stages, systemic chemotherapy remains the primary treatment The chemotherapy agent 5-Fluorouracil (5FU) is commonly used for gastrointestinal cancer, inhibiting thymidylate synthase and impeding DNA synthesis, ultimately curtailing cell metabolism and proliferation [2]. However, its use comes with significant side effects, including chemotherapy-induced diarrhea, intestinal mucositis, hand-foot syndrome, coronary vasospasm, and notably, myelosuppression [3-7]. Consequently, there's a critical need to discover a supplementary drug to enhance 5FU efficacy against gastric cancer cells at lower concentrations.
Nanoparticles, an emerging biotechnology, have made substantial strides in combating various cancers like breast, ovarian, and prostate cancers due to their distinctive physical and chemical properties. Their increased attention in anticancer therapies stems from their favorable biocompatibility, capacity to carry drug particles, and ability to target specific cells. While metal nanoparticles, particularly silver nanoparticles, find extensive use in treating drug-resistant bacteria, mastitis, various cancers' diagnosis and treatment, and disease tracing, research on their application in GC remains limited [8-13].
Gastric cancer, known for its high mortality rates, necessitates the exploration of more effective methods for its early detection. Nanomedicine, owing to its properties at the nanoscale, offers significant advantages in both cancer detection and treatment. Nanoparticles serve as contrasts in MRI/CT imaging, with Superparamagnetic Iron Oxide (SPIO) agents like ferumoxides (Feridex in the USA, Endorem in Europe) and ferucarbotran (Resovist) being the pioneering scientifically approved MR contrast agents [14,15].
In the treatment landscape, drug-loaded nanoparticles such as liposomal Doxorubicin (Doxil), PEGylated liposomal doxorubicin, liposomal paclitaxel, and albumin-bound paclitaxel (Abraxane) have been effectively employed in gastric cancer therapy, notably minimizing the side effects associated with traditional chemotherapeutic agents [16-19]. These nanoparticles are versatile tools, capable of both diagnosing and treating cancers-a concept termed 'theranostics' due to their dual functionality.
Gold Nanoparticles (GNPs) have emerged as a focus in research, particularly for Photothermal Therapy (PTT). Their remarkable ability to absorb visible and Near-Infrared (NIR) light through the Surface Plasma Resonance (SPR) effect leads to rapid conversion of this energy into heat. This method effectively destroys cancer cells by inducing hyperthermia in tumor tissue while sparing normal tissues from damage [20]. This treatment modality can be repeated as necessary and capitalizes on the leaky blood vessels in solid tumors, allowing GNPs to extravasate into the tumor matrix. This accumulation within tumor cells is a passive process termed the Enhanced Permeability and Retention (EPR) effect.
Furthermore, active targeting strategies have been investigated to enhance GNP concentration within tumor cells. Surface modifications of GNPs involve the attachment of antibodies or ligands tailored for receptor, antigen, or carbohydrate targeting [21]. These strategies offer promising avenues for increasing the therapeutic impact of nanoparticles in cancer treatment, paving the way for more precise and effective therapies.
In essence, this study emphasizes the capacity of nanotechnology to enhance conventional cancer treatments and emphasizes the necessity for continued exploration to maximize therapeutic benefits while minimizing undesired outcomes. These results set the stage for future inquiries into the practical application of nanoparticles in treating gastric cancer.
Materials and Methods
Cell culture
The MLN-45 cell line, originating from human gastric cancer at the Pasture Institute of Iran, was cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with Fetal Bovine Serum (FBS). These cells were nurtured within a humidified incubator at 37 degrees Celsius, maintaining a 5 percent CO2 level. Once seeded onto 96-well plates at approximately 5000 cells per well, they were allowed to adhere overnight. To determine the cytotoxic impact and the halfmaximal Inhibitory Concentration (IC50) of 5-Fluorouracil (5FU), varying concentrations (25 mg/dl, 50 mg/dl, 100 mg/dl, and 150 mg/dl) were employed, and the MTT test was performed at 12, 24, 48, and 72-hour intervals following the introduction of the drug. In the subsequent phase, Gold Nanoparticles (GNP) were introduced to the cells in 20-microliter quantities. Subsequently, the MTT test was carried out after exposure durations of 12, 24, 48, and 72 hours to measure the cytotoxic effects initiated by the GNPs. After this exposure, laser irradiation was applied at specific intervals of 12, 24, 48, and 72 hours, using parameters set at 35 J energy, 1 Hz frequency, and 5 minutes duration with a 1064 nm wavelength.
Post each session of laser irradiation, assessments were conducted to determine cell viability. The study concluded following a comprehensive evaluation of the collective impacts of these methodologies. The identified optimal incubation times were 72 hours for 5FU and 24 hours for GNP. Subsequent steps involved cell seeding onto 96-well plates, the application of 5FU at specified concentrations after a 24-hour interval, introduction of 20 micrograms of GNP per well after 72 hours of incubation, and subjecting the cells to laser irradiation following an additional 24-hour incubation with GNP.
MTT test
Each well received 20 μl of MTT solution, a solution consisting of 5 mg/ml in phosphate buffer saline. Following a 4-hour incubation at 37°C, the culture solution was retrieved from each well. To dissolve the formazan crystals, 100 ml of DMSO was added to every well while agitating the plates for 20 minutes. The resulting solution underwent analysis for absorbance using a 96 well microplate reader set at 570 nm wavelength.
Both experiments were conducted in triplicates and in parallel. Cell viability was assessed for different treatments, each compared against an untreated control group. The survival rate was calculated using the formula: (A)test/(A)control × 100, where (A)control refers to the absorption value of the control sample lacking 5FU and GNP, and (A)test represents the absorption value of the treated sample. This calculation allowed for the quantification of cell survival post-treatment compared to the untreated control, enabling the assessment of the impact of different interventions on cell viability.
Statistical analysis
The experiments were replicated thrice, and the data underwent analysis by computing the average and standard deviation. Statistical assessments were conducted utilizing SPSS v.16, and graphical illustrations were generated using Microsoft Excel 2007. Variance Analysis (ANOVA) was executed with a 95% confidence interval following confirmation of normality and consistency among variables. Statistical significance was determined by considering a P-value of ≤ 0.05. For multiple comparisons, Tukey's test was employed.
Results
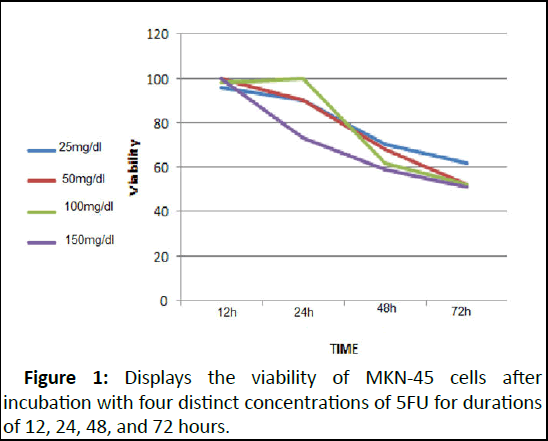
Following a 72-hour incubation period with 5FU, the determined IC50 concentration, inhibiting 50% of cell growth, stood at 50 mg/dl. Beyond this point, elevating the 5FU dosage did not lead to a decline in cell viability. The impact of various 5FU concentrations on cell viability at different time intervals is illustrated in Figure 1.
Figure 1: Displays the viability of MKN-45 cells after incubation with four distinct concentrations of 5FU for durations of 12, 24, 48, and 72 hours.
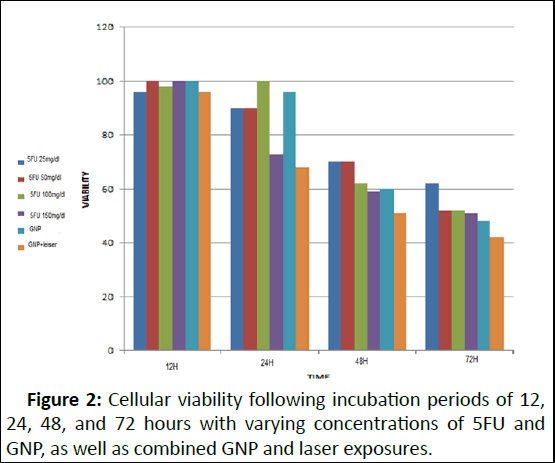
After 72 hours of incubation with 5FU, the most favorable outcome was achieved, and any alterations in dosage beyond 50 mg/dl did not significantly affect cell mortality. In the subsequent phase, the investigation aimed to assess whether GNP induces any cellular toxicity. According to the MTT assay results, GNP's impact on cell viability is contingent upon the duration of exposure to GNP. There was no notable decline in cell viability between 12 and 24 hours of GNP incubation. However, subsequent exposure to GNP for 48 and 72 hours resulted in a substantial 40% and 50% reduction in cell viability, respectively. The subsequent objective was to evaluate GNP's effect on hyperthermia. Post-laser irradiation following 12, 24, 48, and 72 hours of GNP incubation, cell viability stood at 100%, 70%, 50%, and 40%, respectively (Figure 2).
Figure 2: Cellular viability following incubation periods of 12, 24, 48, and 72 hours with varying concentrations of 5FU and GNP, as well as combined GNP and laser exposures.
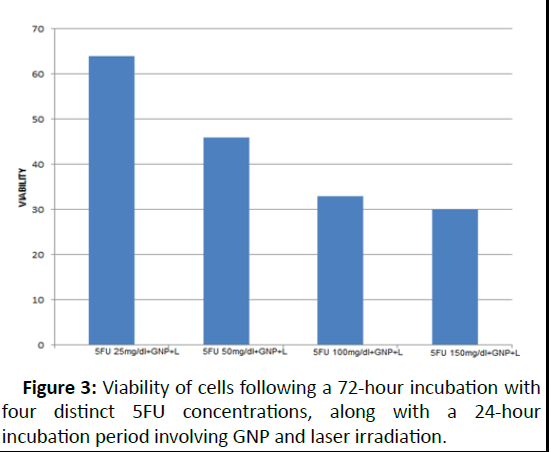
Based on these findings, the optimal incubation period for 5FU, which exhibited the most significant reduction in cell viability, was determined to be 72 hours. As for GNP, the ideal duration was 24 hours of incubation, beyond which the nanoparticles began exerting toxicity in the absence of laser irradiation. In the final phase of analysis, cells were exposed to 5FU at four distinct concentrations for 72 hours. Subsequently,GNP was introduced and allowed to incubate for 24 hours before laser irradiation. The MTT assay results indicated 64 percent viability for 5FU at 25 mg/dl, 46 percent for 5FU at 50 mg/dl, 33 percent for 5FU at 100 mg/dl, and 30 percent for 5FU at 150 mg/dl. These outcomes are depicted in Figure 3.
Figure 3: Viability of cells following a 72-hour incubation with four distinct 5FU concentrations, along with a 24-hour incubation period involving GNP and laser irradiation.
Discussion
The observed impact of 5-Fluorouracil (5FU) on MKN-45 cell viability unveils critical insights into the dosage and duration dynamics governing its cytotoxicity. Our results depict a significant reduction in cell viability at an optimal incubation period of 72 hours with 5FU, resulting in an IC50 concentration of 50 mg/dl. Beyond this concentration, escalating the 5FU dosage failed to elicit further declines in cell viability. This underscores the plateau effect of 5FU beyond a certain concentration, indicating a potential threshold beyond which its cytotoxicity does not intensify.
Figure 1 showcases the nuanced influence of different 5FU concentrations over varied time frames on MKN-45 cell viability. Notably, after 72 hours of incubation with 5FU, the most favorable outcomes were achieved, and any variations in dosage beyond 50 mg/dl did not significantly impact cell mortality. This time-sensitive response highlights the importance of incubation duration in determining the optimal effectiveness of 5FU.
Moreover, our investigation delved into the potential toxicity induced by Gold Nanoparticles (GNP) and their interplay with cell viability. The MTT assay results unveiled a time-dependent effect of GNP exposure on cell viability. Interestingly, the initial 12 to 24 hours of GNP incubation did not yield a substantial decline in cell viability. However, prolonged exposures for 48 and 72 hours resulted in notable reductions of 40% and 50% in cell viability, respectively. Additionally, upon subjecting cells to laser irradiation subsequent to varying GNP incubation periods, a distinct pattern emerged, revealing a decline in cell viability corresponding to prolonged GNP exposure, illustrating the potential impact of GNP-induced hyperthermia. Figure 2 vividly portrays the combined impact of 5FU and GNP incubation periods on cellular viability. These findings facilitated the determination of optimal incubation times for both 5FU and GNP. The most effective period for 5FU, exhibiting substantial reduction in cell viability, was identified as 72 hours. Contrastingly, GNP demonstrated its toxicity after 24 hours of incubation in the absence of laser irradiation.
Finally, in the concluding phase of analysis, cells underwent exposure to varying 5FU concentrations for 72 hours, followed by GNP incubation for 24 hours preceding laser irradiation. The resulting MTT assay outcomes indicated varying cell viabilities across different 5FU concentrations, revealing a dosagedependent effect on cell survival. Figure 3 encapsulates the culmination of these findings, illustrating the viability of cells post-exposure to distinct 5FU concentrations along with subsequent GNP and laser incubation periods.
In essence, our investigation not only delineates the time and dosage-dependent responses of 5FU and GNP on MKN-45 cell viability but also underscores the critical interplay between incubation durations and cytotoxic effects. These insights serve as pivotal guides in optimizing treatment strategies, indicating precise timeframes and dosages to achieve enhanced therapeutic efficacy while mitigating potential adverse effects.
Conclusion
Gastric cancer presents a substantial health issue on a global scale, standing as one of the most common and fatal cancers across the world. Despite the availability of conventional treatment options such as surgery, chemotherapy, and radiotherapy, their effectiveness is hampered by high mortality rates and potential side effects. As a promising alternative, nanomedicine has emerged as a transformative avenue for both diagnosing and treating cancer, with Gold Nanoparticles (GNPs) showcasing multifaceted properties that hold immense potential in this field.
The limitations encountered in current therapeutic approaches, notably chemotherapy using agents like 5- Fluorouracil (5FU), often stem from issues such as drug resistance and adverse effects. Chemotherapeutic agents, while effective, often exhibit toxicities that constrain their doses, impacting their efficacy, particularly in elderly patients diagnosed with gastric cancer. Seeking more efficient and less toxic modalities has driven exploration into nanomedicine, capitalizing on the distinctive properties offered by nanoscale substances. Gold nanoparticles have been extensively studied for their diagnostic and therapeutic applications. These nanoparticles possess unique characteristics, including biocompatibility and modifiable surfaces, rendering them suitable for diverse applications, including Photothermal Therapy (PTT). PTT involves leveraging the absorption of nearinfrared light by GNPs, which converts it into heat, inducing cancer cell death while minimizing harm to healthy tissues.
Our study concentrated on assessing the cytotoxic effects of 5FU, GNPs, and laser irradiation on the MKN-45 human gastric cancer cell line. We observed that incubating cells with 5FU for 72 hours resulted in optimal outcomes, inhibiting 50 percent of cell growth at a concentration of 50 mg/dl. Beyond this concentration, increased dosages did not notably impact cell viability. Subsequently, we investigated the time-dependent toxic effect of GNPs, noting significant reductions in cell viability with longer exposure durations, particularly after 48 and 72 hours. Moreover, our examination of the combined effect of 5FU and GNPs revealed optimized incubation periods of 72 hours for 5FU and 24 hours for GNPs. These optimized periods showed a substantial reduction in cell viability, especially at higher concentrations of 5FU, signifying enhanced cell destruction while minimizing chemotherapy-associated side effects.
Nanotechnology presents promising prospects in cancer therapy, offering multifunctional approaches like combination therapy and simultaneous diagnosis and treatment. However, challenges persist, including the preclinical nature of many studies and the limited specificity of diagnostic markers for gastric cancer. Future research should prioritize investigating the biocompatibility and clinical applicability of nanomaterials, ensuring their safety and effectiveness before transitioning from laboratory experiments to clinical settings.
In conclusion, our study underscores the potential of nanomedicine, particularly GNPs, in enhancing the effectiveness of gastric cancer treatment. The insights gained from observing time-dependent cytotoxic effects provide a valuable pathway for optimizing incubation periods for 5FU and GNPs, paving the way for more efficient and less toxic treatment strategies in the fight against gastric cancer.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, et al. (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71:209–249
[Crossref] [Google Scholar] [PubMed]
- Blondy S, David V, Verdier M, Mathonnet M, Perraud A, et al. (2020) 5-Fluorouracil resistance mechanisms in colorectal cancer: From classical pathways to promising processes. Cancer Sci 111:3142–3154
[Crossref] [Google Scholar] [PubMed]
- Stein A, Voigt W, Jordan K (2010) Review: Chemotherapy-induced diarrhea: Pathophysiology, frequency and guideline-based management. Ther Adv Med Oncol 2:51–63
[Crossref] [Google Scholar] [PubMed]
- Ribeiro RA, Wanderley CW, Wong DV, Mota JM, Leite CA, et al. (2016) Irinotecan-and 5-fluorouracil-induced intestinal mucositis: insights into pathogenesis and therapeutic perspectives. Cancer Chemother Pharmacol 78:881-893
[Crossref] [Google Scholar] [PubMed]
- Lou Y, Wang Q, Zheng J, Hu H, Liu L, et al. (2016) Possible Pathways of Capecitabine-Induced Hand–Foot Syndrome. Chem Res Toxicol 29:1591–1601
[Crossref] [Google Scholar] [PubMed]
- Zafar A, Drobni ZD, Mosarla R, Alvi RM, Lei M, et al. (2021) The Incidence, Risk Factors, and Outcomes With 5-Fluorouracil-Associated Coronary Vasospasm. JACC CardioOncol 3:101–109
[Crossref] [Google Scholar] [PubMed]
- Lunenburg CA, van der Wouden CH, Nijenhuis M, Crommentuijn-van Rhenen MH, de Boer-Veger NJ, et al. (2020) Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene–drug interaction of DPYD and fluoropyrimidines. Eur J Hum Genet 28:508-517
[Crossref] [Google Scholar] [PubMed]
- Sun H, Su J, Meng Q, Yin Q, Chen L, et al. (2016) Cancer-Cell-Biomimetic Nanoparticles for Targeted Therapy of Homotypic Tumors. Adv Mater 28:9581–9588
[Crossref] [Google Scholar] [PubMed]
- Ghanavi J, Mostafavi M, Ghanavi Z, inventors (2016) Method for the synthesis of metallic nano products. United States patent US 9,487,399.
- Jalaledin G, Mehrnaz M, inventors (2011) Method for producing rod-shaped and branched metallic nano-structures by polyol compounds. United States patent application US 12/870,792.
- Mostafavi M, Alizadeh M, Shaabani M, Asadi H (2021) Metallic nanomaterials in cancer theranostics: A review of Iron oxide and Gold-based nanomaterials. J Nanomed Nanotechnol 12:564
- Mostafavi M, Alizadeh M, Shaabani M, Ahad Baqeri S (2018) Engineering plasmonic nanostructures with combining ultrasound and laser pulses. Health Biotechnol Biopharma 2:49–55
- Mostafavi M, Ghanavi J (2012) Nanotechnology in proteomics: Current status, promises and challenges. Arch Adv Biosci 3
- Mostafavi M, Ghanavi J, Shaabani M, Asadi H, Alizadeh M (2021) Application of semiconductor nanomaterials in cancer theranostics. J Nanomed Nanotechnol 12:563–564
- Li R, Liu B, Gao J (2017) The application of nanoparticles in diagnosis and theranostics of gastric cancer. Cancer Lett 386:123-130
[Crossref] [Google Scholar] [PubMed]
- Espelin CW, Leonard SC, Geretti E, Wickham TJ, Hendriks BS (2016) Dual HER2 targeting with trastuzumab and liposomal-encapsulated doxorubicin (MM-302) demonstrates synergistic antitumor activity in breast and gastric cancer. Cancer Res 76:1517-1527
[Crossref] [Google Scholar] [PubMed]
- Cascinu S, Galizia E, Labianca R, Ferrau F, Pucci F, et al. (2011) Pegylated liposomal doxorubicin, 5-fluorouracil and cisplatin versus mitomycin-C, 5-fluorouracil and cisplatin for advanced gastric cancer: a randomized phase II trial. Cancer Chemother Pharmacol 68:37-43
[Crossref] [Google Scholar] [PubMed]
- Xu X, Wang L, Xu HQ, Huang XE, Qian YD, et al. (2013) Clinical comparison between paclitaxel liposome (Lipusu®) and paclitaxel for treatment of patients with metastatic gastric cancer. Asian Pac J Cancer Prev 14:2591-2594
[Crossref] [Google Scholar] [PubMed]
- Sasaki Y, Nishina T, Yasui H, Goto M, Muro K, et al. (2014) Phase II trial of nanoparticle albuminâ?ÂÂbound paclitaxel as secondâ?ÂÂline chemotherapy for unresectable or recurrent gastric cancer. Cancer Sci 105:812-817
[Crossref] [Google Scholar] [PubMed]
- Lee J, Chatterjee DK, Lee MH, Krishnan S (2014) Gold nanoparticles in breast cancer treatment: promise and potential pitfalls. Cancer Lett 347:46-53
[Crossref] [Google Scholar] [PubMed]
- Singh M, Harris-Birtill DC, Zhou Y, Gallina ME, Cass AE, et al. (2016) Application of gold nanorods for photothermal therapy in ex vivo human oesophagogastric adenocarcinoma. J Biomed Nanotechnol 12:481-490
[Crossref] [Google Scholar] [PubMed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences