Cocktail of Infections in a Lupus Patient
Sir Ganga Ram Hospital Rheumatology, Rajinder Nagar, New Delhi, India
- Corresponding Author:
- Jeet Patel
Sir Ganga Ram Hospital Rheumatology
Rajinder Nagar, New Delhi, India
Tel: +919821808588
E-mail: jeet5patel5@gmail.com
Received date: May 28, 2018; Accepted date: July 23, 2018; Published date: July 28, 2018
Citation: Patel J, Duggal L, Jain N, Dhawan S, Kansal S, Bhandari G (2018) Cocktail of Infections in a Lupus patient. J Immunol Microbiol Vol.2 No.2:1
Copyright: © 2018 Jeet Patel, This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Infections have complex interactions with Systemic Lupus Erythematosus (SLE). They can complicate clinical course of lupus at any point of time. Inefficient immune system increases risk of infections in lupus patients in addition to immunosuppressive agents. Uncommon fungi, viruses, parasites and bacteria must be kept in mind while treating lupus patients. This case of Systemic Lupus Erythematosus (SLE) had multiple infections. Amongst them, systemic mucormycosis was the most unusual one while CMV infection was another one. H1N1 influenza, pseudomonas, and E.coli were other co-existing infections which had contributed in mortality of this patient. Systemic mucormycosis can involve different organs depending upon underlying disease and immune status of patient lungs, gastrointestinal tract, paranasal sinuses etc. More than one infection can co-exist in same patient of SLE due to multiple breaks in defence mechanisms of innate and adaptive immune system. Infections and lupus is a dreadful combination and increases morbidity and mortality significantly.
Keywords
Systemic lupus erythematosus (SLE); Haemorrhagic; Anti-malarial; Mucormycosis; Cytomegalovirus (CMV)
Introduction
Infections can present at onset of lupus, can complicate its clinical course and can cause flare of lupus activity. Inefficient immune system increases the risk of infections in lupus patients in addition to immunosuppressive medications. Unusual infections like fungal infections, rarer viral infections or parasitic infections must be kept in mind in addition to usual bacterial infections. Systemic Lupus Erythematosus (SLE) patients are prone for opportunistic infections (up to 40%) due to cumulative intrinsic and extrinsic immune system breaches. Most important factor to restrict infections in SLE patients is the shortest possible use of lowest dose of glucocorticoids (GC) [1]. Urinary tract and respiratory tract are common site for infections while sepsis with or without shock is the major cause of death due to infections.
It is clear that the rate of infections is increased in SLE patients, whatever may be the cause for it: Disease activity, disease duration or GC dose/duration [2,3]. Infections are second common cause of flare after non-compliance of medications in SLE cases (approximately responsible for 27% flares). [4] We here by describe a case of SLE in which we found multiple infections. Amongst them, systemic mucormycosis was the most unusual while cytomegalovirus (CMV) infection was present due to its special association in SLE (because of hypocomplementemia). H1N1 influenza, pseudomonas, and E.coli were other co-existing infections which contributed in adding fuel to failing immune system.
Case History
A 22 year old drug defaulter lady of SLE (polyarthritis, oral ulcers, alopecia, and acute cutaneous lupus, positive antinuclear antibody (ANA) 1:1200 end titre, and antibody to double stranded DNA (anti-dsDNA), and low complements) presented with anorexia, constitutional symptoms and icterus. She had intermittent moderate to high grade fever, cough with expectoration, and swelling & redness over face. She was treated as viral hepatitis outside and did not improve. In addition, she developed vomiting, abdominal pain, generalized body swelling and decreased urine output. On examination she had tachycardia, tachypnoea, hypotension, icterus, pallor, anasarca, mouth ulcer over hard palate, oral thrush, facial swelling with erythematosus hue over cheeks, hepatosplenomegaly and crepitations in both lung fields.
Hemogram showed anaemia, severe leukopenia [Total leukocyte count (TLC)-200], severe thrombocytopenia (Platelet count-4000). Liver function tests showed indirect hyperbilirubinemia, hypoalbuminemia, and normal liver enzymes. Kidney functions were altered [Raised blood urea nitrogen (BUN) S. creatinine]. Anti-HAV IgM was mildly positive while tests for other hepatic virus were negative. S. ferritin was high (1619). Serum Procalcitonin was more than 50 and initial blood, urine, sputum cultures were negative. Anti-dsDNA was high (more than 150) and complements were low. Bone marrow biopsy showed significant histolytic hemophagocytosis suggestive of macrophage activation syndrome. Intravenous immunoglobulin was given (total 2 g/kg over 5 days) in addition to tablet cyclosporine (100 mg/day).
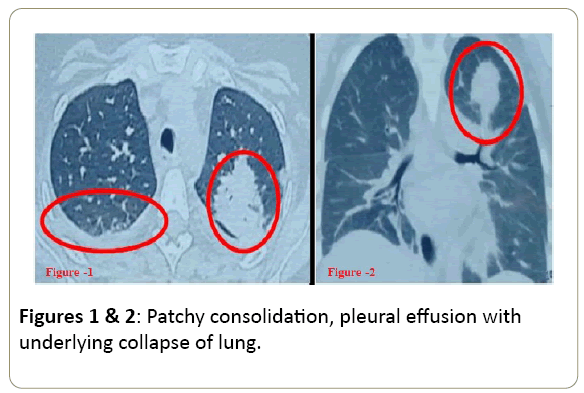
Chest X ray (CXR) revealed bilateral opacities. Real time polymerase chain reaction (RT-PCR) test for H1N1 influenza was sent due to endemicity of H1N1 in India and it came positive. Tablet Oseltamivir was given for 10 days (75 mg twice a day per oral). Computed Tomography (CT) chest revealed patchy consolidation, nodular lesions, and mild pleural effusions. CT Paranasal sinuses (PNS) were normal (Figures 1 and 2). Injectable Amphotericin (AMP-B) was started as an empirical anti-fungal agent along with broad spectrum antibiotics (meropenem teicoplanin). In view of history of fever, jaundice, mouth ulcer, cytopenias, lung lesions, CMV was also kept in mind. CMV PCR was already sent and came positive (26175 copies/dl). Injection Gancyclovir was started intravenously 5 mg/kg twice a day. Patient improved, became afebrile, CXR cleared and TLC improved although platelet were low. Patient was shifted out of intensive care unit (ICU).
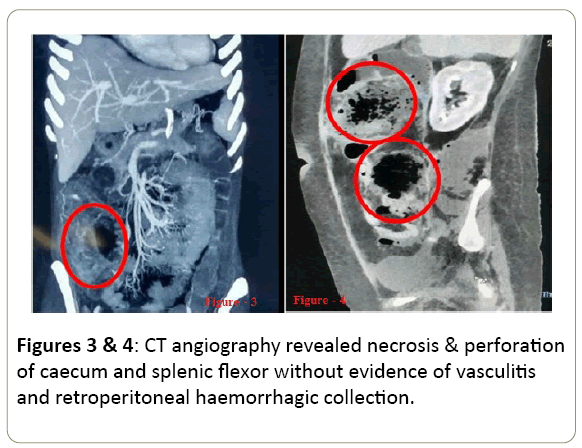
After 14 days, inj. Amphotericin-B was stopped as blood and sputum culture for fungus were negative. After 03 days of stopping injection AMP-B, she developed acute onset severe abdominal pain, massive bleeding P/R, abdominal distension, and hypotension. CT abdominal angiography did not reveal any evidence of vasculitis, albeit it did show evidence of necrosis of the cecum and ascending colon, necrosis and perforation in splenic flexure associated with haemorrhagic collection in the retro peritoneum (Figures 3 and 4). Patient was operated urgently and right hemi colectomy was done. Patient improved, stoma was created. Nasogastric (NG) tube feed was started after Post-Operative Day-2 (POD-2), NG tube was removed after POD-5 and oral feed was started. Tablet cyclosporine was withheld and corticosteroids were continued throughout the period in septic dose (Injection Hydrocortisone 50 mg thrice a day).
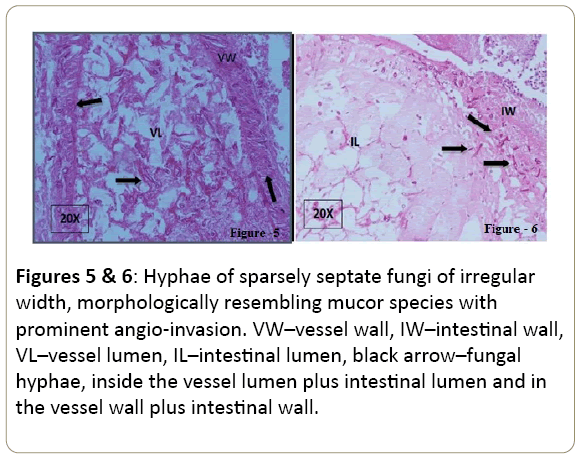
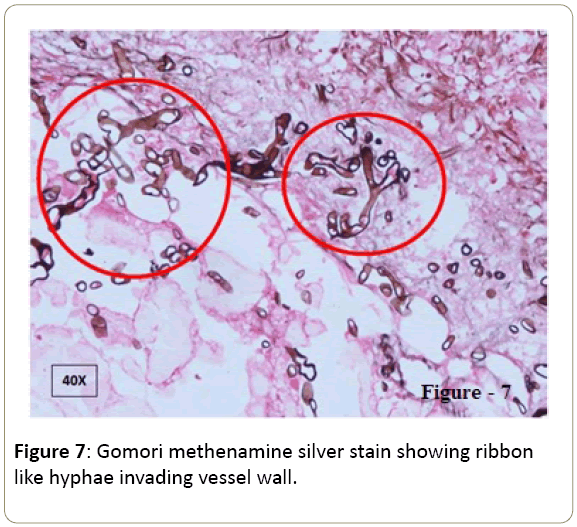
Biopsy specimens of colon showed focal mucosal ulceration, dense mixed inflammatory cell infiltrate, and necrotic foci filled with hyphae of sparsely septate, branching fungi, having irregular width, morphologically resembling mucor species with prominent angio-invasion (Figures 5-7). Injection AMP-B was restarted in addition of syrup Posaconazole. Antibiotics were changed to colistin, tigecyclin, and cefipime. At POD-11, she developed fever and CT abdomen revealed fluid collection in right colonic pouch and pelvis. Two intra-abdominal drains were inserted and pus drained which grew E.coli. Repeat sputum culture grew pseudomonas. Repeat sputum, blood and drain pus culture for fungus did not grow any fungus. At POD-18, she developed multi-organ dysfunction syndrome (MODS) and succumbed ultimately.
Figure 5 and 6: Hyphae of sparsely septate fungi of irregular width, morphologically resembling mucor species with prominent angio-invasion. VW–vessel wall, IW–intestinal wall, VL–vessel lumen, IL–intestinal lumen, black arrow–fungal hyphae, inside the vessel lumen plus intestinal lumen and in the vessel wall plus intestinal wall.
Discussion
Infections are common cause of mortality and morbidity in SLE and significantly increase economic burden on health care system [5]. There is a rising trend of infections in lupus patients like respiratory tract infections, urinary tract infections, skin infections, sepsis and rare opportunistic infections like fungal infections, uncommon viral infections. Analysis of Ward and colleagues showed 52% increase in skin infections needing hospitalization and 190% increase in sepsis.
The rise of infections in lupus patients might be due to increase detection of infections by modern tools and techniques or due to use of stronger immunosuppressive medicines. Lupus patients have twelve times higher risk of infections compared to general population. Inefficient immune system predisposes patients to infections while infections by increasing cellular death, increase auto antigenic debris and ultimately increase autoantibody production and lead to further immunosuppression. Infections and SLE make a self-propagating vicious cycle which makes them both a lethal combination [6]. Complement deficiency is also a risk factor for capsulated organism infections (e.g. pneumococcal pneumonia) and salmonellosis [7]. Lymphopenia breaks Cell Mediated Immunity (CMI) and increases risk for infections where CMI plays a major role e.g. mycobacterial infections, and viral infections while neutropenia increases risk for bacterial infections and fungal infections [8].
55 cases had cryptococcal meningitis in lupus patients and up to 40% were misdiagnosed. At least up to 40% patients had increased lupus activity at the time of cryptococcal infection [9]. A study from northern India showed that 2.6% of 309 lupus patients had multiple infections as in this case. Approximate 25% had infections and tuberculosis was the commonest infection in their study [10].
There was also increase in rare fungal opportunistic infections like mucormycosis, pneumocystis jiroveci pneumonia, aspergillosis etc. Systemic mucormycosis may have different organ involvement depending upon underlying disease and immune status of patient [11,12]. Pulmonary mucormycosis may spread to other organs especially, thoracic cavity and abdominal cavity structures. Chest X ray may show opacities, pleural effusion, prominent hila, etc. CT chest may have crescent sign or halo sign which often confuses with aspergillosis. Detection of fungal hila in specimen is gold standard for diagnosis although detection in BAL fluid, sputum specimen may be carried out when biopsy is not possible. PCR base techniques can also help in diagnosis. Treatment for mucormycosis includes Amphotericin B, and Posaconazole, caspofungin and micafungin. Other antifungal are not effective against mucormycosis while they act well in aspergillosis [13].
CMV infection is notorious one which can complicate clinical course of lupus. CMV has diffuse manifestations that can reflect SLE flares, e.g. hepatitis, thrombocytopenia, vasculitis (especially CNS vasculitis), pancreatitis, colonic perforation, pneumonia and cavitary lung lesions. Gancyclovir is standard of care although duration in SLE patients is still not evaluated properly [14]. This case only shows that infections not only trouble the patient but can even endanger the life in lupus. Sometimes, even after proper use of broad spectrum antibiotics, empirical anti-fungal, anti-tubercular and anti-viral medicines, salvage is difficult due to irreversible damage caused by deadly organisms.
To learn from this case, it is advisable to vaccinate lupus patients against common infectious microbes. Vaccination is recommended to prevent infections in SLE patients, particularly pneumococcal vaccine, annual influenza vaccine, and hepatitis B vaccine [15]. Anti-malarial have shown to protect against infections in addition to sustained proven beneficial effects on overall survival, disease free survival and on cumulative damage [16]. Thus, this case has pointed towards importance of adherence to treatment as patient had history of six months drug default (Table 1).
| Possible Strategies to Prevent Infections in SLE patients | |
|---|---|
| No. | Actions to be taken |
| 1 | Anti-malarial (Hydroxychloroquine) |
| 2 | Treatment adherence, low disease activity & remission |
| 3 | Vaccination |
| 4 | Minimum possible use ( lowest dose & shortest duration) of glucocorticoids |
| 5 | Patient education about disease, health and hygiene |
| 6 | Universal precautions during operative procedures and hospital stay |
| 7 | Justifiable use of stronger immunosuppressive agents |
Table 1: Shows possible measures to decrease mortality/ morbidity due to infections in SLE patients.
Conclusion
More than one infection can co-exist in same patient of SLE due to multiple breaks in defence mechanisms of innate and adaptive immune system. A treating doctor should be aware of possibilities of rarer fungal infections like systemic mucormycosis, Rizopus, histoplasmosis, systemic candidiasis etc. and viral infections like CMV, Epstein Barr Virus (EBV), H1N1 influenza etc. Infections and lupus is a dreadful combination and increases morbidity and mortality significantly. Anti-malarial agents, vaccination and legitimate use of immunosuppressive agents can help to prevent infections.
References
- Al-Rayes H, Al-Swailem R, Arfin M, Sobki S, Rizvi S (2007) Systemic lupus erythematosus and infections: a retrospective study in Saudis. Lupus 16: 755-63.
- Duffy KN, Duffy CM, Gladman DD (1991) Infection and disease activity in systemic lupus erythematosus: a review of hospitalized patients. J Rheumato 18: 1180-4.
- John VS, Vasanthi S, Rathnapriya N (2016) A Study on Bacterial and Fungal Profile of Infections in Patients with SLE. Int J Curr Microbiol App Sci 5: 431-438.
- Kakati S, Teronpi R, Barman B (2015) Frequency pattern and determinants of flare in systemic lupus erythematosus: a study from North East India. Egyptian Rheumatol 37: S55-S59.
- Ginzler EM, Dvorkina O, Wallace DK, Hahn BH (2006) Infections in systemic lupus erythematosus. In: Dubois lupus erythematosus. Philadelphia: Lippincott, Williams & Wilkins 901: 10.
- Doaty S, Agrawal H, Bauer E, Furst DE (2016) Infection and Lupus: Which Causes Which? Curr Rheumatol Rep 18: 1523-3774.
- Tsao CH, Chen CY, Ou LS, Huang JL (2002) Risk factors of mortality for Salmonella infection in systemic lupus erythematosus. J Rheumatol 29: 1214-1218.
- Chu CM, Ng WL, Wu AKL, Cheng VCC, Yuen KJ (2001) Lymphopenia predicts tuberculosis in systemic lupus erythematosus. Am J Resp Crit Care Med :163
- Fang W, Chen M, Liu J, Hagen F, Guo Y, et al. (2016) Cryptococcal meningitis in systemic lupus erythematosus patients: pooled analysis and systematic review. Emerging Micro & Infec 10: 1038-1093.
- Shyam C, Malaviya AN (1996) Infection- in systemic lupus erythematosus: related morbidity a clinico-epidemiological study from northern India. Rheumatol Int 16: 10-3.
- Hung HC, Shen JY, Chen SC, Yeo Kj, Tsao SM, et al. (2015) Pulmonary Mucormycosis in a Patient with Systemic Lupus Erythematosus: A Diagnostic and Treatment Challenge. Infec Dise.
- Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, et al. (2005) Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infe dis 41: 634-653.
- Hackem RY, Langston AA, Graybill JR, Perfect JR, Pedicone LD, et al. (2008) Posaconazole as salvage treatment of invasive fungal infections in patients with underlying renal impairment. J anti chemo 62: 1386-1391.
- Sekigawa I, Nawata M, Seta N, Yamada M, Lida N, et al. (2002) Cytomegalovirus infection in patients with systemic lupus erythematosus. Clin Exp Rheumatol 20: 559-564.
- Murdaca G, Orsi A, Spanò F, puppo F, Durando P, et al. (2016) Vaccine-preventable infections in Systemic Lupus Erythematosus. Human Vacc & Imm 12: 632-643.
- Alarcon GS, McGwin G, Bertoli AM, Fessler BJ, Calvo-Alen J, et al. (2007) Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multi-ethnic US cohort. Ann Rheum Dis 66: 1168-72.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences