Contemporary Profile of Chronic Dialysis Patients Receiving Oral P2Y12 Inhibitors: A Single Center Experience
Rafia Rasu1, Busuyi Olotu1, Margaret L Hansen1, Deepa Raghavan3,4, Milind Phadnis2, Jonathan D Mahnken2 and Nishank Jain3,4*
1School of Pharmacy, University of Kansas, Lawrence, Kansas, USA
2Department of Biostatistics, University of Kansas School of Medicine, Kansas City, Kansas, USA
3Department of Medicine, University of Arkansas for Medical Sciences, Little Rock, Arkansas, USA
4Central Arkansas Veterans Affairs Medical Center, Little Rock, Arkansas, USA
- *Corresponding Author:
- Nishank Jain
MD, MPH, Assistant Professor, University of Arkansas
for Medical Sciences, 4301 W. Markham St, Slot 501
Little Rock, AR 72205, Arkansas, USA
Tel: 501-686-5295
Fax: 501-686-7878
E-mail: NJain2@uams.edu
Received Date: Sep 13, 2017 Accepted Date: Nov 17, 2017 Published Date: Nov 20, 2017
Citation: Rasu R, Olotu B, Hansen ML, Raghavan D, Phadnis M, et al. (2017) Contemporary Profile of Chronic Dialysis Patients Receiving Oral P2Y12 Inhibitors: A Single Center Experience. Jour Ren Med. Vol.1 No.3: 14.
Abstract
Background: Oral P2Y12 inhibitors including clopidogrel, prasugrel, and ticagrelor are FDA-approved for patients presenting with acute coronary syndrome. Due to systematic exclusion of patients with end stage renal disease (ESRD) on outpatient dialysis from major trials, scarce data exist to report contemporary use of these drugs in ESRD patients.
Study design: Retrospective chart review of patient records.
Setting and participants: From 2011-2015, 848 ESRD patients (32%) had prescriptions for oral P2Y12 inhibitors such as clopidogrel, prasugrel, and ticagrelor. Differences in patient characteristics as well as major adverse events (MAEs) defined as a composite of new coronary stent placement, heart valve replacement, coronary artery bypass graft or amputation; and all-cause death, nose and gastrointestinal (GI) bleeding were collected.
Results: Of the 85 patient records that were reviewed, there were 68.2% males, 66.7% whites, 21.4% African Americans and 9% on peritoneal dialysis. There were no differences in baseline characteristics of patients who were prescribed the three drugs. 18 (21.2%) deaths, 57 (67.1%) MAEs, 9 (10.6%) nose bleeds and 13 (15.3%) GI bleeds were identified over 16.5 months follow up. There was no difference in clinical outcome variables between the three groups.
Conclusion: Oral P2Y12 inhibitors were commonly prescribed to ESRD patients despite the inadequacy of data and there were no differences in the profiles of ESRD patients who were prescribed the three drugs or their outcome variables. All-cause death, MAEs, nose and GI bleeds were common in these patients. Future studies are needed to investigate clinical benefits and risks associated with use of P2Y12 inhibitors in this high-risk patient population.
Keywords
Clopidogrel; Prasugrel; Ticagrelor; Oral P2Y12 inhibitors; Dialysis
Introduction
Nearly 600,000 patients with end stage renal disease (ESRD) on outpatient dialysis have coronary heart disease, and onethird experience thrombotic cardiovascular (CV) events such as acute coronary syndrome (ACS) [1,2]. Antiplatelet agents (APA), such as aspirin and P2Y12 inhibitors, are the cornerstone of ACS management [2,3]. In fact, in ESRD patients, oral P2Y12 inhibitors (clopidogrel, prasugrel or ticagrelor) are one of the top fifteen prescribed drugs.1 However, it must be noted that both, efficacy of oral P2Y12 inhibitors in ESRD patients as well as its safety in these patients (who are already predisposed to bleeding), are not established due to systematic exclusion of these patients from the landmark ACS randomized trials [2]. Given the ambiguity and complexity surrounding use of P2Y12 inhibitors in ESRD patients, the goal of this study was to characterize contemporary use of clopidogrel, prasugrel, and ticagrelor in ESRD patients.1 In addition, we report important clinical outcome variables identified in the electronic medical record (EMR) of patients who were prescribed oral P2Y12 inhibitors.
Methods
Study population
The Institutional Review Board approval was obtained. In this retrospective cohort study, we identified a local cohort of ESRD patients from the Healthcare Enterprise Repository for Ontological Narration system (HERON) of the University of Kansas Medical Center (KUMC) between 07.20.2011 and 12.31.2015 using the Informatics for Integrating Biology and the Bedside (i2b2) query and analysis tool (CTSA Award # UL1TR000001) [4]. HERON is the local repository of the KUMC EMR. Since ticagrelor was approved by the FDA in July 2011, all three drugs have been available in the market since 07.20.2011 [5-7]. Therefore, we chose 07.20.2011 as the start date to create a contemporary ESRD cohort and limit bias in the results during the time period when any one of the three APA was unavailable in the market.
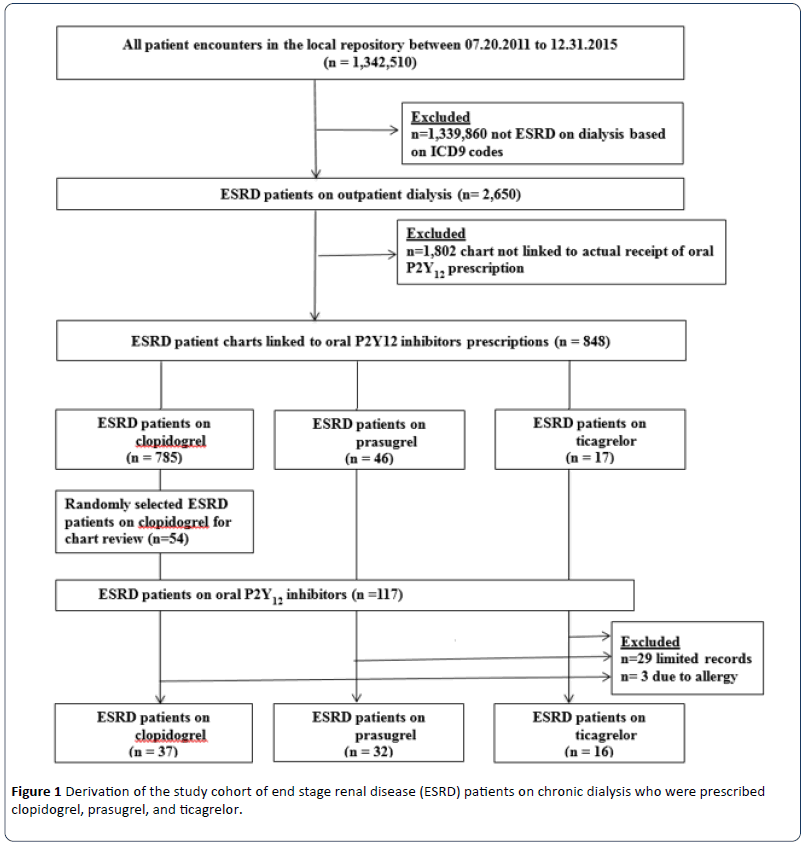
ESRD patients were identified from the HERON repository using the International Classification of Disease 9th Revision (ICD 9) codes 585.6 or V45.11.8. Figure 1 shows the derivation of the study cohort. Among 1,342,510 patients aged 18 years and older identified from the HERON, 2,650 (0.2%) ESRD patients were on outpatient dialysis (hemodialysis or peritoneal dialysis). Of the 2,650 ESRD patients, charts of 848 patients (32%) were linked to the actual receipts of prescriptions for oral P2Y12 inhibitors: 785 patients on clopidogrel (92.6%), 46 patients on prasugrel (5.4%) and 17 patients on ticagrelor (2%). We reviewed EMR of all those who were prescribed prasugrel (n=46) or ticagrelor (n=17). In addition, we randomly selected a convenient sample of 54 (6.9%) of the 785 patients identified on clopidogrel for detailed chart review, thus, yielding a total of 117 ESRD patients on the three oral P2Y12 inhibitors. From the 117 ESRD patients, we further excluded 32 patients due to limited record in the EMR (n=29) or allergy reported from drug use (n=3). A final cohort of 85 ESRD patients on outpatient dialysis who were prescribed oral P2Y12 inhibitors at our academic hospital was identified for detailed chart review.
Baseline data collection
We collected data from the EMR. From the admission encounter when a prescription for an oral P2Y12 inhibitor first appeared in the chart of a patient, we deemed the prescription to be a new prescription and that date to be the index date. Since the three drugs are FDA-approved for patients presenting with acute coronary syndrome (ACS), they are usually prescribed for the first time in the inpatient settings (index admission). Baseline characteristics including demographic information, co-morbidities, medications and laboratory data were derived from the index admission notes or outpatient encounters prior to the index date. If there were multiple encounters for a patient, baseline data were collected from the index admission note. Dialysis vintage was reported in months and reflected the time period between dialysis initiation and the index date. Patients were followed until the end of the observation period.
Clinical variables
The clinical variables were recorded from the index date to the end of the observation period. These included 1) all-cause death; 2) major adverse events (MAE) defined as a composite of new coronary stent placement (coronary stent placement after 5 days of the index date), heart valve replacement, coronary artery bypass graft (CABG), amputation or the number of all-cause hospitalizations defined as the number of inpatient admissions to the university hospital following the index admission; and, 3) bleeding events including GI bleeding requiring hospitalization or nose bleeds during index hospitalization.
Statistical analysis
Baseline comorbidities were coded as binary variables. Descriptive statistics were performed for all study variables. The analysis of variance (ANOVA) was used to compare the mean values of continuous variables such as age, dialysis vintage, and BMI while the Kruskal-Wallis test (the nonparametric version of ANOVA) was used to compare the median values of variables such as follow-up period, number of baseline comorbidities, number of baseline medications, and number of post-index all-cause hospitalizations. In addition, frequencies of baseline characteristics were compared among patients who were prescribed the three drugs using Pearson’s chi square tests. Data analyses were performed using IBM SPSS Statistics Version 23 (SPSS Inc, Chicago, IL). An alpha value was set a priori at <0.05 to be statistically significant.
Results
Prescriptions for clopidogrel, prasugrel and ticagrelor
From 2011-2015, 848 (32%) of the 2,650 ESRD patients were prescribed oral P2Y12 inhibitors: 785 (92.6%) patients on clopidogrel 75 mg/day, 46 (5.4%) patients on prasugrel 10 mg/day and 17 (2.0%) patients on ticagrelor 90 mg twice daily. All these patients were on aspirin 81 mg/day and were prescribed APA during the index admission for ACS.
Demographic information of ESRD patients on oral P2Y12 inhibitors
Of the 85 ESRD patients on oral P2Y12 inhibitors who had detailed chart review, more than two-thirds (n=58, 68.2%) of the chart review patients were male. The mean age of the cohort was 62.7 ± 10.3 years. Two thirds were white (66.7%) and 21.4% were African American. The mean BMI was 30.3 ± 6.4 kg/m2 for the overall cohort. There were no differences in the demographics including age, gender, race, and BMI of patients who were prescribed the three APA (Table 1).
| Variables | All (n=85) |
Clopidogrel (n=37) |
Prasugrel (n=32) |
Ticagrelor (n=16) |
p-value |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, mean (SD) | 62.7 (10.3) | 63.2 (11.9) | 61.5 (9.5) | 64.1 (8.0) | 0.68 |
| Gender, n (%) | |||||
| Female | 27 (31.8) | 12 (32.4) | 9 (28.1) | 6 (37.5) | 0.80 |
| Male | 58 (68.2) | 25 (67.6) | 23 (71.9) | 10 (62.5) | |
| Race, n (%) | |||||
| White | 56 (66.7) | 26 (72.2) | 17 (53.1) | 13 (81.3) | 0.19 |
| African American | 18 (21.4) | 5 (13.9) | 11 (34.4) | 2 (12.5) | |
| Other | 10 (11.9) | 5 (13.9) | 4 (12.5) | 1 (6.3) | |
| BMI, mean (SD) | 30.3 (6.4) | 29.0 (5.9) | 31.6 (6.5) | 30.9 (7.4) | 0.23 |
| Follow-up period in months, median (IQR) | 16.5 (4.0, 34.3) | 16.8 (3.9, 34.0) | 20.8 (6.9, 34.7) | 11.3 (1.0, 30.0) | 0.31 |
| Dialysis type, n (%) | |||||
| Hemodialysis | 77 (90.6) | 34 (91.9) | 28 (87.5) | 15 (93.8) | 0.73 |
| Peritoneal dialysis | 16 (18.8) | 6 (16.2) | 7 (21.9) | 3 (18.8) | |
| Dialysis vintage in months, mean (SD) | 29.8 (34.0) | 33.5 (40.4) | 33.1 (33.0) | 12.4 (11.0) | 0.25 |
| Baseline comorbidities, n (%) | |||||
| Number of baseline comorbidities, median (IQR) |
5 (3, 7) | 5 (3, 7) | 5 (4, 7) | 5.5 (3, 6) | 0.72 |
| Diabetes mellitus | 60 (70.6) | 27 (73.0) | 24 (75.0) | 9 (56.3) | 0.37 |
| Hypertension | 83 (97.6) | 36 (97.3) | 32 (100.0) | 15 (93.8) | 0.39 |
| CHF | 38 (44.7) | 20 (54.1) | 11 (34.4) | 7 (43.8) | 0.26 |
| PVD | 29 (34.1) | 15 (40.5) | 11 (34.4) | 3 (18.8) | 0.31 |
| Amputation | 13 (15.3) | 5 (13.5) | 5 (15.6) | 3 (18.8) | 0.89 |
| CAD/CABG | 74 (87.1) | 30 (81.1) | 30 (93.8) | 14 (87.5) | 0.29 |
| Previous coronary stent | 42 (49.4) | 15 (40.5) | 19 (59.4) | 8 (50.0) | 0.30 |
| MI | 33 (38.8) | 13 (35.1) | 15 (46.9) | 5 (31.3) | 0.48 |
| Previous bleed | 16 (18.8) | 4 (10.8) | 9 (28.1) | 3 (18.8) | 0.19 |
| Atrial fibrillation | 14 (16.5) | 4 (10.8) | 5 (15.6) | 5 (31.3) | 0.18 |
| Stroke/TIA | 14 (16.5) | 12 (32.4) | 2 (6.3) | 0 (0.0) | 0.01 |
| Baseline medications, n (%) | |||||
| Number of baseline medications, median (IQR) | 5 (4, 7) | 5 (4, 6.5) | 6 (5, 7) | 5 (4, 6.75) | 0.52 |
| Beta-blockers | 65 (76.5) | 30 (81.1) | 24 (75.0) | 11 (68.8) | 0.61 |
| ACE/ARB | 43 (50.6) | 19 (51.4) | 18 (56.3) | 6 (37.5) | 0.47 |
| Statin | 62 (72.9) | 28 (75.7) | 24 (75.0) | 10 (62.5) | 0.58 |
| Warfarin | 6 (7.1) | 4 (10.8) | 2 (6.3) | 0 (0.0) | 0.36 |
Abbreviations: ACE-I: Angiotensin-Converting Enzyme Inhibitors; ARB: Angiotensin Receptor Blockers; BMI: Body Mass Index; CABG: Coronary Artery Bypass Grafting; CAD: Coronary Artery Disease; CHF: Congestive Heart Failure; IQR: Interquartile Range; MI: Myocardial Infarction; PVD: Peripheral Vascular Disease; SD: Standard Deviation; TIA: Transient Ischemic Attack.
Table 1: Demographic and baseline characteristics of ESRD patients who were prescribed clopidogrel, prasugrel and ticagrelor during hospital admission for acute coronary syndrome.
Almost 91% (n=77) were on chronic hemodialysis, and only 9% (n=8) were on peritoneal dialysis. There was no difference between dialysis modality and the type of P2Y12 inhibitor prescribed: 91.9% of patients on clopidogrel, 87.5% on prasugrel and 93.8% ticagrelor were on hemodialysis, (p=0.73). In addition, dialysis vintage was similar between the three groups: 33.5 months for patients who were prescribed clopidogrel, 33.1 months for prasugrel, and 12.4 months for ticagrelor (p=0.25).
Baseline comorbidities and concomitant medication use
For the overall cohort (n=85), a median (IQR) of 5 (3,7) baseline comorbidities was reported (Table 1). However, there were no differences in the comorbid disease burden between the treatment groups (p=0.72). More than two-thirds (70.6%) of the cohort was diabetic and most of the patients (97.6%) had hypertension. Prevalence of baseline cardiovascular disease was high in the cohort, 44.7% had congestive heart failure, 87.1% had either coronary artery disease or previous CABG and 49.4% had previous coronary stents. However, there were no between group differences in the baseline comorbidities (Table 1). The median (IQR) number of baseline medications documented during the index admission for the cohort was 5 (4,7). Only 76.5% of the patients were on betablockers, whereas 7.1% were on warfarin. There were no between group differences in the use of baseline medications (Table 1).
Clinical variables
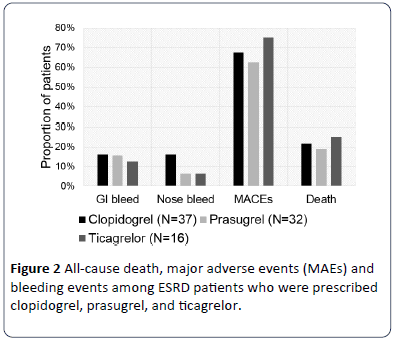
There were 18 deaths (21.2%) during the median (IQR) follow up of 16.5 (4.0, 34.3) months: 8 deaths in the clopidogrel group, 6 in prasugrel group and 4 in the ticagrelor group (p>0.05). In the overall cohort, 57 patients (67.1%) encountered MAE, including eight (9.4%) new coronary stent placements and 11 (12.9%) procedures related to either HVR, CABG or amputation occurred during the follow up. Furthermore, we found 13 (15.3%) episodes of GI bleeds and 9 (10.6%) episodes of nose bleeds during the follow up period (p>0.05) (Figure 2). In the exploratory analyses, we did not observe any differences in outcomes between the three drugs.
Discussion
We report contemporary prescription practices of oral P2Y12 inhibitors in ESRD patients at our university hospital. Our major findings are: 1) 32% of the ESRD patients on dialysis were prescribed oral P2Y12 inhibitors between 2011 and 2015 and 92.6% of these prescriptions were clopidogrel; 2) there were no major differences in baseline characteristics including demographics, dialysis modality, dialysis vintage, baseline comorbidities and baseline medication use of patients who were prescribed the three drugs; and, 3) among those who were prescribed oral P2Y12 prescriptions following admissions for ACS, all-cause deaths, MAE, and bleeding events were common during the short-term follow up and there were no differences in the outcomes between three drugs.
Of the three oral P2Y12 inhibitors, prasugrel and ticagrelor are FDA approved for use in patients experiencing ACS, whereas clopidogrel has an additional indication for patients with stable CAD [2,8,9]. There are important pharmacokinetic and pharmacodynamic differences between the three oral P2Y12 inhibitors [10-12]. Clopidogrel is a prodrug. It requires 2-step metabolism by cytochrome P450 (CYP450) enzymes to become active before binding to P2Y12 receptor on the platelet surface [3,10]. Theoretically, this multistep process renders clopidogrel less favorable for inhibiting platelet aggregation in CKD patients because: a) Genetic polymorphisms in CYP450 enzymes result in high inter- and intra-individual variability in clopidogrel effects; and, b) Uremic toxins can alter CYP450 system and thus affect availability of clopidogrel active metabolite [2]. Newer oral P2Y12 inhibitors such as prasugrel and ticagrelor compared to clopidogrel have more desirable pharmacological properties. Prasugrel requires only 1-step to become metabolically active whereas ticagrelor is an active drug. The resulting higher bioavailability as well as the reduced inter- and intra-individual variability in their antiplatelet effects are likely responsible for the superior antiplatelet efficacy of newer oral P2Y12 inhibitors versus clopidogrel [13-15].
There is growing popularity of newer oral P2Y12 inhibitors in patients experiencing ACS [16,17]. However, no study has reported efficacy, safety or prescribing trends of oral P2Y12 inhibitors in ESRD patients [2]. ESRD is a special clinical condition with multiple defects in hemostatic pathways that increase risk of thrombotic CV events compared to the general population [2,18,19]. In our study, approximately one-third of our ESRD cohort was prescribed oral P2Y12 inhibitors after admission for acute coronary syndrome. Majority (92.6%) of the study patients received prescriptions for clopidogrel, 5.4% for prasugrel and 2.0% for ticagrelor. A recent study in the general population analyzed EMR from several hospitals across the USA and found 77% of all patients undergoing percutaneous coronary intervention (PCI) and/or diagnostic coronary angiography (n=37,964) received clopidogrel, 13% received prasugrel and 10% received ticagrelor as of June 2013 [20]. Studies from Michigan and Australia found similar distribution of these agents [4,21]. We were unable to find studies that compared differences in prescription allocation ratios of the three drugs in ESRD patients. However, we anticipate that the newer oral P2Y12 inhibitors will gain popularity in this high-risk patient population despite lack of evidence.
We found no differences in baseline characteristics including demographics, dialysis modality, dialysis vintage, baseline comorbidities and baseline medication use of patients who were prescribed the three drugs. A recent study in the general population reported that compared to patients receiving clopidogrel, majority of patients receiving ticagrelor were white, younger and had fewer comorbidities [17]. This may suggest that patients who receive newer oral P2Y12 inhibitors may be working and may have better insurance providers compared to those who received prescriptions for clopidogrel. Since >93% of the chronic dialysis patients are covered by Medicare in the USA, these differences in baseline demographics of patients who were prescribed the three drugs were not noted in our study patients [17]. Moreover, demographics of our study cohort were similar to the national ESRD cohort except age, gender and presence of diabetes mellitus [21]. Our study patients compared to the U.S. Renal Data Sharing (USRDS) cohort were older (62.7 years versus 56.9 years), comprised of more males (68.2% versus 56.3%), included fewer African Americans (21.4% versus 35.2%) and had higher prevalence of diabetes mellitus (70.6% versus 43.6%) [22].
We found 21.2% of the ESRD patients who were prescribed oral P2Y12 inhibitors died during a short follow up of 16.5 months. Almost two-thirds of hemodialysis patients and half of peritoneal dialysis patients have cardiovascular disease, with <60% survival rate 2-years post-PCI [23,24]. Therefore, mortality rates in our study patients are similar to the national cohort. Notably, these rates are significantly higher than post- PCI mortality rates in the general population, and underline the urgent need for studies to examine cardiovascular and/or mortality benefits of oral P2Y12 inhibitors post-PCI in this highrisk patient population. Approximately two-thirds of our study patients experienced MAE and 20% experienced bleeding events (GI or nose bleeding), numbers that are six- and fivefold higher than that reported in the general population, respectively [25,26]. Only 7.1% were on concomitant warfarin therapy. In the exploratory analyses, we did not observe any differences in outcomes between the three drugs. Recent RCTs in the general population (TRITON-TIMI and PLATO trials) reported higher efficacy of newer oral P2Y12 inhibitors compared to clopidogrel in reducing mortality and future CV events among patients presenting with ACS [25,26]. However, these trials enrolled few patients with mild to moderate CKD and excluded patients with advanced CKD and ESRD, precisely those with highest risk of thrombotic CV events. Among a small subgroup of participants that had mild CKD (defined as creatinine clearance of <60 ml/min), use of ticagrelor over clopidogrel was associated with 23% reduction in mortality and future CV events without any increased risk of bleeding [26,27].
Our pilot study has several strengths. The study protocol followed the best practices recommended for conducting retrospective chart review. Chart review of patient record is still considered to be a gold standard. Data abstractors were trained before starting the data abstraction. In addition, variables were adequately operationalized before starting the study and we used a standardized abstraction form for collecting study variables. Furthermore, we were systematic in including eligible patients.
Our study has several limitations that are generally associated with retrospective chart review. Selection bias, confounding by indication and lead-time bias are known limitations of the retrospective studies. Therefore, in this pilot study, we have carefully interpreted our results. In addition, due to lack of data on various confounders, we have not investigated any associations between drug exposure and outcomes and avoided over-interpretation of our data. Despite these limitations, we believe that our findings are meaningful as this patient population is systematically excluded from major studies and data remain scarce in this topic. Second, this is a pilot study examining contemporary use of oral P2Y12 inhibitors in a small sample of ESRD patients from a single center. Therefore, our study may be under-powered to detect meaningful differences and limit its generalizability. In addition, we were not able to determine the temporal drug exposure of patients who were prescribed oral P2Y12 inhibitors. We report small number of clinical events linked to the charts of the ESRD patients after being prescribed oral P2Y12 inhibitors. As a result, our analyses is exploratory and not be powered to detect differences between the drug prescription at index admission and clinical outcomes. Finally, we were not able to collect information regarding the type of stent, the number of vessels intervened and the type of ACS with certainty due to poor documentation in the EMR and missing data. We chose not to include this information in the manuscript. We believe this pilot data will lay the foundation for further studies that evaluate the comparative efficacy and safety of these increasingly used agents in this high-risk population.
Conclusion
Oral P2Y12 inhibitors are commonly prescribed to ESRD patients although there is insufficient data to support their overall use as well as inadequate guidance regarding individual agent choice in this patient population. In contemporary practice, clopidogrel was the predominant agent used although it is likely we will see a surge in use of the other agents. There were no differences in the profiles of ESRD patients who were prescribed these drugs or clinical outcomes associated with the three agents, but all-cause death, MAEs, nose and GI bleeds were common in these patients. There is a pressing need for further studies to investigate clinical benefits and risks associated with use of P2Y12 inhibitors in this growing high-risk patient population.
Grant Support
This study was supported by the American Heart Association Scientist Development Grant 16SDG31000045 (NJ) and the intramural grant from University of Kansas School of Pharmacy (RR). The views expressed here are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the American Heart Association.
References
- US Renal Data System (2014) USRDS 2014 Annual Data Report. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
- Jain N, Reilly RF (2016) Oral P2Y12 receptor inhibitors in hemodialysis patients undergoing percutaneous coronary interventions: Current knowledge and future directions. Semin Dial 29: 374-381.
- Jain N, Hedayati SS, Sarode R, Banerjee S, Reilly RF (2013) Antiplatelet therapy in the management of cardiovascular disease in patients with CKD: what is the evidence? Clin J Am Soc Nephrol 8: 665-674.
- Waitman LR, Warren JJ, Manos EL, Connolly DW (2011) Expressing observations from electronic medical record flow sheets in an i2b2 based clinical data repository to support research and quality improvement. AMIA Annu Symp Proc 2011:1454-1463.
- US Food and Drug Administration (2011) FDA approves blood-thinning drug Brilinta to treat acute coronary syndromes.
- US Food and Drug Administration (2009) FDA approves Effient to reduce the risk of heart attack in angioplasty patients.
- US Food and Drug Administration (2012) FDA approves generic versions of blood thinner Plavix.
- Shireman TI, Phadnis MA, Wetmore JB (2014) Antihypertensive medication exposure and cardiovascular outcomes in hemodialysis patients. Am J Nephrol 40: 113-122.
- Washam JB, Herzog CA, Beitelshees AL (2015) Pharmacotherapy in chronic kidney disease patients presenting with acute coronary syndrome: a scientific statement from the american heart association. Circulation 131: 1123-1149.
- Mullangi R, Srinivas NR (2009) Clopidogrel: review of bioanalytical methods, pharmacokinetics/pharmacodynamics, and update on recent trends in drug-drug interaction studies. Biomed Chromatogr 23: 26-41.
- Dobesh PP, Oestreich JH (2014) Ticagrelor: Pharmacokinetics, pharmacodynamics, clinical efficacy, and safety. Pharmacotherapy 34: 1077-1090.
- Williams ET, Jones KO, Ponsler GD (2008) The biotransformation of prasugrel, a new thienopyridine prodrug, by the human carboxylesterases 1 and 2. Drug Metab Dispos 36: 1227-1232.
- Siller-Matula JM, Akca B, Neunteufl T (2016) Inter-patient variability of platelet reactivity in patients treated with prasugrel and ticagrelor. Platelets 27: 373-377.
- Storey RF, Melissa TS, Lawrance R (2009) Ticagrelor yields consistent dose-dependent inhibition of ADP-induced platelet aggregation in patients with atherosclerotic disease regardless of genotypic variations in P2RY12, P2RY1, and ITGB3. Platelets 20: 341-348.
- Angiolillo DJ, Fernandez-Ortiz A, Bernardo E (2007) Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am Coll Cardiol 49: 1505-1516.
- Sandhu A, Seth M, Dixon S (2013) Contemporary use of prasugrel in clinical practice: insights from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium. Circ Cardiovasc Qual Outcomes 6: 293-298.
- Karve AM, Seth M, Sharma M (2015) Contemporary use of ticagrelor in interventional practice (from Blue Cross Blue Shield of Michigan Cardiovascular Consortium). Am J Cardiol 115: 1502-1506.
- Jain N, Li X, Adams-Huet B (2016) Differences in whole blood platelet aggregation at baseline and in response to aspirin and aspirin plus clopidogrel in patients with versus without chronic kidney disease. Am J Cardiol 117: 656-663.
- Leblond F, Guevin C, Demers C, Pellerin I, Gascon-Barre M, et al. (2001) Downregulation of hepatic cytochrome P450 in chronic renal failure. J Am Soc Nephrol 12: 326-332.
- Fan W, Plent S, Prats J, Deliargyris EN (2016) Trends in P2Y12 inhibitor use in patients referred for invasive evaluation of coronary artery disease in contemporary US practice. Am J Cardiol 117: 1439-1443.
- Yudi MB, Clark DJ, Farouque O (2016) Clopidogrel, prasugrel or ticagrelor in patients with acute coronary syndromes undergoing percutaneous coronary intervention. Intern Med J 46: 559-565.
- United States Renal Data System (2016) The 2015 United States Renal Data System Report. Chapter 1: Incidence, prevalence, patient characteristics, and treatment modalities.
- United States Renal Data System (2015) The 2015 United States Renal Data System Report. Chapter 6: Mortality.
- United States Renal Data System (2015) The 2015 United States Renal Data System Report. Chapter 9: Cardiovascular disease in patients with ESRD.
- Wiviott SD, Braunwald E, McCabe CH (2007) Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 357: 2001-2015.
- James S, Budaj A, Aylward P (2010) Ticagrelor versus clopidogrel in acute coronary syndromes in relation to renal function: results from the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation 122: 1056-1067.
- Basra SS, Tsai P, Lakkis NM (2011) Safety and efficacy of antiplatelet and antithrombotic therapy in acute coronary syndrome patients with chronic kidney disease. J Am Coll Cardiol 58: 2263-2269.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences