Compartment Syndrome: A Cornerstone in Critical Care Management
Ghada Shalaby Mahran1 and Mostafa Samy Abbas2*
1Department of Critical Care nursing, Faculty of nursing, Assiut University, Egypt
2Department of Anaesthesia and intensive care, Assiut university hospital, Faculty of medicine, Assiut University, Egypt
- *Corresponding Author:
- Abbas MS
Department of Critical Care nursing
Assiut University, Egypt, Germany
Tel: + 201003060187
E-mail: mostafasamy@aun.edu.eg
Received date: October 30, 2017; Accepted date: December 11, 2017; Published date: December 18, 2017
Citation: Mahran GS, Abbas MS (2017) Compartment syndrome: A cornerstone in critical care management. J Anaesthesiol Crit Care. Vol 1 No.1:2
Copyright: ©2017 Mahran GS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Abstract
Intra-abdominal Hypertension (IAH) doesn't just influence abdominal organs as well as influences different organ systems. It affects the respiratory system, hemodynamics, and even cerebral perfusion. IAH is a paramount determinant of the compliance of the respiratory system, and practical consequences for mechanically ventilated patients are important. IAH influences our traditional filling pressures, and volumetric preload indices better reflect the true preload status in IAH. We review the most recent literature in pathophysiology and causes of IAH and Abdominal Compartment syndrome (ACS) to improve management of critically ill patients.
Keywords
Critical care; Abdominal compartment syndrome; Intra-abdominal hypertension; Trauma
Introduction
A compartment syndrome is a state with increased pressure in a limited anatomical space that adversely influences the circulation and threatens the function and viability of the tissues there in. Such a syndrome may occur within any enclosed space that is subject to distension. The classic example is an extremity compartment syndrome following trauma to the major inflow or outflow vessels of the lower limb, or as a result of primary pathology within the compartment itself [1]. Other confined anatomical spaces mostly accompanied with compartment syndromes are the cranial cavity (epidural/subdural hematoma), the orbital globe (glaucoma), and the kidney capsule (postischemic oliguria) [2].
Abdominal Compartment Syndrome
The abdominal compartment syndrome (ACS) is generally defined as a state of adverse physiologic consequences and serious organ dysfunction resulting from sustained acute increase in intra-abdominal pressure (IAP) over 20 mm Hg (with or without an abdominal perfusion pressure (APP) less than 60 mm Hg), that most obviously affects the cardiovascular, respiratory and renal systems. It is uniformly fatal if untreated [1,3,4].
Intra-abdominal pressure
The world society of abdominal compartment syndrome (WSACS) consensus definitions have defined intra-abdominal pressure (IAP) as the pressure inside the abdominal cavity, measured at end-expiration in the relaxed patient in the supine position, which is normally less than 10 mm Hg. Intra-abdominal pressure (IAP) fluctuates with respiration and is easily manipulated by activity and changes in position in normal individuals. In a healthy person, the normal range of the IAP is between the 0 and 5 mm Hg and it significantly depends on Body Mass Index (BMI) [1,4]. Different factors, such as coughing, sneezing, loud singing, defecation and weight lifting may cause IAP to increase drastically for a while and then return easily to baseline measurement [5].
Intra-abdominal hypertension
Intra-Abdominal hypertension (IAH) is defined as a sustained or repeated pathological elevation in IAP greater than 12 mm Hg and it is a frequent though somewhat under-recognized occurrence in critically ill patients [1,6].
12 mm Hg is the accepted upper limit of IAP by the World Society, reflecting the expected elevation in normal pressure from clinical conditions that apply external pressure against the peritoneal cavity or diaphragm, including obesity and chronic obstructive pulmonary disease [7].
The critical level of IAP for most of patients may be some place between 10 and 15 mm Hg. This pressure leads to diminished microcirculation blood flow and provokes the development of organ dysfunction and failure. If not discovered and treated early, the elevation of IAP naturally leads to abdominal compartment syndrome [8]. Hence, Intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS) are important causes of morbidity and mortality in the critically ill [9].
Abdominal perfusion pressure
Abdominal perfusion pressure (APP) is a measure of the relative adequacy of abdominal blood flow [5]. It indicates the pressure available for perfusion of intra-abdominal organs [10]. The abdominal perfusion pressure (APP), is possibly a more accurate indicator of visceral perfusion, as well as a theoretical end point for resuscitation with a greater area under the receiver operator curve than the mean arterial or IAP alone. It is a superior to other common endpoints such as pH, base deficit, lactate and urine output, although further validation is required [10].
The abdominal perfusion pressure, similar to the usual concept of cerebral perfusion pressure, is defined as the difference between the mean arterial pressure (MAP) and the IAP and implies that as the IAP rises, the perfusion of organs or vessels in or near the abdomen decreases even in the absence of a drop in MAP [11]. The APP in patients with IAH or ACS should be kept at 60 mm Hg or greater [5].
Historical Aspects of (IAH) and (ACS)
There are three big eras in the history of ACS. The first is the evolution in the understanding of the pathophysiology of the compartment syndrome in general (The first case of the muscular compartment syndrome was described by Hamilton in 1850). The second was the era of the experimental studies for measuring the IAP. The third is the era of the understanding and treatment of the basic problem which was started with the work of Ogilvie [12] who performed the first laparotomies and described the beneficial impact of this process in the management of giant abdominal war wounds [4].
The real story of ACS started in the second part of the XIX century. First, in 1863 Marey of Paris published his experiences about the elevated abdominal pressure's effects and highlighted the effects that IAH produces on the thorax. In 1865 the first measurement of intra-abdominal pressure through the rectum was done in Germany. In 1890, Heinricius demonstrated that ACS was fatal to animals because of impairment of respiration, decreasing cardiac output [13].
In-between 1870 and 1900 many attempts were done to measure IAP and to study its effect to vital functions. In 1911 Emerson [14] published his article titled "The intra-abdominal pressure". In 1940 the first laparostomy was performed to decrease the elevated IAP. The first description of the importance of staged abdominal closure was published in 1948. In 1951 Baggot claimed that abdominal closure in case of distension can lead to death. In the 70's several investigators carried out trials on laparoscopy and effects of pneumoperitoneum on IAP [4].
The term ACS was used for the first time by Fietsam et al. [15] in the late 1980s to describe the pathophysiologic effect resulting from IAH secondary to aortic aneurysm surgery. Hence the first definition of ACS was finally coined [13]. In spite of being described over 100 years earlier, the new-era abdominal compartment syndrome (ACS) was described for the first time as a ‘new’ clinical entity in the 1980s in emergency surgery patients [9]. In 1984, Harman et al. showed the evidence of the drawbacks of IAP on renal function and significance of decompression in these cases [16].
A long time after the first acknowledgement of intraabdominal hypertension (IAH) in the eminent studies during the 18th and 19th century, it has been discovered again as a medical problem in patients after Harman et al.’s [16] publication of 1984, which audits the intra-abdominal pressure (IAP) as an indication for relaparotomy in patients that had surgery for complicated abdominal aortic aneurysms [8].
The two articles that had opened the decade of ACS were published in 1995 and 1996. In the previous ten years, several teams started to investigate the monitoring techniques of intraabdominal pressure. due to the extraordinary interest and as a response to the requirement to unify and standardize the definitions, classifications and protocols for management and monitoring of intra-abdominal hypertension and abdominal compartment syndrome (ACS), The first worldwide conference was organized 6-8 December 2004, in Noosa (Queensland) in Australia and WSACS (World Society of Abdominal Compartment Syndrome) was established at the same time.
Incidence of intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS) (Table 1)
| Population | IAH | ACS |
|---|---|---|
| Medical | 18-78% | 4-36% |
| Surgical | 32-43% | 4-8% |
| Trauma | 2-50% | 0.5-36% |
| Burn | 37-70% | 1-20% |
| Pediatric | *** | 0.6-19% |
| *** not available | ||
Table 1 Incidence of intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS).
While the precise incidence of intra-abdominal hypertension (IAH) and ACS is unclear due to the varying definitions and methodology of measurement, these processes are common in the critically ill patients [1]. The incidence of IAH and ACS reflects the acuity of the population and also the clinician’s diligence in monitoring IAP [17].
In the intensive care unit (ICU), the prevalence of IAH on admission ranges between 31 and 58.8% and the incidence increases with the length of ICU stays [18]. This is consistent with the rate of IAH in those with severe burns (36.7%-70%), injuries as a result of trauma (2%-50%) and major abdominal procedures (31.5-40.7%) [1].
Table 1 Incidence of intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS).
An institution with both liberal fluid resuscitation protocol and routine IAP monitoring documented an incidence of 8% for secondary ACS and an incidence of 6% for primary ACS in patients with severe trauma who are presented with shock. When compared with patients with primary ACS, secondary IAH has also been shown to happen afterword in a patient’s hospital admission and to be associated with aggressive and prolonged increase of IAP and thus a higher mortality [17].
Classification of Abdominal Hypertension
Mild abdominal hypertension
Acute sustained increase of IAP of 10-20 mm Hg: With wellcompensated physiologic effects. Operative therapy may not be required.
Moderate abdominal hypertension
Acute sustained increase of IAP of 21-35 mm Hg: Therapy is essential. An intervention like operative abdominal decompression may be essential.
Severe abdominal hypertension
Acute sustained increase >35 mm Hg: Operative abdominal decompression is a must. Other pathologic conditions associated with elevated IAP may be stratified into acute and chronic abdominal hypertension.
Grading of Abdominal Hypertension
The world society of abdominal compartment syndrome (WSACS) has developed grades of IAH [19] (Table 2):
| Grade I | 12-15 mm Hg |
| Grade II | 16-20 mm Hg |
| Grade III | 21-25 mm Hg |
| Grade IV | >25 mm Hg |
Table 2: Grades of abdominal hypertension.
Types of Abdominal Hypertension
Acute abdominal hypertension
It is a pathologic condition of brief increase in abdominal pressure that may be advanced to the ACS that requires operative decompression. Acute abdominal hypertension also can be seen in 18% of elective laparotomies and in up to 40% of emergency cases of laparotomy [2].
Causes [4]
• Spontaneous: Peritonitis, abdominal abscess, bowel obstruction, ruptured aortic aneurysm, tension pneumoperitoneum, acute pancreatitis, mesenteric thrombosis.
• Postoperative: Peritonitis, abscesses, bowel obstruction, intra-abdominal haemorrhage.
• Trauma: Intraperitoneal and retroperitoneal haemorrhage, visceral oedema after cardiopulmonary resuscitation.
• Iatrogenic: Laparoscopy, abdominal closure under distension.
Chronic abdominal hypertension
Chronic and slow elevation of IAP can be compensated by the human organism and the abdomen adapts for increased load, so ACS won't develop in this case [4].
Chronic abdominal hypertension is a pathologic condition of enduring raised abdominal pressure that may lead to impairment in physiologic function and that thus decompression may be beneficial. Some examples are massive ascites and congestive heart failure, huge abdominal tumors, chronic peritoneal dialysis, gravid uterus, and obesity [2].
Causes and Risk Factors of IAH
Prevention is the key to avoid the bad effects of IAH; therefore, early recognition of the risk factors and signs of IAH is essential improve outcomes in intensive care [20].
It is essential to screen all patients for IAH while admission to a critical care unit; or at least all those with risk factors for IAH/ ACS. Furthermore, any patient with a clinical deterioration should undergo immediate IAP monitoring [1].
IAH/ACS has been regularly described in patients with severe trauma. The incidence of ACS in trauma patients with emergency laparotomy ranges from 5% to 14% according to the severity of trauma and the amount of applied abdominal packing. IAH/ACS may also be seen in those with shock requiring aggressive fluid resuscitation; the incidence of IAH/ACS is 0.07% of all admissions after trauma and 9% of shocked trauma patients. IAP more than 20 mm Hg can be observed in 2% of all trauma cases admitted to ICUs. Thus the risk factors for IAH and ACS are severe abdominal trauma, as well as increased markers of metabolic derangement, and shock [20,21].
IAH occurs in 59% to 78% and ACS in 27% to 56% of those with severe acute pancreatitis. Both primary (due to abdominal collections and inflammation) and secondary (due to fluid resuscitation) IAH is often observed and this could explain the high prevalence of IAH/ACS in patients with severe acute pancreatitis [20].
IAH/ACS is reported in severely burned patients and is now considered a fatal complication of thermal injury. Massive swelling and ascites due to systemic inflammation associated with massive fluid resuscitation causes elevated IAP in such patients. IAP ˃20 mm Hg is frequent in patients with burns >40%, especially in case of inhalational injury, poorly guided fluid resuscitation or burns of the abdominal wall [20].
The term ACS was introduced by Harman et al. [16] in 1984, who reported ACS post aortoiliac surgery. A 32% incidence in liver transplant patients was found [22]. 46% of emergency gastrointestinal surgery patients requiring intensive care had IAP ≥ 18 mm Hg [20,23].
Other factors such as recent abdominal surgery, sepsis, organ failure, mechanical ventilation, and change in body position have all been reported in with increase in IAP. The use of positive endexpiratory pressure (PEEP) or the presence of auto-PEEP during mechanical ventilation (MV) can act as a predisposing factor for the increase in IAP [24].
In heart surgery, both cardiopulmonary bypass (CPB) and offpump procedures are associated with factors potentially leading to IAH. Compromised bowel capillary endothelium by ischaemiareperfusion, inflammatory mediator’s injury or splanchnic hypoperfusion may enhance third space losses and cause IAH [25].
Physiological Factors Impacting on Intra- Abdominal Pressure (IAH) [4,10,19,26]
Factors related to diminished abdominal wall compliance
• Prone position
• High BMI, central obesity
• Pregnancy
• Mechanical ventilation
• Acute respiratory failure
• PEEP or auto PEEP
• Basal pneumonia
• Pneumoperitoneum
• Abdominal surgery with tight abdominal closures
• Pneumatic garments
• Abdominal wall bleeding or abdominal hematoma
• Burns with extensive abdominal scars
Factors related to increased intra-abdominal contents
• Gastro-paresis
• Gastric distension
• Ileus
• Volvulus Abdominal hematoma
• Intra-abdominal or retroperitoneal hematoma
• Damage control laparotomy
• Liver dysfunction with ascites
• Bowel pseudo obstruction
• Abdominal infection (peritonitis, pancreatitis)
• Hemoperitoneum
• Pneumoperitoneum
• Major trauma
• Excessive inflation during laparoscopy
• Peritoneal dialysis
Factors related to capillary leak and fluid resuscitation
• Acidosis (pH below 7.2)
• Poly transfusion (10 units of blood/24 h)
• Coagulopathy
• Massive fluid resuscitation
• Pancreatitis
• Oliguria
• Major trauma/burns
• Damage control laparotomy
• Hypothermia (core temperature below 33°)
• Multiple transfusions/trauma (>10 units in 24 h)
• Severe sepsis or bacteremia
• Septic shock
• Massive fluid resuscitation
Types of ACS
WSACS categorizes conditions that cause ACS as primary (surgical), secondary (medical), and recurrent [5]:
• Primary or “surgical”, ACS may be seen with illness in the abdomino-pelvic region that either precedes, or follows, surgical or angiographic intervention. This may be considered “classic” ACS. Those patients have intra- or retro-peritoneal bleeding, organ injury, damage control surgery (e.g. packing of liver haemorrhage), pelvic fractures with severe bleeding and organ transplantation. Those patients are frequently requires early surgical or radiological intervention. (Example of Primary conditions: peritonitis, pancreatitis, bowel obstruction & haemorrhage, trauma) [1,4,17].
• Secondary or “medical”, ACS is a unique entity because it occurs in patients without a primary intra-peritoneal injury or intervention. It is related to bowel, abdominal wall, and retroperitoneal edema and ascites induced by fluid resuscitation. Medical ACS does not originate from the abdominal cavity, it is caused by surgical procedure (As: abdominal closure under tension, huge abdominal hernias, surgeries for bowel obstruction) [4].
• Tertiary or “recurrent”, ACS occurs despite the prophylactic or therapeutic surgical, or medical, treatment of primary or secondary ACS. Classic examples include patients with the persistence of ACS after a surgical decompression procedure, a completely new episode of ACS after a temporary abdominal closure had been removed and the abdominal fascia re-approximated, or even with an already open abdomen if visceral swelling is further provoked. [17]. Other risk factors include episodes of sepsis requiring aggressive fluid resuscitation, tertiary peritonitis arising in the frozen abdomen and potential ischemia/reperfusion injury Although there different etiologies of primary, secondary and tertiary ACS, the requirement for both an altered numerical component (IAP) and corresponding clinical sequelae are absolute when diagnosing ACS [1,5,17].
Methods of Measurement of IAP [19]
In analogy with the paradigm “when you don’t take a temperature you can’t find a fever”, one can state that “if you don’t measure IAP you cannot diagnose IAH or ACS” [13].
A recommendation by the WSACS is to measure IAP each 4 to 6 hours in critically ill patients who demonstrate at least one of risk factors to develop IAH or ACS [19].
Physiological indices, such as blood pressure, ECG, heart rate and oxygen saturation, are monitored as a routine in all patients in intensive care units, While measurements of (IAP) are seldom used as a routine monitoring. Early monitoring of IAP, and its proper treatment if raised, may have reduced the progression from IAH to ACS [20].
Direct measurement of IAP with a solid micro transducer placed in the abdominal cavity is not indicated in intensive care units because of its invasiveness. The use of indirect methods has advantages due to its less invasiveness, more cost efficient, and easier to use. Indirect measurement of IAP is done through the natural openings of the abdominal cavity: transesophageal intragastric; transvaginal intrauterine; transanal intra-rectal; intra-vesical transurethral, percutaneous transfemoral to the subdiaphragmatic inferior vena cava. The guidelines of the (WSACS), recommend the use of the intravesical method [27,28].
Conditions for a reproducible IAP measurement
1. Expressed in mmHg (1 mm Hg =1.36 cm H2O)
2. Measured at end-expiration
3. Performed in supine position
4. Zeroed in the mid-axillary line at the iliac crest
5. Priming volume <25 ml of saline
6. Measured 30-60 s after instillation
7. Measured in the absence of active abdominal muscle contractions
Pathophysiology
The mortality and morbidity of IAH and ACS are high and may reach 100% for unattended ACS. Deleterious effects of increased intra-abdominal pressure not limited to the abdomen but also impact the pressure balances on other organ systems [29,30].
IAH can cause serious complications in any organ (Table 3). Without effective therapy/intervention it can causes multiorgan failure (MOF) by affecting cardiovascular, respiratory, central nervous system and renal function. De Waele et al. [31] published that there was a 94% incidence of respiratory, 94% cardiovascular and 89% of renal failure among patients with IAH (where IAP >12 mm Hg) [4].
| IAP=0-9 mm Hg | IAP=10-15 mm Hg | IAP=16-25 mm Hg | IAP=26-40 mm Hg |
|---|---|---|---|
| Cytokines release | Circulation of the abdominal wall decreases with 42% | Significant reduction of splanchnic circulation and venous return | "Hemodynamic collapse” |
| Increased capillary permeability | Significant reduction in the circulation of other abdominal organs | Increase in systemic vascular resistance (SVR), central venous pressure (CVP), peak airway pressure (PAWP) | Fatal acidosis |
| Fluid content of the “third space” expands | Local acidosis | Total respiratory capacity (TRC), vital capacity (VC) lowered due to pulmonary compression | Hypoxia hypercapnia |
| Decreased venous return as well as preload | Free radical release | Hypoxia hypercapnia | Anuria |
| Early effects on the central nervous system | Bacterial translocation through intestinal mucosa | Circulation of intestinal mucosa decreases with 61% | Circulation of coeliac artery is reduced to 58% |
| Increasing acidosis | Circulation of superior mesenteric artery is reduced to 39% | ||
| Renal failure: Oliguria, anuria | Circulation of renal artery is reduced to 30% | ||
| Disturbance of central nervous system | Circulation of abdominal muscles lowered with 80% (infection, wound-healing disturbances) |
Table 3: Complications of IAH.
Complications
Cardiovascular system
Decrease in cardiac output and a tamponade-like picture occurs due to compression of the heart and major vessels because of IAH. Central venous pressures (CVPs) and pulmonary artery wedge pressures (PAWPs) are also elevated. Those elevations lead physicians to predict that a patient is volume loaded [5,29]. The patient is in an increased risk for thromboembolism due to stasis in deep venous circulation [4,30,32,33].
Increased IAH adversely affect the parameters of cardiac output (i.e., preload, afterload, and contractility). Changes in right ventricular mechanics affect contractility. An elevation in intra-thoracic pressure due to IAH also increases pulmonary vascular resistance and right ventricular afterload. This leads to right ventricular dilatation that pushes the intra-ventricular septum into the left ventricle. This decreases left ventricular filling. The increased workload increases myocardial oxygen consumption and demand. As a compensation, systemic vascular resistance increases to maintain arterial pressure. Moreover, the direct compression of the abdominal aorta, due to IAH, further increases systemic vascular resistance and the left ventricular afterload [5,30,33].
The direct compression of the femoral veins due to IAH increases both venous stasis and the development of deep vein thrombosis [5].
Respiratory function
The relationship between intra-abdominal pressure (IAP) and respiratory function was demonstrated firstly in 1863. Today the negative effects of IAH on respiratory system have been investigated in different studies. Development of IAH decreases chest wall compliance and functional residual capacity, shifts the end-expiratory position of the diaphragm, and leads to development of atelectasis. Thus, it impairs gases exchange [27].
The lung, thoracic cage, and the abdominal cavity comprise a closed system with the diaphragm as the connecting interface. If abdominal and diaphragmatic pressures are increased, the pleural pressure may be changed and a decrease in total lung capacity, compliance, volumes may follow. The adjustments in the PEEP applied to the patient could be transmitted to the abdomen, that leads to an increase in IAP [30,34].
Atelectasis, pneumonia
As the abdomen distension pushes the diaphragm upward, encroaching on the thoracic cavity. Around 50% of the IAP is transmitted across the diaphragm impairing respiration and ventilation. Pulmonary changes may be one of the first signs of ACS. Subnormal expansion of the lungs reduces inhaled tidal volume, leading to hypoxemia. Conversely, carbon dioxide is retained, leading to respiratory acidosis [5,29].
Mechanical ventilation in patients with IAH increases peak airway and plateau pressures. Ventilated patients with abdominal hypertension require increased airway pressure to deliver a fixed tidal volume. Pneumonia is a typical early complication in abdominal hypertension from diffuse peritonitis. Ventilation/perfusion mismatch results and arterial blood gas readings show hypoxemia, hypercarbia, and acidosis [4,29].
Ventilation & respiratory failure
Because of the increased IAP the diaphragm will be shifted to cranial direction on both sides causing the reduction of pulmonary volume and cardiac function leading to acidosis and hypoxia [2,4].
Patients with IAH are in need for mechanical ventilation and have more difficulty in weaning. The major reason is the cephalad displacement of the diaphragm leading to a reduction of the functional residual capacity. In addition, reduction of chest wall compliance causes atelectasis. Therefore, patients with IAH may need a different ventilator strategy and more specific treatment considering IAH such as positive endexpiratory pressure against IAP [18,32].
Renal function
The deleterious effects of the increased (IAP) on the function of kidneys and other organs have been known for century before [11,29].
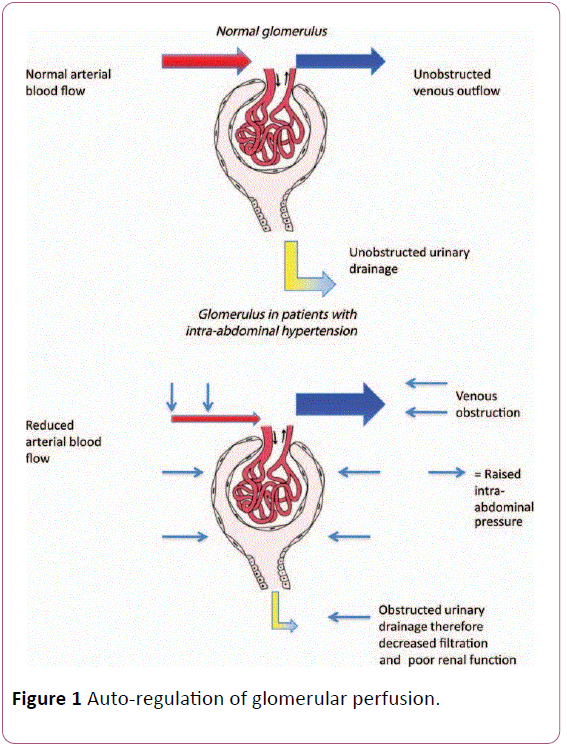
Kidney is very sensitive to elevations in IAP [32]. Because it is located deep within the posterior retroperitoneal space, the kidney is especially subjected to the deleterious effects of increased IAP resulting in AKI (IAH-AKI). Renal perfusion is impaired in patients with IAH because of decreased cardiac output (to a variable extent), that results in a reduction in arterial blood flow to the kidney. Kidney perfusion pressure can be defined as MAP minus IAP. Therefore, in theory, decreased kidney function can be prevented by either decreasing IAP or increasing MAP. Ulyatt suggested that filtration gradient (FG) is a very appropriate parameter to explain AKI associated with IAH. FG represents the balance between hydrostatic forces in glomerular capillaries that promote fluid transfer into Bowman space and oncotic forces that promote transfer into glomerular capillaries. In normal physiologic states, hydrostatic pressure in Bowman space (and therefore in the proximal tubules) is negligible, promoting glomerular filtration; accordingly, glomerular filtration pressure can be approximated as equal to kidney perfusion pressure and thus equal to MAP-IAP. However, in the presence of IAH, Bowman space and proximal tubular pressure will be close to IAP; therefore, FG can be approximated as FG_MAP–(2_IAP), assuming that auto-regulation of glomerular perfusion is not present [31] (Figure 1).
The markers of renal insufficiency due to ACS are that oliguria progresses into anuria and diuretics have no significant impact. Urinary sodium is low, reflecting a “pseudo pre-renal” condition [32]. Renal impairment, as indicated by an elevated level of serum creatinine, may not be obvious until 2 to 3 days later to IAH [5].
Outflow obstruction caused by compression of the renal vein, causes renal dysfunction. Elevation of plasma antidiuretic hormone may represent another important factor [2,29]. IAP 15-20 mm Hg results in oliguria, and above 30 mm Hg causes anuria [4,29].
Effects on liver function
The vascular liver is extremely susceptible to IAH. Persistent pressures as low as 10 mm Hg can reduce hepatic perfusion and impair liver function. In case of varices, the same pressure may increases variceal stress and the possibility of rupture. Both the hepatic arterial and the portal venous blood flow could decrease with high IAP. This change in blood flow leads to decreased glucose metabolism, mitochondrial malfunction, and reduced lactate clearance by the liver. Decreased lactate clearance leads to lactic acidosis [5].
Intestinal vascular resistance as an important determinant of portal blood flow may be complicated by abdominal hypertension in trauma patients as they are susceptible for shock induced changes. Hepatic synthesis of acute-phase protein, immunoglobulin, and factors of the other host defense systems are assumed to be impaired by decreased hepatic flow and further compromise response to massive trauma and diffuse peritonitis [2].
Gastrointestinal function
IAH causes diminished gut perfusion because of its effect on the splanchnic organs. That results in ischemia, acidosis of the mucosal bed, capillary leak, intestinal edema and translocation of gut bacteria. As IAP advances, pressure is placed on the arteries, capillaries, and veins in the abdominal cavity. The increased pressure may lead to diminished arterial blood flow to the organs and resistance to drainage into the veins [5,32].
Intramucosal acidosis results due to the diminished oxygenation to the gut. The ischemic intestine becomes more permeable to the intestinal contents as it loses its protective mucosal barrier. Edema in the intestinal wall causes further increases in IAP. An IAP of up to 20 mm Hg causes decreased mesenteric perfusion by 40% and pressures up to 40 mm Hg can reduce mesenteric perfusion by about 70%. The improvement in IAH can cause ischemia reperfusion injury and release inflammatory cytokines to other organs, initiating a multisystem organ failure. The reduced blood flow to the abdominal wall caused by increased IAP causes poor healing and possible dehiscence of abdominal surgical wounds [5].
Lymphatic flow in the thoracic duct significantly decreases with IAH and promptly increases after abdominal decompression [2]. Reduced intestinal perfusion predisposes to translocation of bacteria to the regional lymph nodes [2]. This microbial translocation is a predisposing factor for sepsis. Also there is a high risk for stress ulcers because of the loss of the mucosal barrier [5].
Splanchnic circulation
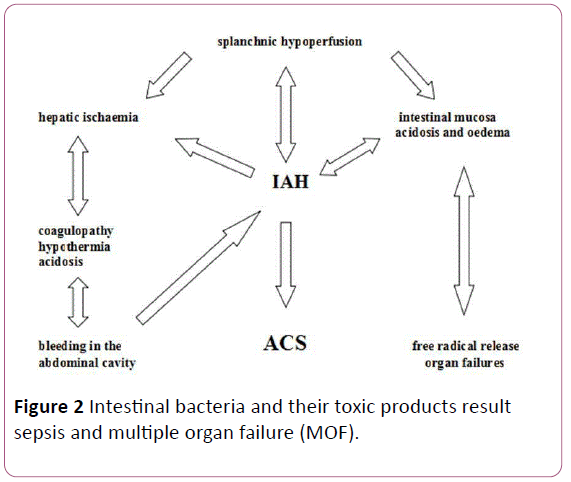
IAP above 15 mm Hg decreases the splanchnic, mesenteric, and hepatic perfusion. It is a well-known and proved fact that splanchnic and hepatic circulation has an auto regulation. The renin-angiotensin system, the HABR (hepatic arterial buffer response) and vasopressin are the basis of this auto regulation. Though numerous clinical studies proved that this complex system could compensate the consequences of insufficient arterial circulation, venous return, decreased preload due to the increased IAP only for few hours. When it works out it causes irreversible destruction of the intestinal mucosa and the liver. The reduced circulation results in a mucosal ischaemia and bacterial translocations. Blood flow to abdominal viscera is reduced by elevation in IAP. Splanchnic ischemia impairs subsequent intestinal barrier function and gastrointestinal motility. Intestinal bacteria and their toxic products result sepsis and multiple organ failure (MOF) (Figure 2).
Endotoxins or exotoxins cause a chain reaction of mediators that can damage either the pathogen or the human organism and if it becomes irreversible can cause death. This progressive clinical syndrome is a manifestation of multiple organic dysfunction or failure (ARDS: Adult Respiratory Distress Syndrome, Renal Failure; DIC: Disseminated Intravascular Coagulation). If it progresses the symptoms of multiple organ failure and septic shock with hypotonia will appear [4,18].
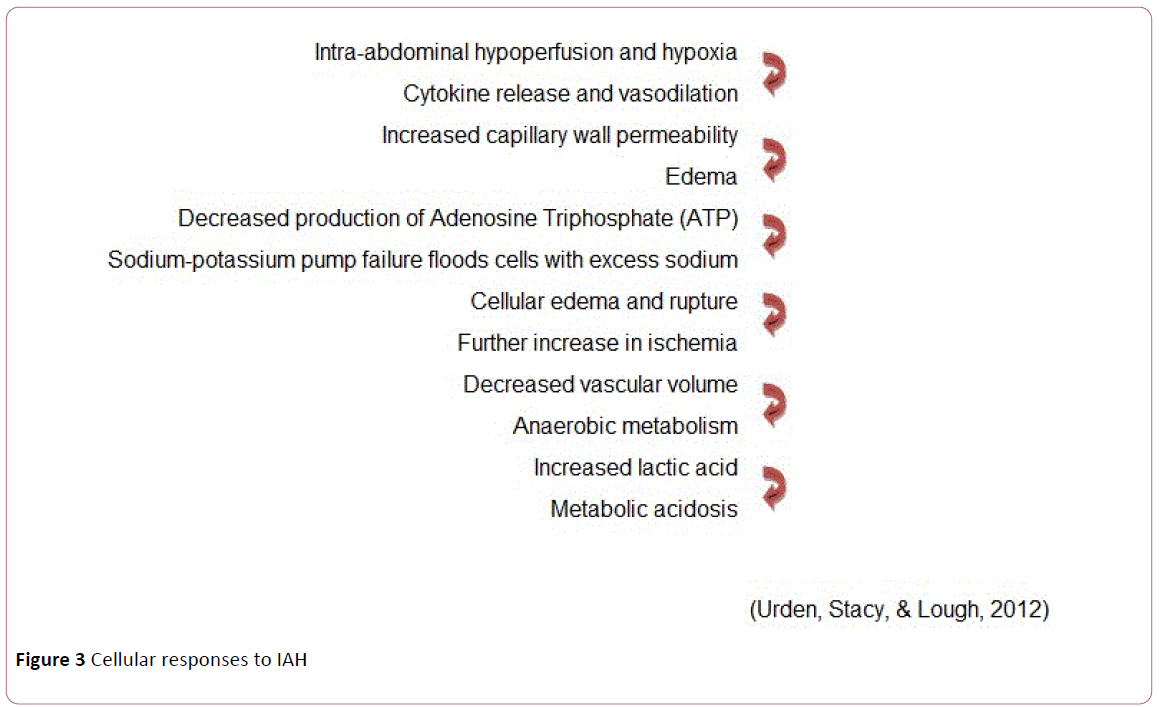
Cellular responses to IAH (Figure 3) [35]
Intracranial pressure (ICP)
Idiopathic intracranial hypertension increased in chronic abdominal hypertension [2]. The Cerebrospinal fluid and the brain’s venous drainage both leave the brain via the jugular vein. The increase in IAP is transferred into the thoracic compartment that puts back pressure on the jugular veins leading to increased intracranial pressure. In patients with increased intracranial pressure, the effects of IAH causes a further increase in the pressure in the cranium and decrease cerebral perfusion pressure [5].
Management of IAH and ACS
Non-surgical management
The World Society of Abdominal Compartment Syndrome suggests that any patient with two or more risk factors should be monitored for their intra-abdominal pressure and a proactive approach to intervention followed. Initial management can be classified into:
Lowering intra-abdominal pressure
Supine positioning and passing a nasogastric tube to decompress the stomach moderately impact decreasing intraabdominal pressure; however, the former procedure could increases the risk of. Enemas, flatus tubes, aperients, and prokinetic agents may benefit. Coughing, straining, and ventilator desynchrony all elevate intra-abdominal pressure, so adequate sedation is essential and a period of muscular paralysis may be important [26,36,37].
Organ support & fluids resuscitation management
Initial fluid resuscitation should be aimed at restoring normovolaemia to optimize cardiac output. Trends and response to fluid challenges may more beneficial than monitoring central venous pressure and pulmonary artery wedge pressures. Stroke volume variation and stroke volume index may be useful guides to fluid resuscitation [26,37].
Fluid overload may be detrimental in itself. Abdominal perfusion pressure of 60 mm Hg should be targeted during fluid resuscitation and inotropic support. Renal replacement therapy may be important and should be considered in all patients with commencing renal dysfunction. It may be appropriate to initiate early replacement rather than persist with large volume fluid resuscitation, with an elevated incidence of secondary abdominal compartment syndrome [36,37].
Lung protective strategies should be used. Enteral feeding should be continued if possible to maintain gut integrity, as the feed volume does not contribute significantly to intra-abdominal pressure; however, many patients may have primary or secondary gastrointestinal failure as guided with a four-hourly gastric aspiration [26,38].
Surgical management
Abdominal decompression by laparotomy (the ‘open abdomen’), has been shown to improve mortality in patients with abdominal compartment syndrome. The timing of surgical decompression has vital role. Temporary closure should be a primary event in patients thought to be at increased risk of abdominal compartment syndrome at the time of any intraabdominal procedure. In others who have developed abdominal compartment syndrome and where nonsurgical methods have failed, decompression should be performed as an emergency procedure [1,26,38].
Recent advances in temporary abdominal closure (TAC) techniques in managing open abdomen help to achieve many benefits without incurring much complication. The ideal TAC technique serves as a barrier, thus preventing evisceration and contamination. It assists with evacuation of abdominal fluid and decreases bowel edema. It prevents adhesions and avoids repeated damage to the bowel, fascia, or skin due to exposure. Furthermore, it allows easy access to the abdominal cavity, avoids damage to the fascial edges, prevents fistula formation and allows fistula isolation if present, and prevents abdominal wall retraction while allowing for expansion of abdominal contents to prevent the development of ACS. Different TAC techniques were categorized based on the definitions described by Boele van Hensbroek et al. (Table 4) [39].
| TAC Technique | Description |
|---|---|
| NPWT | A perforated plastic sheet is positioned to cover the intestine, a polyurethane sponge, or damp surgical towels/pads are placed on top, between the fascial edges. The wound is covered with an airtight seal and is centrally pierced by a suction drain, which is connected to a pump and fluid collection system. Self-made variations of this technique (using towels/gauzes) are commonly referred to as Barkers’ ‘‘Vacuum Pack’’. Commercial available systems include VAC Abdominal Dressing (KCI), Renasys NPWT (S&N), Avance (Mo¨lnlycke), and ABThera Open Abdomen Negative Pressure Therapy System (KCI). |
| NPWT with continuous fascial traction | Modification of NPWT, using a mesh or sutures sutured to the fascial edges, which can be tightened with every NPWT system change |
| Dynamic Retention Sutures | Extraperitoneally placed large, non-absorbable sutures through all layers of the abdominal wall, including the skin. Sutures can be gradually tightened. May be combined with a NPWT system. Commercial available systems include ABRA Abdominal Wall Closure System (Canica Design) |
| Wittmann patch (‘artificial burr’) | Two Velcro pieces are sutured to the fascial edges and facilitate gaining access to the abdominal cavity and gradual re-approximation of the abdominal wall. May be combined with a NPWT system. |
| Bogota bag | A sterile irrigation bag is sutured between the fascial edges. It can be reduced in size to approximate the fascial edges |
| Mesh | An absorbable or non-absorbable mesh is sutured between the fascial edges (usually ‘inlay’). The mesh can potentially be tightened gradually. Non-absorbable meshes can be removed or left in place. |
| Zipper | A mesh with a zipper is sutured between the fascial edges. It is comparable to mere mesh placement but allows for a more easy access to the abdominal cavity. |
| Loose packing | The fascial defect is covered by standard wound dressing. |
| TAC: Temporary Abdominal Closure; NPWT: Negative Pressure Wound Therapy | |
Table 4 Description of temporary abdominal closure techniques.
Key elements for abdominal decompression
Anaesthetic management of these patients should reflect that they are critically ill. There are four key elements related to patients with abdominal compartment syndrome [26].
• Pharmacokinetics/dynamics: Patients with ACS are highly sensitive to the cardiac depressant effects of induction agents due to liver dysfunction, altered drug handling, altered volume of distribution, and hypovolaemia. A decreased dose of drug and careful induction with invasive monitoring is important [26,38].
• Sudden decrease in intra-thoracic pressure: As the abdomen is opened, the intra-abdominal pressure rapidly equilibrates with atmospheric pressure. There is a consequent reduction in the intra-thoracic pressure. A dramatic elevation in respiratory compliance may occur, with the potential of ‘over ventilation’ and damage to lung parenchyma due to barotrauma and volutrauma. Therefore, close monitoring of airway pressures/tidal volumes should be paid [26].
• Sudden decrease in systemic vascular resistance: On opening the abdomen, afterload will be decreased as may cardiac output and arterial pressure. This may be profound, resulting in sudden cardiac arrest. Further fluid loading and/or vasopressors may be essential, and resuscitation drugs and equipment should be close at hand [26].
• Reperfusion injury: Finally, on opening the abdomen, previously ischemic areas of bowel and viscera may once again be perfused, leading to a systemic reperfusion insult with risk of myocardial depression, arrhythmias, and, on occasion, cardiac arrest. With those patients, senior anaesthetic staff should be available and extreme care and vigilance should be taken at induction of anaesthesia and on opening of the abdomen. Resuscitation drugs and equipment should be immediately available [26,40]. However, many of these reperfusion syndromes are hardly seen any more with much earlier decompression of the abdomen and not waiting till the IAP are massive and the ACS is full-blown.
The world society of abdominal compartment syndrome stated the following management [29]
Therapies to improve abdominal wall compliance
• Sedation and analgesia
• Neuromuscular blockade
• Consider supine position <20°- Avoid prone position
• Remove constrictive dressings and abdominal eschars
Therapies to evacuate intraluminal contents
• Nasogastric/colonic decompression
• Promotility agents
• Enemas
• Colonoscopic decompression
Evacuation of abdominal collections
• Percutaneous drainage
• Paracentesis
Management of fluids [15]
• Restriction of fluids/permissive hypotension in trauma
• Negative fluid balance
• Use of diuretics/albumin
• Haemodialysis/ultrafiltration
• Organ support and reducing capillary leak
• Maintain APP_60 mm Hg with vasopressors
• Optimize ventilation, alveolar recruitment
• Antibiotic therapy in septic patients
Collaborative management
Once IAH has been detected in a susceptible patient, the goal is to decrease the IAP to 15 mm Hg or less, maintains the APP at 60 mm Hg or greater, and prevent ACS [5]:
• The WSACS has developed a medical management algorithm [41] according to the causes of IAH and patient condition.
• The algorithm is set in a stepwise approach.
• Many of the recommendations are within the domain of bedside nurses while others are for physicians and advanced practice nurses.
• Recommendations include monitoring and recording daily bowel movements and implementing a bowel protocol before being constipated.
• Patients who are paralyzed, unconscious or sedated should be checked daily for faecal impactions.
• Abdominal radiographs and computed tomography reports should be reviewed for evidence of impacted faeces.
• Maintaining the patency of the nasogastric tube and the rectal tube is important.
• The amounts administered for enteral feeding should be decreased or feedings should be discontinued if residuals are greater than accepted levels.
• IAH should be re-evaluated as a possible cause of increases in residual volumes.
• For patients who can eat, gas-producing foods should be minimized or eliminated.
• Recommendations for evacuating intra-abdominal spaceoccupying lesions are part of the purview of physicians or advanced practice nurses.
• Bedside nurses ensure that the diagnostic studies are safely carried out and assist with any bedside interventional procedures.
• Positioning patients to achieve stability has been a mainstay of acute and critical care nurses’ practice.
• Recommendations to improve abdominal wall compliance include avoiding the prone position and elevating the head of bed more than 20° [36,37].
• Raising the head of the bed is a conflict with the recommendations of the ventilator bundle to prevent ventilator-associated pneumonia, which calls for elevating the head of the bed at least 30°.
• One way to compromise is to place patients in a reverse Trendelenburg position. However, when IAP is measured, patients must be supine with the head of the bed flat.
• The recommendations to improve abdominal wall compliance are interdisciplinary.
• Debriding of abdominal eschar and removal of constrictive abdominal dressings are advised.
• Patients who are in pain or are agitated should be given adequate doses of analgesics and sedatives and should be assessed for relief.
• As a last resort, patients may need to be intubated and given paralytic agents to decrease the effects of muscle contraction on IAP.
• Fluid replacement is a known risk factor for IAH, especially if a patient has capillary leak.
• Monitoring and recording daily intake and output and assessing cumulative fluid balance are important nursing actions in managing these patients.
• The recommendations for optimizing fluid administration are to avoid excessive fluid administration and to aim for a goal of an equal or negative fluid balance by the third day in the ICU.
• If nurses have a fluid replacement protocol to follow and the protocol includes an option to use colloids or crystalloids; the colloids should be chosen.
• The last category in the medical management algorithm is optimizing systemic and regional perfusion.
• Again, goal-directed fluid replacement is recommended.
• If the APP cannot be maintained at 60 mm Hg or greater with fluids, inotropes or vasopressors can be given. This category includes the need for hemodynamic monitoring to guide fluid replacement. IAH causes fictitious elevations in the CVP and PAWP. To negate this effect, the WSACS2 recommends using the following correction formula:
• CVP corrected=CVP measured - (IAP/2)
• PAWP corrected=PAWP measured - (IAP/2)
Summary
Intra-abdominal Hypertension (IAH) does not only affect abdominal organs but also affects different organ systems. Rising or sustained IAP may lead to a poor prognosis, and the development of ACS. So, Monitoring, early identification and treatment of IAP could prevent the development of ACS and improve prognosis in critically ill patients.
References
- Ball CG, Kirkpatrick AW (2007) Intra-abdominal hypertension and the abdominal compartment syndrome. Scand J Surg 96: 197-204.
- Wittmann DH, Iskander GA (2000) The compartment syndrome of the abdominal cavity: A state of the art review. J Intensive Care Med 15: 201-220.
- Bajouh O, Assidi M (2013) Unexpected Abdominal Compartment Syndrome Following a Diagnostic Hysteroscopy. Middle East J Sci Res 16: 919-921.
- Bodnár Z (2012) Intra-abdominal hypertension and abdominal compartment syndrome in critically ill surgical patients (special findings in severe acute Pancreatitis). In; Prof. Luis Rodrigo Saez (Ed.) Pancreatitis - Treatment and Complications. In Tech, pp: 163-180.
- Lee RK (2012) Intra-abdominal hypertension and abdominal compartment syndrome: A comprehensive overview. Crit Care Nurse 32: 19-31.
- Hill L, Hill B, Miller M, Michell WL (2011) The effect of intra-abdominal hypertension on gastro-intestinal function. S Afr J Crit Care 27: 12-19.
- Maerz L, Kaplan L (2008) Abdominal compartment syndrome. Crit Care Med 36: S212.
- Arabadzhiev G, Ivanov V, Peeva K (2014) Intra-abdominal hypertension and secondary abdominal compartment syndrome in medical patients–complication with a high mortality. Trakia J Sci 12: 202-207.
- De Waele JJ, Malbrain ML, Kirkpatrick AW (2015) The abdominal compartment syndrome: Evolving concepts and future directions. Crit Care 19: 211.
- Hunt L, Frost SA, Hillman K, Newton PJ, Davidson PM (2014) Management of intra-abdominal hypertension and abdominal compartment syndrome: a review. J Trauma Manag Outcomes 8: 2.
- Mohmand H, Goldfarb S (2011) Renal dysfunction associated with intra-abdominal hypertension and the abdominal compartment syndrome. J Am Soc Nephrol 22: 615-621.
- Ogilvie W (1940) The late complications of abdominal war-wounds. Lancet 236: 253-257.
- Malbrain ML (2012) Different techniques to measure intra-abdominal pressure (IAP): Time for a critical re-appraisal. Intensive Care Med 30: 357-371.
- Emerson H (1911) Intra-abdominal pressures. Arch Intern Med 7: 754-784.
- Fietsam Jr R, Villalba M, Glover JL, Clark K (1989) Intra-abdominal compartment syndrome as a complication of ruptured abdominal aortic aneurysm repair. Am Surg 55: 396-402.
- Harman PK, Kron IL, Nolan SP (1984) The measurement of intra-abdominal pressure as a criterion for abdominal re-exploration. Ann Surg 199: 28-30.
- Ball CG, Kirkpatrick AW, McBeth P (2008) The secondary abdominal compartment syndrome: Not just another post-traumatic complication. Can J Surg 51: 399-405.
- Kyoung KH, Hong SKN (2015) The duration of intra-abdominal hypertension strongly predicts outcomes for the critically ill surgical patients: A prospective observational study. World J Emerg Surg 10: 22.
- Sugrue M, De Waele JJ, De Keulenaer BL, Roberts DJ, Malbrain ML (2015) A user’s guide to intra-abdominal pressure measurement. Anaesthesiol Intensive Ther 47: 241-251.
- Starkopf J, Tamme K, Blaser AR (2012) Should we measure intra-abdominal pressures in every intensive care patient? Ann Intensive Care 2: S9.
- Holodinsky JK, Roberts DJ, Ball CG, Blaser AR, Starkopf J, et al. (2013) Risk factors for intra-abdominal hypertension and abdominal compartment syndrome among adult intensive care unit patients: A systematic review and meta-analysis. Crit Care 17: R249.
- Biancofiore G, Bindi ML, Romanelli A, Boldrini A, Consani G, et al. (2003) Intra-abdominal pressure monitoring in liver transplant recipients: A prospective study. Intensive Care Med 29: 30-36.
- Bozeman MC, Ross CB (2012) Intra-abdominal hypertension and abdominal compartment syndrome in association with ruptured abdominal aortic aneurysm in the endovascular era: vigilance remains critical. Crit Care Res Pract 151650.
- Morejón CdDS, Barbeito TOT (2012) Effect of mechanical ventilation on intra-abdominal pressure in critically ill patients without other risk factors for abdominal hypertension: An observational multicenter epidemiological study. Ann Intensive Care 1: S22.
- Dalfino L, Sicolo A, Paparella D, Mongelli M, Rubino G, et al. (2013) Intra-abdominal hypertension in cardiac surgery. Interact Cardiovasc Thorac Surg 17: 644-651.
- Berry N, Fletcher S (2012) Abdominal compartment syndrome. Continuing Educ Anaesth Crit Care Pain 12: 110-117.
- Gaidukov KM, Raibuzhis EN, Hussain A, Teterin AY, Smetkin AA, et al. (2013) Effect of intra-abdominal pressure on respiratory function in patients undergoing ventral hernia repair. World J Crit Care Med 2: 9-16.
- Lopes AM, Nunes A, Niza MM, Dourado A (2016) Intra-abdominal pressure is influenced by body position? Am J Clin Med Res 4: 11-18.
- Cheatham ML (2009) Abdominal compartment syndrome: Pathophysiology and definitions. Scand J Trauma Resusc Emerg Med 17: 10.
- Falcão ALE, de Oliveira DG (2011) Intra-abdominal hypertension associated with acute lung injury: Effects on intracranial pressure. Rev Bras Ter Intensiva 23: 117-119.
- De Waele JJ, De Laet I, Kirkpatrick AW, Hoste E (2011) Intra-abdominal hypertension and abdominal compartment syndrome. Am J Kidney Dis 57: 159-169.
- Asencio CM, Fleiszig ZB (2015) Intra-abdominal hypertension and abdominal compartment syndrome in acute pancreatitis. In; Prof. Luis Rodrigo (Ed.) Acute and Chronic Pancreatitis, In Tech.
- Yang C, Yang Z, Chen X, Liu T, Gou S, et al. (2015) Inverted U-shaped relationship between central venous pressure and intra-abdominal pressure in the early phase of severe acute pancreatitis: A retrospective study. PloS One 10: e0128493.
- Torquato JA, Lucato JJJ, Antunes T, Barbas CV (2009) Interaction between intra-abdominal pressure and positive-end expiratory pressure. Clinics 64:105-112.
- Urden LD, Stacy KM, Lough M (2010) Critical care nursing. Elsevier.
- Al-Dorzi HM, Tamim HM, Rishu AH, Aljumah A, Arabi YM (2012) Intra-abdominal pressure and abdominal perfusion pressure in cirrhotic patients with septic shock. Ann Intensive Care 2: S4.
- Kirkpatrick AW, Ball CG, D'Amours SK, Zygun D (2008) Acute resuscitation of the unstable adult trauma patient: Bedside diagnosis and therapy. Can J Surg 51: 57-69.
- An G, West MA (2008) Abdominal compartment syndrome: A concise clinical review. Crit Care Med 36: 1304-1310.
- Hensbroek PBV, Wind J, Dijkgraaf MG, Busch OR, Goslings JC (2009) Temporary closure of the open abdomen: A systematic review on delayed primary fascial closure in patients with an open abdomen. World J Surg 33: 199-207.
- Kirkpatrick A, Roberts D, De Waele J, Jaeschke R, Malbrain M, et al. (2013) Pediatric Guidelines Sub-Committee for the World Society of the Abdominal Compartment Syndrome. Intra-abdominal hypertension and the abdominal compartment syndrome: Updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med 39: 1190-1206.
- Cheatham ML (2009) Non-operative management of intra-abdominal hypertension and abdominal compartment syndrome. World J Surg 33: 1116-1122.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences