ISSN : 0976-8505
Der Chemica Sinica
Ciprofloxacin hydrochloride as a Potential inhibitor of Copper Corrosion in 1M HNO3
S Ouattara, PM Niamien*, EB Avo Bilé and A Trokourey
Laboratory of Physical Chemistry, Félix Houphouët Boigny University, Abidjan, Côte d'Ivoire
Abstract
Ciprofloxacin hydrochloride has been tested as an inhibitor of copper corrosion in 1.0 M nitric acid, using mass loss technique and DFT (Density Functional Theory) calculations. The experimental studies have been performed in the concentration range of 0.05 mM to 1 mM and the temperature range of 308 K to 328 K. The results show that the inhibition efficiency of the studied molecule is concentration and temperature dependent. The fractions of surface coverage and the related concentrations were used to fit adsorption isotherms including, Langmuir, El-Awady, Temkin, Freundlich and Flory Huggins. Though the Langmuir isotherm was found to be the best isotherm, it could not be applied strictly because of deviation from the assumptions used to derive this isotherm. So, the appropriate isotherm was the modified Langmuir isotherm. To solve the ambiguity usually associated with the characterization of the adsorption processes of organic compounds, the Dubinin-Radushkevitch isotherm and that of Adejo-Ekwenchi were used. Furthermore, DFT calculations based on B3LYP functional and 6-31G (d) basis set were used to derive the molecular properties of ciprofloxacin. The relationships between the inhibition efficiency and some molecular descriptors have been discussed. The Fukui functions were calculated and used to locate the sites of electrophilic and nucleophilic attacks via the dual descriptor. Quantum chemical studies corroborate experimental results.
Keywords
Copper, Nitric acid, Ciprofloxacin hydrochloride, Corrosion inhibition, Mass loss, DFT, Chemical descriptors
Introduction
Due to its good mechanical and electrical properties [1] copper is used in many applications, including, electronics, ware production, sheets, tubes, etc. It is resistant to many chemicals; however, it is known [2,3] that in aggressive media such as acid ones, it undergoes corrosion. Corrosion processes are important matters in industries and academic fields. Many methods have been used to protect metals from it. The use of corrosion inhibitors of the best known methods to combat metal dissolution due to the low cost and easy use [4-6]. Inhibitors have great acceptance in the industries due to their excellent anti-corrosive properties. However, many of them cause damage to the environment. Thus, many researchers lead their works [7-10] toward eco-friendly inhibitors like organic molecules. These organic compounds [11] contain nitrogen, sulphur and/or oxygen atoms. Moreover, many heterocyclic compounds [12,13] have been revealed to be effective inhibitors for metal corrosion in acidic media. The protection of the metal is achieved by adding a small quantity of a chemical compound in its nearby environment. Generally, the inhibition mechanism [14] leads to a uniform film, which as a coating, acts as a physical barrier which isolate the metal from its environment.
Many papers have documented the use of medicinal compounds such as penicillin G [15], nizoral [16], cefixime [17] riboflavin [18], pyridoxine hydrochloride [19], etc.
The aim of the present paper is to investigate experimentally and theoretically, the inhibiting properties of ciprofloxacin hydrochloride against copper corrosion in 1.0 M nitric acid solution, using mass loss technique and DFT studies.
Materials and Methods
Mass loss method
For mass loss measurements, the copper was of chemical composition in percentage Pb: 0.005, Al: 0.006, Fe: 0.006, S: 0.002 and the remainder Cu: 99.8. A sample of 10 mm in length and 2.2 mm in diameter was used. The samples were polished with different grade emery papers up to 4/0 grade, cleaned with acetone, washed with doubly distilled water and dried. The cleaned samples were weighed before immersion in the nitric acid solution for 1 h in the absence or in the presence of various concentrations of ciprofloxacin hydrochloride (molecular formula: C17H18FN3O3, HCl) from Sinopharm Chemical Reagent Co, at different temperatures. The mass loss method [20] is the most used method of inhibition assessment due to the simplicity and reliability of the measurements; this technique forms the baseline method of measurements in many corrosion monitoring programs. All tests were made in aerated solutions and were run triplicate to guarantee the reliability of the results. The corrosion rate (W) was calculated according to the equation below:
W=(m1-m2)/St (1)
Where m1 and m2 are respectively the mass (in g) before and after immersion in the test solution, S is the total surface of the sample (in cm2) and t is the immersion time (h). The inhibition efficiency IE (%) is calculated, using the relation below:
IE(%)=(wo-w/wo) × 100 (2)
In equation (2), wo and w are respectively the corrosion rates of copper in the absence and the presence of the tested compound.
Computational method
In order to obtain more information about the molecular descriptors and the inhibitory action of ciprofloxacin, quantum chemical calculations were performed, using Density Functional Theory (DFT). The ground state energy and the physical properties of ciprofloxacin have been calculated, using the Gaussian 03W [21] package. The molecular structure was optimized to a minimum without symmetry restrictions, using B3LYP exchange correlation functional of Lee, Yang and Parr [22,23] associated with 6-31G (d) basis set [24].
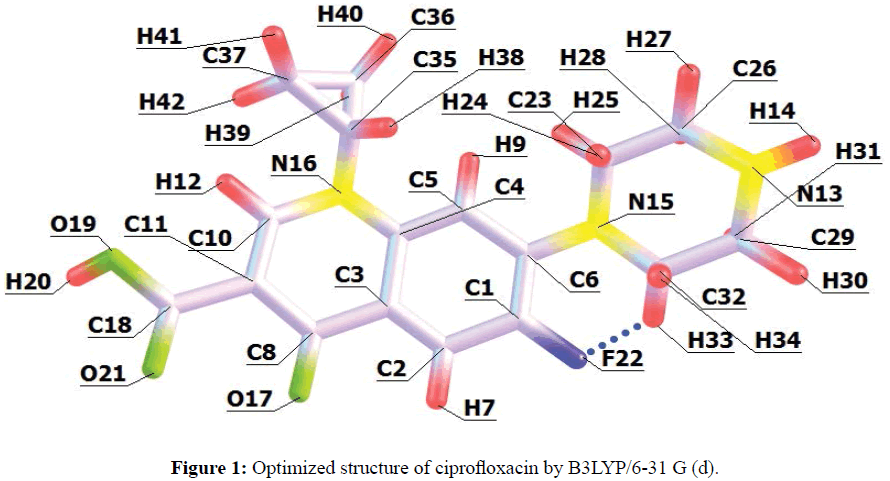
Quantum chemical calculations were carried out in the gas phase to ascertain if there is a clear relationship between the molecular descriptors of ciprofloxacin and its inhibition efficiency. The optimized minimum energy geometrical configuration of ciprofloxacin is shown in Figure 1.
DFT [25] has been found to be successful in providing theoretical insights into the chemical reactivity and selectivity of molecules via chemical concepts like electronegativity (χ), hardness (η), softness (S), electrophilicity index (ω) and local reactivity descriptors including Fukui functions 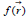 and the local softness
and the local softness 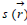 .
.
Density functional theory (DFT) states that changes in electronic energy dE[ρ(r)] are related to changes in the number of electrons N and changes in the external potential v(r) felt by the electron distribution (which refers to the nuclear position in chemical systems):
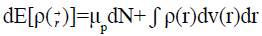 (3)
(3)
According to Parr et al. [26], the chemical potential μp is linked with the first derivative of the energy in respect with the number of electrons and therefore with the negative of the electronegativity by the following equation:
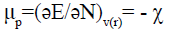 (4)
(4)
Where μp is the electronic chemical potential, E is the total energy, N is the number of electrons, v(r) is the external potential of the system.
The second partial derivative of the energy with respect to the number of electrons has been defined as hardness (η):
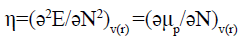 (5)
(5)
This quantity [27] measures both the stability and the reactivity of the molecule.
According to Koopman’s theorem [27], the ionization potential (I) and the electron affinity (A) of the inhibitors are calculated using the following equations:
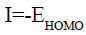 (6)
(6)
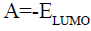 (7)
(7)
The electronegativity (χ) [28] which measures the power of an atom or group of atoms to attract electrons towards itself can then be written as:
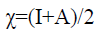 (8)
(8)
The chemical hardness (η) [29] which expresses the resistance of an atom to charge transfer is estimated using the equation below:
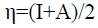 (9)
(9)
The inverse of the hardness known as softness (S) [29] measures the capacity of an atom or group of atoms to receive electrons; it is estimated by the equation:
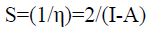 (10)
(10)
The fraction of electrons transferred from the inhibitor molecule to the metallic surface was calculated using the following equation [30]:
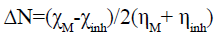 (11)
(11)
Where χM, ηM, χinh and ηinh are respectively the electronegativity and hardness of the metal and the inhibitor. In our study, the theoretical values of electronegativity χcu=4.98eV [31] and hardness ηcu=0 [30] have been used for copper.
The global electrophilicity index, introduced by Parr [32] is given by the equation below:
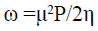 (12)
(12)
This index [32] measures the propensity of chemical species to accept electrons. A good nucleophile is characterized by a low value of ω whereas a good electrophile is characterized by a high value of ω.
Fukui function [33] is one of the widely used local density functional descriptors to model chemical reactivity and site selectivity; it is defined as the derivative of the electron density 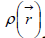 with respect to the total number of electrons N in the system, at constant external potential
with respect to the total number of electrons N in the system, at constant external potential 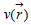 acting on an electron due to all the nuclei in the system:
acting on an electron due to all the nuclei in the system:
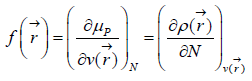 (13)
(13)
The condensed Fukui functions are calculated using Yang and Mortier [34] procedure based on a finite difference method:
 (14)
(14)
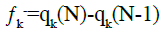 (15)
(15)
Where qk is the electronic population of atom k in the molecule. The functions ƒk+ and ƒk- are respectively, related to nucleophilic attack and electrophilic attack.
Recently, some authors [35] have proposed a dual descriptor (Δƒ(r)), which is defined as the difference between the nucleophilic and electrophilic Fukui functions:
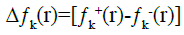 (16)
(16)
If Δƒk(r)>0, then the site is favoured for a nucleophilic attack, whereas if Δƒk(r)<0, then the site may be favoured for an electrophilic attack.
Results and Discussion
Mass loss method
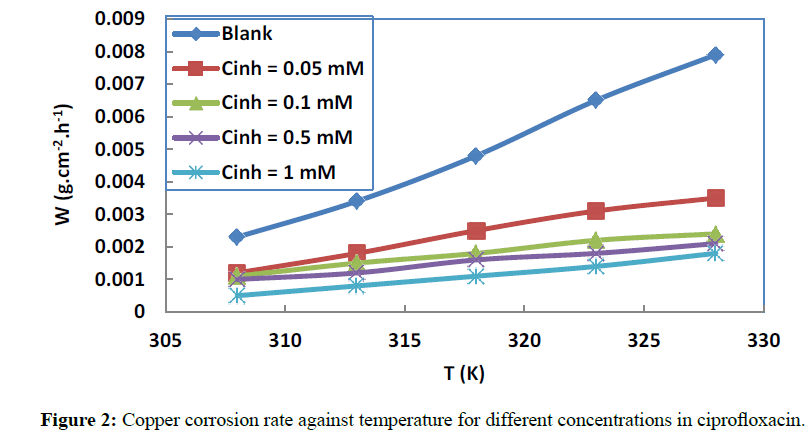
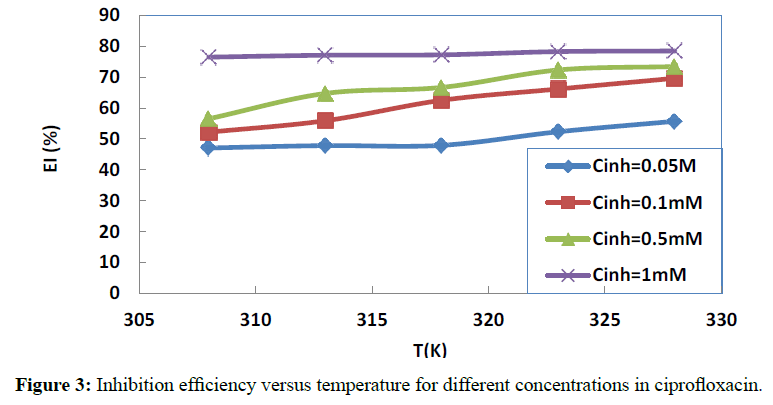
The effect of the addition of ciprofloxacin at various concentrations on copper corrosion in 1 M HNO3 aqueous solutions for different temperatures is depicted in Figures 2 and 3.
It is clear from these data that corrosion rate decreases and inhibition efficiency increases when the concentration in the studied molecule increases. These results revealed that copper corrosion is decelerated in the presence of ciprofloxacin at all the concentrations used in this study. The extent of the inhibitory action depends on the amount of ciprofloxacin present.
This behaviour can be explained by the increased adsorption process and a progressive coverage protection of the copper surface when the concentration of the molecule increases. We also note that the increase in temperature leads to an increase in inhibition efficiency, showing that the adsorption of the studied molecule increases when the temperature increases. This observation is suggestive of chemical adsorption [36] and can be attributed to the formation of a complex film between the molecule and copper ions Cu2+ by electron transfer.
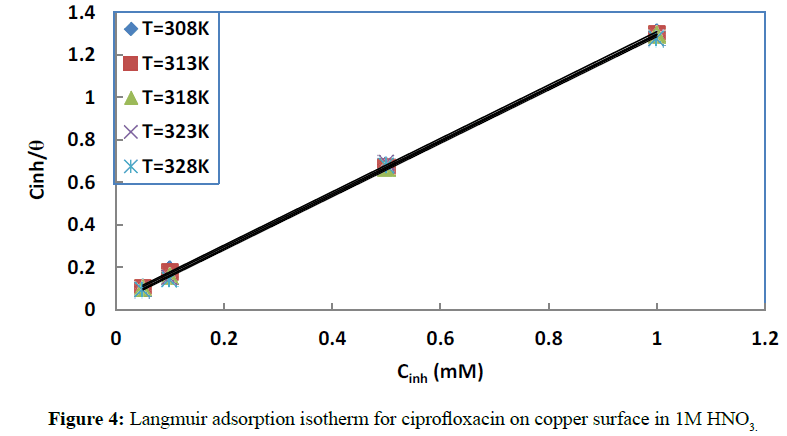
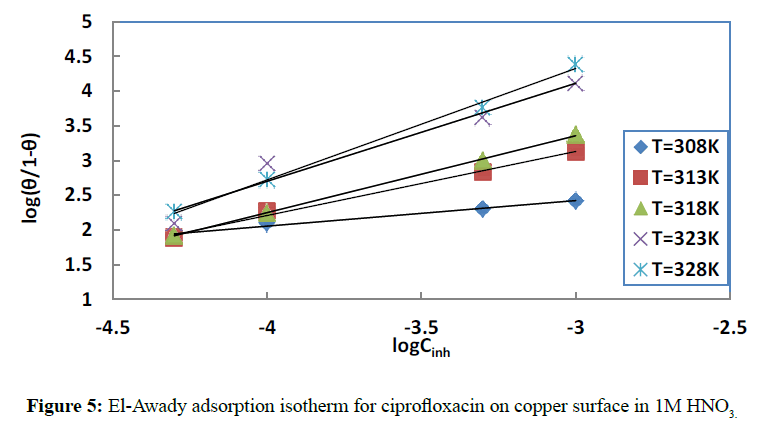
To get information on the adsorption mechanism, it is necessary to derive the adsorption isotherm [37] that characterizes the metal-inhibitor/environment system. Attempts were made to fit the coverage rate values θ to some well-known adsorption isotherms including Langmuir (Figure 4), El-Awady (Figure 5), Temkin, Flory Huggins and Freundlich isotherms. Adsorption [38] is regarded as a substitution process between the organic inhibitor in aqueous phase and the water molecules on the metal surface:
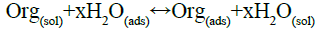 (17)
(17)
Where, Org(sol), Org(ads), H2O(sol), and H2O(ads) are respectively the organic molecule and water molecule in the aqueous solution and adsorbed on the metal surface. The common general adsorption equation form [39] is given by the following relation:
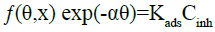 (18)
(18)
Where ƒ(θ,x) is the configuration factor, which depends on the physical model adopted and the assumptions made in deriving the isotherm. The parameter x represents the number of water molecules replaced by one adsorbed inhibitor molecule. α is the molecular interaction parameter which accounts for the lateral interaction between adsorbed species [40], whilst Cinh is the inhibitor concentration and Kads is the adsorption equilibrium constant. The best fits were obtained with Langmuir and El-Awady isotherms (Table 1).
| T(K) | Langmuir Isotherm | El-AwadyIsotherm | ||||
|---|---|---|---|---|---|---|
| R2 | Slope | Intercept | R2 | Slope | Intercept | |
| 308 | 0.999 | 1.2537 | 0.0546 | 0.994 | 0.437 | 1.8084 |
| 313 | 0.999 | 1.2498 | 0.0478 | 0.934 | 0.3881 | 1.6306 |
| 318 | 0.999 | 1.257 | 0.038 | 0.999 | 0.3331 | 1.3958 |
| 323 | 0.999 | 1.2534 | 0.037 | 0.998 | 0.3947 | 1.7306 |
| 328 | 0.999 | 1.253 | 0.0316 | 0.999 | 0.3546 | 1.6186 |
Table 1: Regression parameters of Langmuir and El-Awady isotherms.
The El-Awady adsorption isotherm [41] is given by the following equation:
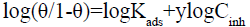 (19)
(19)
Where Kads=K1/y; in our work for all temperatures, 1/y>1, suggesting [42] a monolayer adsorption and one inhibitor molecule will occupy more than one active site.
Though the isotherms of Temkin, Freundlich and Flory Huggins have not the highest values of correlation coefficient, they can be taken into account to get useful information about the type of adsorption, such as lateral interactions in the adsorbed layer, heterogeneity of the metal surface, number of water molecules replaced by one molecule of the inhibitor. Table 2 gives the equations of these adsorption isotherms and the related information.
| Name | Equation | Information |
|---|---|---|
| Temkin | θ=2.303/ƒ[log Kads+logCinh] | Ƒ>0:Repulsionbetweenadsorbed molecules. |
| Freundlich | logθ= log Kads+nlogCinh | 0<n<1: heterogeneity of the metal surface |
| Flory-Huggins | Log(θ/Cinh)=logKads+xlog(1-θ) | x-3 (one molecule of inhibitor replaces 3 water molecules) |
Table 2: Temkin, Freundlich and Flory-huggins equations and related information.
The Langmuir isotherm is characterized by the equation below:
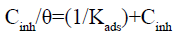 (20)
(20)
For this isotherm [43], a slope greater than unity could signify that one of each inhibitor molecule occupies more than one adsorption site; there are interactions between adsorbed molecules on the metal surface, or the adsorption heat (enthalpy) changes with increasing surface coverage. A slight deviation from the ideal conditions (all the adsorption sites are equivalent) occurs. So, the Langmuir model cannot be applied rigorously. Hybrid isotherm may be used as a way out, namely the modified Langmuir [44] (or Villamil) isotherm which equation is given below:
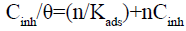 (21)
(21)
A compensation factor “n” is introduced into the conventional Langmuir equation. This factor leads to the effective coverage rate (nθ).
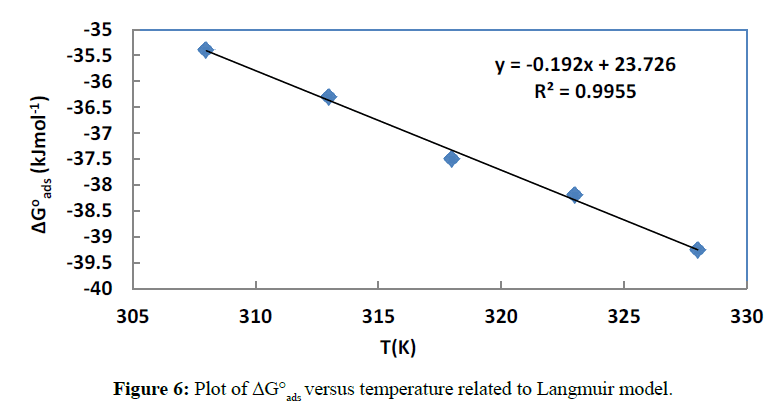
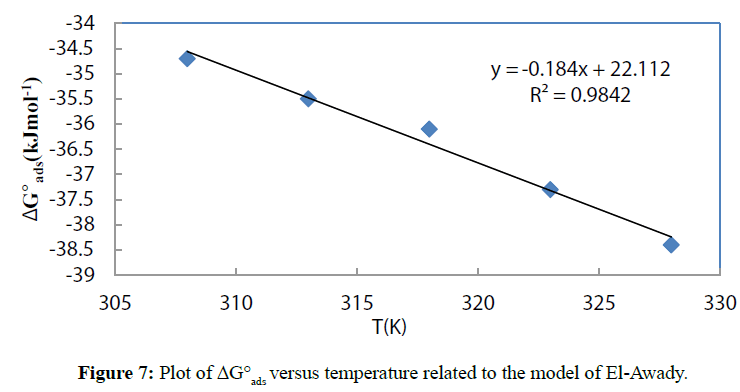
The equations (19) and (21) of the appropriate isotherms were then used to calculate the equilibrium constant values which are related [45] to the change in the free adsorption energy:
 (22)
(22)
Where ΔG°ads is the change in free adsorption energy, R is the universal gas constant, T is the absolute temperature and 55.5 is the concentration of water in the solution in molL-1. The obtained values are listed in Tables 3 and 4. For the two isotherms, the values of ΔG°ads are negative at all the studied temperatures, suggesting [46] a spontaneous adsorption of ciprofloxacin on the copper surface.
| T(K) | Kads(M-1) | DG°ads(KJmol-1) | DH°ads(KJmol-1) | DS°ads(Jmol-1K-1) |
|---|---|---|---|---|
| 308 | 18315.0 | -35.4 | 23.7 | 192 |
| 313 | 20920.5 | -36.3 | ||
| 318 | 26315.8 | -37.5 | ||
| 323 | 27027.0 | -38.2 | ||
| 328 | 31645.6 | -38.6 |
Table 3: Thermodynamic functions related to the modified Langmuir adsorption isotherm.
| T(K) | Kads(M-1) | DG°ads(KJmol-1) | DH°ads(KJmol-1) | DS°ads(Jmol-1K-1) |
|---|---|---|---|---|
| 308 | 13747.2 | -34.7 | 22.1 | 184 |
| 313 | 15903.6 | -35.6 | ||
| 318 | 15414.6 | -36.1 | ||
| 323 | 24243.5 | -37.3 | ||
| 328 | 36692.7 | -38.4 |
Table 4: Thermodynamic functions related to El-Awady adsorption isotherm.
Generally, values of ΔG°ads up to -20 kJmol-1 [47] suggests a physisorption mechanism due to electrostatic interaction between the charged molecules and the charged metal. A chemisorption mechanism [47] is concerned when the values of ΔG°ads are around or lower than -40 kJmol-1: it is the result of charges sharing or electrons transfer from the organic molecule to the metal surface to form a coordinate bond. In our case ΔG°ads values range from -39 to -34 kJmol-1: both physisorption and chemisorption exist. The change in standard adsorption enthalpy ΔH°ads and the change in standard adsorption entropy ΔS°ads have been assessed using the thermodynamic basis equation:
 (23)
(23)
The plot of ΔG°ads versus temperature gives ΔH°ads and ΔS°ads respectively as intercept and negative of the slope. Figures 6 and 7 give the plots for the two appropriate adsorption isotherms.
Figure 7: Plot of ΔG°ads versus temperature related to the model of El-Awady.
The values of ΔH°ads and ΔS°ads are listed respectively in Tables 3 and 4. For all the temperatures studied, ΔH°ads is positive, suggesting [48] an endothermic adsorption process, which supports a chemical adsorption of the molecules on the metal surface. The obtained values of ΔS°ads are positive, showing that disorder takes place during the inhibitor adsorption process, probably [49] due to desorption of water molecules.
The resolution of the ambiguity usually associated with the characterization of adsorption process of organic molecules as inhibitors of metal corrosion in acidic media was achieved using both Dubinin-Radushkevitch [50] and Adejo- Ekwenchi [51] isotherms.
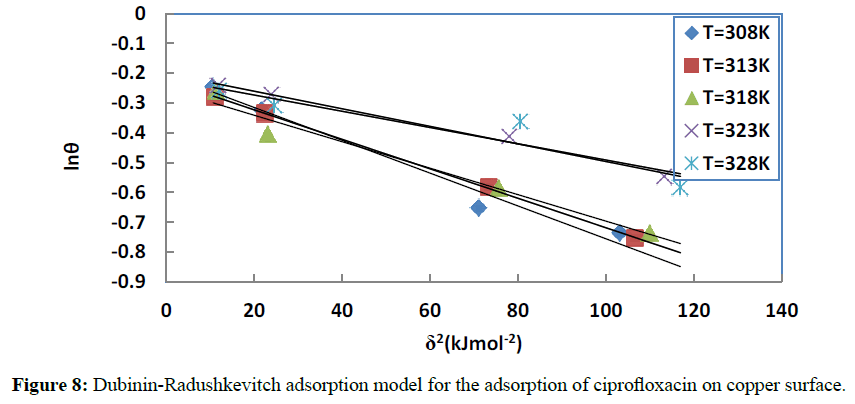
The experimental data were fitted to the Dubinin-Raduskhevitch adsorption isotherm to explain the mechanism of adsorption onto the copper surface. This model is based on the following equation [50]:
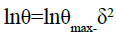 (24)
(24)
Where θmax is the maximum surface coverage and δ is the Polanyi potential which is given by:
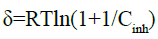 (25)
(25)
In this equation, R is the universal gas constant, T is the absolute temperature and Cinh is the concentration in gL-1 of the inhibitor. Figure 8 gives the plots of lnθ versus δ2. The obtained parameters are collected in Table 5.
Figure 8: Dubinin-Radushkevitch adsorption model for the adsorption of ciprofloxacin on copper surface.
| T(K) | R2 | A(kJ-2mol2) | Em(KJmol-1) |
|---|---|---|---|
| 308 | 0.966 | 0.0055 | 9.5 |
| 313 | 0.999 | 0.0049 | 10.1 |
| 318 | 0.964 | 0.0044 | 10.7 |
| 323 | 0.989 | 0.0030 | 12.9 |
| 328 | 0.900 | 0.0027 | 13.6 |
Table 5: The Dubinin-Radushkevitch isotherm parameters.
The mean adsorption energy Em which represents the energy transferred by 1 mol of adsorbate from the infinity (bulk solution) to the surface of the adsorbent is given by:
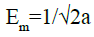 (26)
(26)
The magnitude of the mean energy [52] gives information about the type of adsorption; Em values less than 8 kJ mol-1 indicate physical adsorption. In our case the values range from 9.5 to 13.6 kJ mol-1, showing a chemisorption process for all the studied temperature.
The Adejo-Ekwenchi isotherm was also used to distinguish between physisorption and chemisorption. This isotherm is based on the following equation:
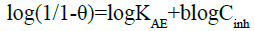 (27)
(27)
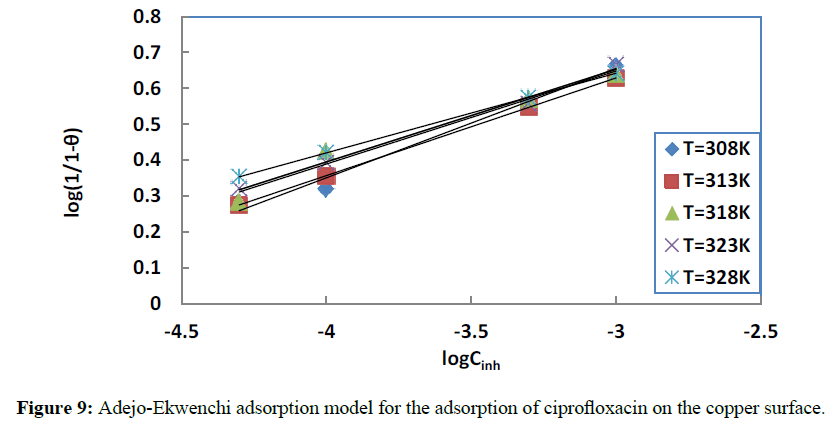
The evolution of the values of the parameter determines [51] the type of adsorption: decrease in values with rise in temperature signifies physisorption, while increase or fairly constant values indicate chemisorption. Figure 9 shows the plots of the studied isotherm. Table 6 gives the obtained values of this parameter.
Figure 9: Adejo-Ekwenchi adsorption model for the adsorption of ciprofloxacin on the copper surface.
| T(K) | R2 | b | Intercept |
|---|---|---|---|
| 308 | 0.982 | 0.30 | 1.5698 |
| 313 | 1.000 | 0.27 | 1.4419 |
| 318 | 0.971 | 0.26 | 1.4250 |
| 323 | 0.993 | 0.26 | 1.4272 |
| 328 | 1.000 | 0.22 | 1.3075 |
Table 6: Parameters of the isotherm of Adejo-Ekwenchi.
From Table 6, it is clear that the parameter b is fairly constant when the temperature increases, confirming the chemisorption process of the adsorption of ciprofloxacin on the copper surface.
In order to calculate the activated parameters of the corrosion reaction including the activation energy (Ea), the change in activation enthalpy (ΔHa*) and the change in activated entropy (ΔSa*) in the absence and presence of ciprofloxacin at different concentrations, the Arrhenius equation (28) and the transition state equation (29) were used:
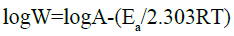 (28)
(28)
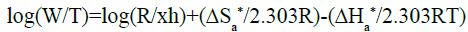 (29)
(29)
Where R is the universal gas constant, T is the absolute temperature, A is the Arrhenius préexponentiel factor, h is the Planck’s constant and x is the Avogadro’s number.
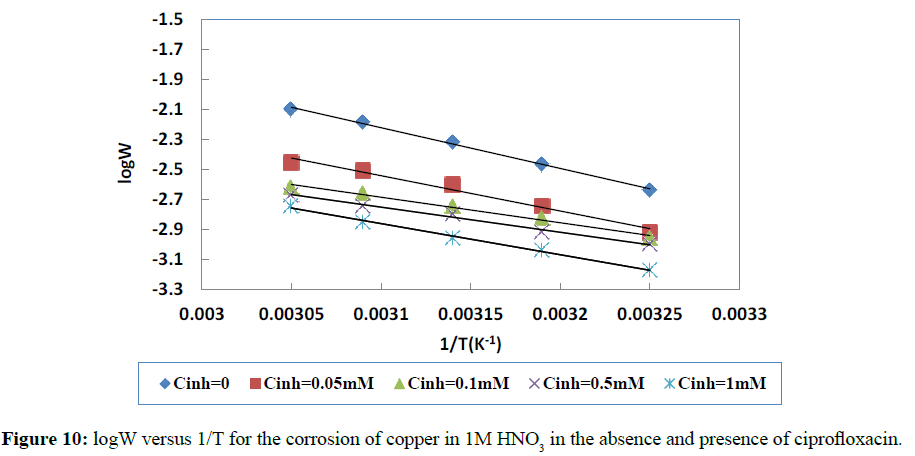
Plotting the logarithm of the corrosion rate (W) versus the reciprocal of the absolute temperature leads to a straight line with
(-Ea/2.303R) as slope. Figure 10 depicts log W versus 1/T.
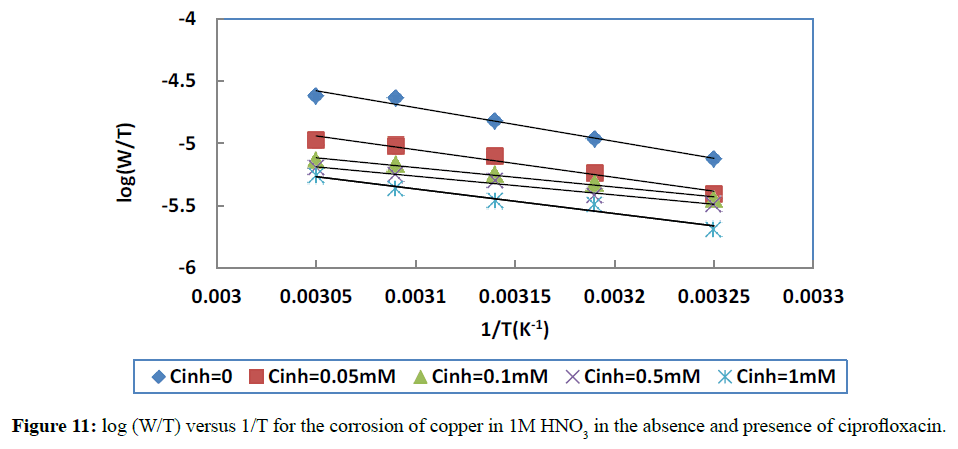
The plot of log(W/T) versus 1/T leads to a straight line which allows determining ΔHa * from the slope (ΔHa */2.303R) and ΔSa * from the intercept (log R/xh+ ΔSa */2.303R) (Figure 11). All the activation parameters are listed in Table 7.
| Concentration (mM) | Ea(KJmol-1) | DH°ads(KJmol-1) | DS°ads(Jmol-1K-1) |
|---|---|---|---|
| 0 | 51.95 | 51.87 | -126.9 |
| 0.05 | 45.00 | 42.39 | -162.7 |
| 0.1 | 32.57 | 29.91 | -284.2 |
| 0.5 | 32.05 | 28.74 | -209.1 |
| 1 | 39.72 | 37.84 | -183.0 |
Table 7: Activation parameters of the corrosion of copper in 1 M HNO3 in the absence and presence of different concentrations of ciprofloxacin.
From Table 7, it is clear that the values of the activation energy Ea of the inhibited solutions are lower than that of the uninhibited solution, suggesting [53] a chemical adsorption process. The decrease in Ea values in the presence of the inhibitor is indicative [54] of an appreciable increase in the adsorption process of the inhibitor on the copper surface with increase in temperature. The increase in adsorption leads to decrease in corrosion rate due to the lower exposed surface area of the metal towards the nitric acid solution.
The values of the change in activation entropy ΔSa * are negative for both inhibited and uninhibited solutions, what shows that the activation complex in the rate determining step is an association rather than dissociation, meaning [55] that a decrease disorder takes place on going from reactants to the activated complex.
The change in activation enthalpy (ΔHa *) is positive for the inhibited and the uninhibited system, indicating an endothermic dissolution; (ΔHa *) values are lower in the presence of the inhibitor, supporting [56] the formation of copper ions (Cu2+: [Ar] 3d9) with vacant energy level close to the metal, what favours the transfer of electrons from the inhibitor to the metal to form a complex barrier which protects the metal from the aggressive environment.
DFT studies
The molecular parameters calculated are EHOMO (the energy of the highest occupied molecular orbital), ELUMO (the energy of the lowest unoccupied molecular orbital), the energy gap (ΔE), the dipole moment μ, the electronegativity χ, the hardness η, and the electrophilicity index (ω). All these parameters are listed in Table 8.
| Parameter | Value |
|---|---|
| EHOMO (eV) | -5.660 |
| ELUMO (eV) | -1.413 |
| ΔE (eV) | 4.247 |
| I (eV) | 5.660 |
| A(eV) | 1.413 |
| χ (eV) | 3.536 |
| η(eV) | 2.123 |
| S (eV-1) | 0.471 |
| μ (Debye) | 10.780 |
| ΔN | 0.340 |
| ω(eV) | 2.945 |
Table 8: Molecular properties of ciprofloxacin calculated by B3LYP/6-31G (d).
EHOMO and ELUMO are very popular quantum parameters; they determine [57] the way the molecule interacts with other species. HOMO acts as an electron donor, whereas LUMO acts as electron acceptor. The frontier molecular theory [58] states that the formation of a transition state is due to an interaction between HOMO and LUMO orbitals of the reactants. The obtained value of EHOMO (-5.660 eV) can be considered as a high value when compared to values reported in the literature [59-61], indicating the tendency of ciprofloxacin to donate electrons to the empty molecular orbital of copper ions. For the calculated ELUMO (-1.413 eV), the low value compared with that reported in the literature [60-62] indicates the ability of the studied molecule to accept electrons.
The energy gap (ΔE=ELUMO-EHOMO) is an important parameter related to the reactivity of the organic molecules. Lower values of energy gap [63] lead to good inhibition efficiency because the energy to remove an electron from the last occupied orbital is low. In our case the obtained value (4.247 eV) is low when compared with that of many molecules reported [64,65] in the literature.
The ionization energy (I) is one of the fundamental descriptors of chemical reactivity of molecules. High ionization energy signifies chemical inertness (high stability) and small ionization energy [66] expresses high reactivity of the molecule. In our study, the low value (I=5.660 eV) shows that ciprofloxacin can easily give electrons to copper.
The molecular stability and reactivity is measured by both the absolute hardness (η) and the absolute softness (S). The chemical hardness [67] indicates the resistance towards the deformation or polarization of the electron cloud of the molecules under small perturbations. In our work, the low value of hardness (η=2.123 eV) and relative high value of softness (S=0.471 eV-1) when compared [65,68] to values obtained for many molecules. in the literature shows the ability of ciprofloxacin to give electrons to copper.
The dipole moment (μ) is an important electronic parameter, used for the prediction of the direction of the corrosion inhibition process. This parameter [69] results from the non-uniform distribution of charges on the atoms in a molecule. It is generally agreed [70] that the adsorption of high polar compounds possessing high dipole moment on the metal surface should lead to better inhibition. The dipole moment of ciprofloxacin (μ=10.780 Debye) is high when compared with the values obtained for many other molecules in the literature [70,71], indicating higher inhibition efficiency.
The number of electrons transferred (ΔN=0.340) [72] shows that the inhibition efficiency resulting from electron donation agrees with the Lukovits study.
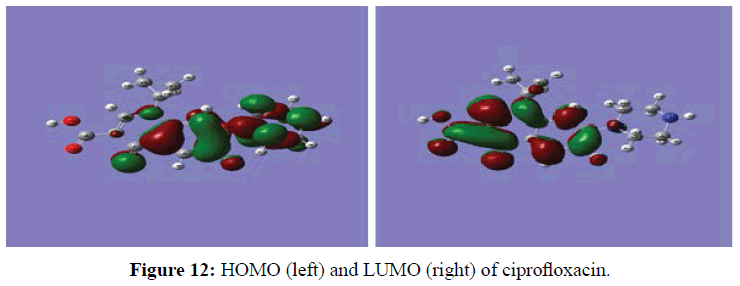
The global electrophilicity index () is the measure of the electrophilic tendency of a molecule (energy lowering process on soaking electrons from potential donors). In our case the obtained value (ω=2.945 eV) [73] can be considered as a high value when referred to literature, indicating that the molecule can receive electrons from copper. In order to rationalize the reactivity of individual atoms in the molecule, the local parameters of Fukui have been computed. Calculated values of qN+1, qN, qN-1, ƒk +, ƒk- and Δƒr) are presented in Table 9. The HOMO and LUMO orbitals are given in Figure 12.
| Atom | qk(N+1) | qk(N) | qk(N-1) | ƒk+ | ƒk- | (ƒk+)-(ƒk-) |
|---|---|---|---|---|---|---|
| 1C | 0.310957 | 0.267440 | 0.393364 | 0.043517 | -0.125924 | 0.169441 |
| 2C | -0.305356 | -0.189363 | -0.257790 | -0.115993 | 0.068427 | -0.184420 |
| 3C | 0.029745 | 0.020639 | 0.042703 | 0.009106 | -0.022064 | 0.031170 |
| 4C | 0.319823 | 0.336240 | 0.374073 | -0.016417 | -0.037833 | 0.021416 |
| 5C | -0.302020 | -0.168447 | -0.248514 | -0.133573 | 0.080067 | -0.213640 |
| 6C | 0.254815 | 0.298647 | 0.321610 | -0.043832 | -0.022963 | -0.020869 |
| 7H | 0.140455 | 0.194364 | 0.233235 | -0.053909 | -0.038871 | -0.015038 |
| 8C | 0.353484 | 0.247422 | 0.410538 | 0.106062 | -0.163116 | 0.269178 |
| 9H | 0.111818 | 0.151980 | 0.194552 | -0.040162 | -0.042572 | 0.002410 |
| 10C | -0.088413 | 0.124856 | 0.048059 | -0.213269 | 0.076797 | -0.290066 |
| 11C | -0.065583 | 0.006826 | -0.047252 | -0.072409 | 0.054078 | -0.126487 |
| 12H | 0.128250 | 0.215285 | 0.233105 | -0.087035 | -0.017820 | -0.069215 |
| 13N | -0.553704 | -0.573640 | -0.545817 | 0.019936 | -0.027823 | 0.047759 |
| 14H | 0.282303 | 0.283913 | 0.344757 | -0.001610 | -0.060844 | 0.059234 |
| 15N | -0.498084 | -0.607289 | -0.439329 | 0.109205 | -0.167960 | 0.277165 |
| 16N | -0.532714 | -0.715470 | -0.555288 | 0.182756 | -0.160182 | 0.342938 |
| 17O | -0.580581 | -0.426503 | -0.428081 | -0.154078 | 0.001578 | -0.155656 |
| 18C | 0.504014 | 0.413233 | 0.578146 | 0.090781 | -0.164913 | 0.255694 |
| 19O | -0.641210 | -0.601090 | -0.600962 | -0.040120 | -0.000128 | -0.039992 |
| 20H | 0.361651 | 0.383697 | 0.430880 | -0.022046 | -0.047183 | 0.025137 |
| 21O | -0.535445 | -0.375339 | -0.417409 | -0.160106 | 0.042070 | -0.202176 |
| 22F | -0.332938 | -0.329283 | -0.252404 | -0.003655 | -0.076879 | 0.073224 |
| 23C | -0.101839 | -0.065500 | -0.169992 | -0.036339 | 0.104492 | -0.140831 |
| 24H | 0.116212 | 0.136438 | 0.208349 | -0.020226 | -0.071911 | 0.051685 |
| 25H | 0.144590 | 0.143205 | 0.187114 | 0.001385 | -0.043909 | 0.045294 |
| 26C | -0.133437 | -0.107957 | -0.138777 | -0.025480 | 0.030820 | -0.056300 |
| 27H | 0.117173 | 0.136840 | 0.191284 | -0.019667 | -0.054444 | 0.034777 |
| 28H | 0.113376 | 0.123006 | 0.156712 | -0.009630 | -0.033706 | 0.024076 |
| 29C | -0.134824 | -0.107625 | -0.146269 | -0.027199 | 0.038644 | -0.065843 |
| 30H | 0.119413 | 0.140632 | 0.194053 | -0.021219 | -0.053421 | 0.032202 |
| 31H | 0.117171 | 0.128291 | 0.162800 | -0.011120 | -0.034509 | 0.023389 |
| 32C | -0.122961 | -0.098987 | -0.174970 | -0.023974 | 0.075983 | -0.099957 |
| 33H | 0.164907 | 0.177149 | 0.210514 | -0.012242 | -0.033365 | 0.021123 |
| 34H | 0.115331 | 0.140573 | 0.209652 | -0.025242 | -0.069079 | 0.043837 |
| 35C | 0.032420 | 0.013632 | -0.027312 | 0.018788 | 0.040944 | -0.022156 |
| 36C | -0.307340 | -0.271397 | -0.316871 | -0.035943 | 0.045474 | -0.081417 |
| 37C | -0.325006 | -0.295129 | -0.327692 | -0.029877 | 0.032563 | -0.062440 |
| 38H | 0.128105 | 0.175471 | 0.198516 | -0.047366 | -0.023045 | -0.024321 |
| 39H | 0.166583 | 0.175486 | 0.186850 | -0.008903 | -0.011364 | 0.002461 |
| 40H | 0.125626 | 0.157038 | 0.184445 | -0.031412 | -0.027407 | -0.004005 |
| 41H | 0.129868 | 0.165029 | 0.202242 | -0.035161 | -0.037213 | 0.002052 |
| 42H | 0.173365 | 0.175687 | 0.197177 | -0.002322 | -0.021490 | 0.019168 |
Table 9: Mulliken atomic charges, Fukui functions and dual descriptor of ciprofloxacin.
The analysis of Table 9 shows that according to the Fukui functions and the dual descriptor, N (16) (in the LUMO region) which has the highest value of ƒk + and a positive value of Δƒk(r) is the site of nucleophilic attack.
It can also be seen that C (23) (located in the HOMO region) with the highest value of ƒk- and a negative value of Δƒk(r) is the site of electrophilic attack. The analysis of Table 9 shows that according to the Fukui functions and the dual descriptor, N (16) (in the LUMO region) which has the highest value of ƒk + and a positive value of Δƒk(r) is the site of nucleophilic attack.
It can also be seen that C (23) (located in the HOMO region) with the highest value of ƒk - and a negative value of Δƒk(r) is the site of electrophilic attack.
Conclusion
From our study, the following conclusion can be drawn:
- Ciprofloxacin is a good inhibitor for copper corrosion in 1M HNO3 for the studied temperatures and concentrations and the inhibition efficiency increases with increasing concentration and increasing temperature.
- The thermodynamic adsorption and activation parameters support a chemical adsorption mechanism;
- The modified adsorption isotherm and that of El-Awady are the appropriate isotherms
- The Dubinin-Radushkevich isotherm and that of Adejo-Ekwenchi confirm the chemical adsorption process;
- The calculated molecular descriptors show reasonably good correlation with the inhibition efficiency of the studied molecule;
- The Fukui functions and the dual descriptor show that N (16) and C (23) are respectively the nucleophilic and the electrophilic attacks sites.
References
- Marcus Ph (2011) Corrosion mechanisms in theory and practice. 3rd Edn, CRC Press.
- Kuznetsov YI, Agafonkina MO, Shikhaliev HS, Andeeva NP, Potapov AY (2014) Adsorption passivation of Copper by triazoles in neutral aqueous solution.JCorrosScale Inhib3: 137-138.
- Wang H, Wu Q, Li CM, Gu N (2013) Copper corrosion inhibition by polyaspartic acid and imidazole. MaterCorros64: 347-352.
- Al-Otaibi MS, Al-Mayouf AM, Khan M, Moussa AA, Al-Mayzroa SA, et al. (2014) Corrosion inhibitory action of some plant extracts on the corrosion of mild steel in acidic media.Arab J Chem7: 340-346.
- Obot IB, Obi-Egbedi NO, Umoren SA (2009) Antifungal drugs as corrosion inhibitors for Aluminium in 0.1M HCl. Corros Sci51: 1868-1875.
- Yildirim A, Çetin M (2008) Synthesis and evaluation of new long alkyl side chain acetamide, isoxazolidine and isoxazoline derivatives as corrosion inhibitors. Corros Sci50: 155-165.
- Ngem NA, Kandile NG, Badr E AA, Mohamed MA (2012) Gravimetric and electrochemical evaluation of environmentally friendly non-ionic corrosion inhibitors for carbon steel in 1M HCl. Corros Sci 65: 94-103.
- Abdel-Gaber AM, Abd-El-Nabey BA, Khamis E (2011) A natural extract as scale and corrosion inhibitor for steel in brine solution. Desalination 278: 337-342.
- Salasi M, Sharabi T, Roayaei E, Aliofkhazrei M (2007) The electrochemical behavior of environment-friendly inhibitors of Silicate and phosphate in corrosion control of carbon steel in soft-water media. Mater Chem Phys104: 183-190.
- Radojac K, Berkovic S, Vorkapic Furac J (2008) Natural honey and black radish juice as tin corrosion inhibitors.Corros Sci50: 1498-1504.
- Wang X, Yang H, Wang H (2011) An investigation of benzimidazole derivatives as corrosion inhibitor for mild steel in different concentration HCl solution. Corros Sci53: 113-121.
- El-Sayed A (1997) Phenothiazine as inhibitor of the corrosion of cadmium in acidic solution. J Appl Electrochem27: 193-200.
- Schmitt G (1984) Application of inhibitors for acid media: Report prepared for the European Federation of Corrosion working party on inhibitors.Br Corrosion19: 165-176.
- Sanyal B (1981) Organic compounds as corrosion inhibitors in different environment: A review. Prog Org Coat 9: 165-236.
- Eddy NO, Odoemelam SA, Ekwumemgbo P (2009) Inhibition of the corrosion of mild in H2SO4 by penicillin G. Sci Res Essays4: 33-38.
- Obot IB, Obi-Egbedi NO (2010) Inhibition of aluminium corrosion in hydrochloric acid using nizoral and effect of iodide ion addition.E-Journal of Chemistry 7: 837-843.
- Naqvi I, Salami AR, Naveed S (2011) Cefixime: A drug as efficient corrosion inhibitor for mild steel in acidic media. Int J Electrochem Sci 6: 146-161.
- Tigori MA, Bony FN, Niamien PM, Yapo AJ, Trokourey A (2016) Experimental and theoretical studies ou riboflavin’s behaviour against copper corrosion in 1M HNO3. Arch Appl Sci Res8: 18-32.
- Tigori MA, Bony FN, Niamien PM, Yapo AJ, Trokourey A (2016) Effect of pyridoxine hydrochloride on copper corrosion in 1 M HNO3. J Chem Biol Phys Sci6: 1201-1216.
- Bouklah M, Hammouti B, Lagrenée M, Bentiss F (2006) Thermodynamic properties of 2, 5 bis (4-methoxyphenyl)-1, 3, 4-Oxadiazole as a corrosion inhibitor for mild steel in normal sulphuric acid medium. CorrosSci48: 2831-2842.
- Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA (2003) Gaussian 03, Revision B.05, Gaussian, Pittsburgh PA.
- Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation energy formula into a functional of the electron density. Phys Rev37: 785-789.
- Miehlich B, Savin A, Stoll H, Preuss H (1989) Results obtained with correlation energy density functionals of Becke and Lee, Yang and Parr. Chem Phys Lett 157: 200-206.
- Peterson GA, Bennett A, Tensfeldt TG, Al-Laham MA, Shirley WA (1988) A complete basis set model chemistry. The total energies of closed shell atoms and hybrids of the first row elements. J Chem Phys89: 2193-2218.
- Parr RG, Yang W (1986) Density Functional Theory of Atoms and Molecules, Oxford University Press: Oxford.
- Parr RG, Donally RA, Levy M, Palke WE (1978) Electronegativity: The density functional view point.J Chem Phys68: 3801-3807.
- Parr RG, Pearson RG (1983) Absolute hardness companion parameter to absolute negativity. JAm Chem Soc 105: 7512-7516.
- Pauling L (1960) The nature of the chemical bond, Cornell University Press, Ithica, New York.
- P Senet (1997) Chemical hardness of atoms and molecules from frontier orbitals. Chem Phys Lett275: 527-532.
- Sastri VS, Perumareddi JR (1997) Molecular orbital theoretical studies of some organic corrosion inhibitors. Corros Sci 53: 617-622.
- Pearson RG (1986) Absolute electronegativity and hardness correlated with molecular orbital theory. Proc Natl Acad Sci83: 8440-8441.
- Parr RG, Szentpaly L, Liu S (1999) Electrophilicity index. J Am Chem Soc121: 1922-1924.
- Fukui K (1982) Role of frontier orbitals in chemical reactions.Science 218: 747-754.
- Yang W, Mortier WJ (1986) The use of global and local molecular parameters for the analysis of the gas-phase basicity of amines. J Am Chem Soc108: 5708-5711.
- Morell C, Grand A, Toro-Labbé A (2006) Theoretical support for using Δf(r) descriptor. Chem Phys Lett425: 342-346.
- Popova A, Sokolova E, Raicheva S, Christov M (2003) AC and DC study of temperature effect on mild steel corrosion in acidic media in the presence of Benzimidazole derivatives. Corros Sci 45: 33-58.
- Kendig M, Jeanjacquet S (2002) Cr (VI) and Ce (III) inhibition of oxygen reduction on copper.J Electrochem Soc149: B47-B51.
- Bockris OM, Swinkels AJ (1964) Adsorption of n-Decylamine on solid metal electrodes. J Electrochem Soc111: 736-743.
- Martinez S (2003) Inhibitory mechanism of mimosa tannin using molecular modelling and substitutional adsorption isotherms. Mater Chem Phys77: 97-102.
- Shibli S, Saji V (2005) Co-inhibition characteristics of Sodium Tungstate with potassium iodate on mild steel corrosion. Corros Sci47: 2213-2224.
- El-Awady AA, Abd-El-Nabey BA, Aziz SG (1992) Kinetic-thermodynamic and adsorption isotherms analyses for inhibition of the acid corrosion of steel by cyclic and open-chain amines.J Electrochem Soc139: 2149-2154.
- Essien EA, Umoren SA, Essien EE, Udoh AP (2012) Preparation and evaluation of cucumeropsis mannii naud. seed oil metallic soap as driers in gloss paint. J Mater Environ Sci3: 477-484.
- Shaban SM, Aiad I, El-Sukkary MM, Soliman EA, El-Awady MY (2015) Inhibition of mild steel corrosion in acidic medium by vanillin cationic inhibitor. J Mol Liq203: 20-28.
- Villamil RFV, Corio P, Rubin JC, Agostinho SML (1999) Effect of sodium dodecylsulfate on copper corrosion in sulphuric acid media in the absence and presence of Benzotriazole.J Electroanal Chem 472: 112-119.
- Khamis E (1990) The effect of temperature on the acidic dissolution of steel in the presence of inhibitors. Corros Sci 46: 476-484.
- Cano E, Polo JL, La Iglesia A (2004) A study on the adsorption of benzotriazole on copper in hydrochloric acid using the inflection point of the isotherm. Adsorption 10: 219-225.
- AliSA, El-Shareef AM, Al Gamdi RF, Saeed TM (2005) The isoxazolidines: the effects of Steric factor and hydrophobic chain length on the corrosion inhibition of mild steel in acidic medium. Corros Sci 47: 2659-2678.
- Durnie W, Marco RD, Jefferson A, Kinsella B (1999) Development of a structure-activity relationship for oil field corrosion inhibitors. J Electrochem Soc146: 1751-1756.
- Banerjie G, Malhotra SN (1992) Contribution to Adsorption study of aromatic amines on mild steel surface from HCl solutions by impedance, UV and Raman spectroscopy. Corros Sci 48: 10-15.
- Huston ND, Yang RT (1997) Theoretical basis for the dubinin-radushkevitch adsorption isotherm. Adsorption 3: 189-195.
- Adejo SO, Ekwenchi MM (2004) Proposing a new empirical adsorption isotherm known as Adejo-Ekwenchi isotherm. IOSR-JAC6: 66-71.
- Noor EA (2009) Potential of aqueous extract of Hibiscus Dabdariffa leaves for inhibiting the corrosion ofaluminium in alkaline solution. J Appl Electrochem 39: 1465-1475.
- Ashassi-Sorkhabi H, Shaabani B, Seifzadeh D (2005) Corrosion inhibitor of mild steel by some Schiff base compounds in hydrochloric acid. Appl Surf Sci239: 154-164.
- Dehri I, Ozcan M (2006) The effect of temperature on the corrosion of mild Steel in acidic media in the presence of some sulphur containing organic compounds.Mater Chem Phys 98: 316-323.
- Abd-El-Rehim SS, Refaey SAM, Taha F, Saleh MB, Ahmed RA (2001) Corrosion inhibition of mild steel in acidic medium using 2-amino thiophenol and 2-cyanomethylbenzothiazole. J Electrochem Soc 31: 429-435.
- Johnson HE, J Leja (1965) On the potential-pH diagrams of the Cu-NH3-H2O and Zn-NH3-H2O systems. J ElectrochemSoc 112: 638-641.
- Musa AY, Kadhum AH, Mohamad AB, Rohma AB, Mesmari H (2010) Electrochemical and quantum chemical calculations on 4,4-dimethyloxazolidine-2-thione as inhibitor for mild steel corrosion in hydrochloric acid. J Mol Struct969: 233-237.
- Gece G, Bilgic S (2009) Quantum chemical study of some cyclic nitrogen compounds as corrosion inhibitors of steel in NaCl media.Corros Sci51: 1876-1878.
- Obot IB, Obi-Egbedi NO, Umoren SA (2009) The synergistic inhibitive effect and some quantum chemical parameters of 2, 3- diaminonaphtalène and iodide ions on the hydrochloric acid corrosion of aluminium. Corros Sci 51: 276-282.
- Yadav DK, Maiti B, Quraishi MA (2010) Electrochemical and quantum chemical studies of 3,4-dihydropyrimidin-2(1H)-ones as corrosion inhibitors for mild steel in hydrochloric acid solution. Corros Sci 52: 3586-3598.
- Bentiss F, Lebrini M, Lagrenée M, Traisnel M, Elfarouk A, et al. (2009) The influence of some new 2,5-disubstituted 1, 3, 4- thiadiazoles on the corrosion behaviour of mild steel in 1M HCl solution: A C impedance study and theoretical approach. Elecrochem Acta52: 6865-6872.
- Zarrouk A, Hammouti B, Zarrok H, Warad I, Bouachrine M (2011) N containing organic compound as an effective corrosion inhibitor for copper in 2M HNO3: Weight loss and quantum chemical study. Der Pharma Chemica3: 263-271.
- M Şahin, G Gece, F Karcı, S Bilgic (2008) Experimental and theoretical study of the effect of some heterocyclic compound on the corrosion of low carbon steel in 3.5% NaCl medium. J Appl Electrochem38: 809-815.
- Awad MK, Mustafa MR, Abo Elnga MM (2010) Computational simulation of the molecular structure of some triazoles as inhibitors for the corrosion of metal surface. J Mol Struc Theochem 959: 66-74.
- Udhayakala P, Rajendiran TV, Gunasekaran S (2012) Theoretical approach to corrosion efficiency of some pyrimidine derivatives using DFT method. J Comput Meth Mol Des2: 1-15.
- Rajak SK, Islam N, Ghosh DC (2011) Modeling of the chemico-physical process of protonation of molecules entailing some quantum chemical descriptor. JQuantum information Science1: 87-95.
- Khodaei-Tehrani M, Niazi A (2015) Quantum chemical studies on the corrosion inhibition of some hector bases on mild steel in acidic medium. Oriental Journal of Chemistry31: 423- 429.
- Rajendran M, Keerthika K, Kowsalya M, Devapiriam D (2005) The study of the role of structural parameters as corrosion inhibition efficiency of thiadiazole derivatives using DFT method. J Chem Biol Phys Sci 5: 3937-3947.
- Gece G (2008) The use of quantum chemical methods in corrosion inhibitor studies. Corros Sci50: 2981-2992.
- Bereket G, Hür E, Ogretir C (2002) Quantum chemical studies on some imidazole derivatives as corrosion inhibitors for iron in acidic medium. J Mol Struc Theochem 578: 79-88.
- Kay K, Senthilkumar AN, Tharini K (2016) Quantum chemical study on the corrosion inhibition of N-benzylpiperidine-4-one Oxime. Adv Appl Sci Res 7: 150-154.
- Lukovits I, Kalman E, Zucchi F (2001) Corrosion inhibitors-Correlation between electronic structure and efficiency. Corros Sci57: 3-8.
- Udhayakala P, Maxwell SA, Rajendiraa TV, Gunasekaran S (2013) DFT study on the adsorption mechanism of some phenyltetrazole substituted compounds as effective corrosion inhibitors for mild steel. Der Pharma Chemica 5: 111-124.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences