ISSN : 0976 - 8688
Der Pharmacia Sinica
Chemo Selective Synthesis and Evaluation of Antimicrobial Activity of Novel 5-hydroxyindole Derivatives
Ramesh S Gani1*, Karabasanagouda T2, Shwetha Kumari MP1, Raju K Chalannavar3, Ravindra B Malabadi3, Ravindra B Chougale4, Saraswati Masti4 and Deepak Kasai5
1Department of Industrial Chemistry, Mangalore University, Mangalore-574199, India
2Sequent Scientific Limited, 120, A&B, Industrial Area, Baikampady, Mangalore-575011, India
3Department of Applied Botany, Mangalore University, Mangalore-574199, India
4Department of Chemistry, Karnataka University, Dharwad-580003, India
5Department of Materials science, Mangalore University, Mangalore-574199, India
Abstract
The paper presents a Novel C-5 monosubstituted chemoselctive Indole derivatives, its Schiff base were prepared and characterized (15 examples). The present perspective of the study was to synthesis chemoselctive 5-hydroxy indole derivatives and investigates the role of electronic effect of substituents variation on the antimicrobial activity of Schiff bases. A comparative study of inhibition values were done to get high potent antimicrobial active molecule. The electron withdrawing and donating substituents showing high antifungal and antibacterial activity. This is due to the effect of electron withdrawing groups such as, NO2 and electron donating group OH. This variation is due to change in inductive effect of substituents.
Keywords
Schiff base, Substituents, Antimicrobial activity, Chemo selective, Indole
Introduction
The effect of azomethine (>C=N) group can be extensively studied because, which involves the formation of a hydrogen bond through the azomethine group (>C=N-) with the active centres of cell constituents resulting in interferences with the normal cell process [1,2]. Schiff bases are versatile ligands and known to be synthesized from the condensation of an amino compound with carbonyl compounds. In azomethine derivatives, the C=N linkage is essential for biological activity, several azomethines were reported to possess remarkable antibacterial, antifungal, anticancer and diuretic activities. There has been increasing emphasis on the screening of new and more effective antimicrobial drugs with low side effects. Nitro, halo derivatives of Schiff bases were reported to have antimicrobial, antitumor and antifungal activities of various Schiff bases have also been reported. Some derivative of Schiff base & Beta-Lactam acts as good antimicrobial agents [3]. Synthesis, characterization and structural activity relationship of Schiff bases have been studied worldwide, as it is proven that C=N linkage in Schiff bases is an essential feature for bioactivity [4]. Schiff bases have been reported to possess noteworthy antibacterial [5], antifungal [6], anticancer [7], urease inhibition [8] and antioxidant [9-14] properties. The Indole ring has become an important structural requirement in many pharmaceutical drugs because of the structural diversity of biologically active indoles and their derivatives [15]. The important physical and biological properties of Schiff bases directly related to the presence of intermolecular hydrogen bonding and a proton transfer equilibrium. A new Schiff base of 5-chloro-3-phenyl-1H-indole-2-carboxyhydrazide and 3-formyl-2-hydroxy-1H-quinoline (HL) and its Cu (II), Co (II), Ni (II), Zn (II), Cd (II) and Hg (II) complexes have been synthesized, characterized and the Schiff base HL acts as tridentate (ONO) chelating agent coordinate with metal ions were showing good antimicrobial activity [16]. The presence of sulphur atoms in the compounds improves biological activity possibly due to its specific interference with enzymes having sulfhydryl groups at their active sites [17]. The chelation of Schiff base tends to make the ligand act as a more powerful and potent bacterial agent [18]. This inspires synthetic chemists to search for new Schiff bases compounds. Study of effect of substituents of Schiff base and their antimicrobial activity is of current our prime priority and interest. Based on these findings and in continuation of our research work on coordination chemistry, we describe the synthesis of a novel Schiff base by using 1-cyclopropyl-3-ethoxycarbonyl-2-methylindole-5(1-methoxyacetic acid hydrazide) with different substituted aromatic aldehyde. Further, a comparative study of inhibition values of the different Schiff base containing indole moiety were done to investigate the effect of substituent on the antimicrobial activity of Schiff base subsequently to get high potent antimicrobial active molecule for future studies.
Materials and Methods
Experimental section
All chemicals used in the present study were of analytical grade purchased from Sigma, Aldrich and Merck chemical Co. All the solvents were used after distillation. TLC sheet used with silica coated on alumina (60 F254, Merck, Germany) and visualized in UV light. IR spectra were recorded on the FT-IR Perkin Elmer spectrum BX spectrophotometer. NMR spectra were obtained by using Bruker NMR instrument 400 MHz. The Mass spectra were recorded from JEOL SX 102/DA-6000 spectrometer using m-nitrobenzyl alcohol as matrix.
Preparation of 1-Cyclopropyl-3-ethoxycarbonyl-5-hydroxy-2-methyl Indole (1)
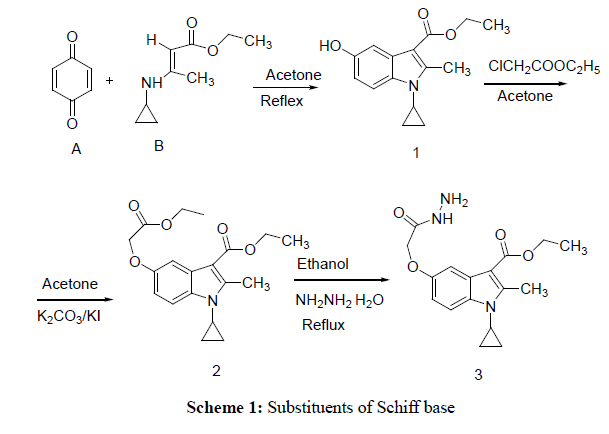
In the present investigation 1-cyclopropyl-2-methyl-3-ethoxycarbonyl-5-hydroxy indole (1) was prepared by adopting the Nenitzescu reaction [19]. The mixture of p- benzoquinone (A) (23.0 g/0.213 mol), 150 ml dry acetone and 3-Ethyl (cyclopropyl amino) butanate (B) (30 g /0.1775 mol) were stirred under room temperature for 1 h, and then it was heated for 6 hour on steam bath. Progress of reaction monitored by TLC using mobile phase (ethyl acetate– Hexane (4:1). The organic phase was evaporated to dryness under reduced pressure and the residue was purified by crystallization from acetone.
Preparation of 1-cyclopropyl-2-methyl-3-ethoxycarbonyl-5-(1-ethoxycarbonyl-1-methoxy)-indole (2)
1-Cyclopropyl-3-ethoxycarbonyl-5-hydroxy-2-methyl Indole (1) (2 0g, 0.0772 mol) and 150ml dry acetone were taken in a round bottom flask, the mixture was cooled to 10-15°C. Chloroethyl acetate (12 g, 0.0926 mol), potassium carbonate (53g, 0.38 mol), a pinch of potassium iodide were added. The reaction mixture was further heated to reflux for 8 h. After the completion of reaction and the reaction mass was cooled to room temperature and filtered. Filtrate containing product and the organic phase was evaporated to dryness under reduced pressure and then added 25 ml of fresh acetone to the residue and filtered. The crude product washed with chilled acetone, dried recrystallized from suitable solvent
Preparation of 1-cyclopropyl-3-ethoxycarbonyl-2-methylindole-5-(1-methylethoxyaceticacidhydrazide (3)
The mixture of compound 2 (20 g, 0.0579 mol), 99% hydrazine hydrate (15 ml), ethanol (200 ml) and pyridine (1 drop) were stirred for 1 hour at room temperature, then heated on a boiling water bath for 4-5 h and was concentrated to half volume and left overnight. The separated solid was filtered, washed with little ethanol, recrystallized from suitable solvent.
Preparation of Schiff Base HL (8a-l)
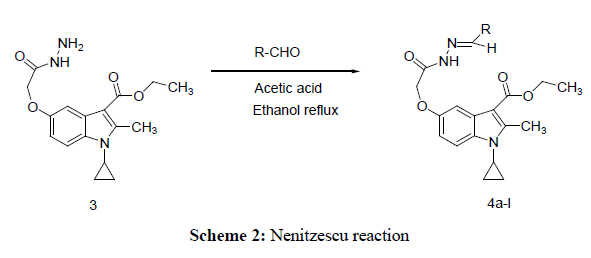
Equimolar mixture of 1-cyclopropyl-3-ethoxycarbonyl-2-methylindole-5-(1-methylethoxyaceticacidhydrazide.(3) (0.001 mol) and different aromatic aldehyde (0.001 mol) with catalytic amount of glacial acetic acid (1-2 drops) in ethanol (40 mL) was refluxed on a water bath for about 7-8 h. The reaction was monitored by TLC. The pale yellow solid separated was filtered, washed with little ethanol, dried and recrystallized from dioxane.
(1): Yield obtained was 45%. Melting point 187-195°C. Molecular formula: C15H17NO3, Molecular weight: 259; IR (KBr) (Vmax, cm−1): 1657 (C-3 ester C=O), 3263(OH); 1H NMR (400 MHz, CDCl3/TMS) VH:1.1-198 (m 4H) (cyclopropyl methylene protons), 1.35 (t, 3H), (C-3-ester CH3),2.7 (s, 3H), (C-2-CH3), 3.2 (m, 1H),(N-CH), 4.2 (q, 2H), (C-3-OCH2), 5.1 (s, 1H), (C-5-OH), 6.66 (d, 1H), (Ar-C-7 H), 7.33 (s, 1H,)(Ar-C-4 H), 8.93 (d, 1H), (Ar-C-6H) ppm. MS (ESI): (M+H), 260.
(2): Yield was 90%, Melting Point, 57-59°C. Molecular formula,C19H23NO5, Molecular weight. 345.39 IR (KBr) (Vmax, cm−1): 1695 and 1673 (C-5 ester C=O), (C-3 ester C=O), 3263(OH); 1H NMR (400 MHz, CDCl3/TMS) VH:1.1-198 (m 4H) (cyclopropyl methylene protons), 1.35 (t, 3H), (C-3-ester CH3), 2.7 (s, 3H), (C-2-CH3), 3.2 (m, 1H), (N-CH), 5.1 (s, 1H), (C-5-OH), 6.66 (d, 1H), (Ar-C-7 H), 7.33 (s, 1H,)(Ar-C-4 H), 8.93 (d, 1H), (Ar-C-6H), 5.1 and 4.8(q 2H, C-5 O-CH2 and (q, 2H, C-3-O-CH2) ppm.
(3): Melting point.156-160°C. Yield: obtained was 80%, Molecular formula, C17H21N3O4, Molecular weight. 331.37: IR (KBr) (Vmax, cm−1): 1685 and 1663 (C-3 ester C=O), (C-5 amide C=O), 3315,3375, (NH/NH2); 1H NMR (400 MHz, CDCl3/TMS) VH:1.1-198 (m 4H) (cyclopropyl methylene protons), 1.35 (t, 3H), (C-3-ester CH3), 2.7 (s, 3H), (C-2-CH3), 3.2 (m, 1H),(N-CH), 5.1 (s, 1H), (C-5-OH), 6.66 (d, 1H), (Ar-C-7 H), 7.33 (s, 1H,)(Ar-C-4 H), 8.93 (d, 1H), (Ar-C-6H), 4.35(q 2H, (q, 2H, C-3-O-CH2), 8.12(s,1H amide) ppm.
(8c): (Veratraldeyhyde)
Yield 82%, Melting Point, 159-162°C. Molecular formula, C26H29N3O6, Molecular weight. 479: IR (KBr) (Vmax, cm−1): 1680 and 1613 (C-5 ester C=O), (C-3 ester C=O), 3080(NH); 1H NMR (400 MHz, CDCl3/TMS) VH:1.1-198 (m 4H) (cyclopropyl methylene protons),1.35 (t, 3H), (C-3-ester CH3), 2.7 6(s, 3H), (C-2-CH3), 3.2 (m, 1H), (N-CH). All aromatic protons appear in the range of 6.9-8.2 δ. 4.28 (q, 2H,) C-3-O-CH2) 3.7(s,6H)(6 protons of two OCH3 groups) 11.48(s,1H)(NH protons) imine proton exhibit at 8 ppm.5.1(s,2H)(C-OCH2) ppm. MS (ESI): (M+H), 480. (M-OCH2CH3), 434.
(8d): (Nitroverataldehyde): Yield 67%, Melting Point, 213-215°C. Molecular formula, C26H28N4O8, Molecular weight. 492.:IR (KBr) (Vmax, cm−1): 1708 and 1663 (C-5 ester C=O), (C-3 ester C=O), 3331(NH); 1 H NMR (400 MHz, CDCl3/TMS) VH:0.98-2.3 (m 4H) (cyclopropyl methylene protons), 1.35 (t, 3H), (C-3-ester CH3), 2.7 6(s, 3H), (C-2-CH3), 3.2 (m, 1H), (N-CH). All aromatic protons appear in the range of 6.8-8.5 δ. 4.25 (q, 2H, C-3-O-CH2) 3.9(s,6H)(6 protons of two OCH3 groups) 8.8(s,1H)( imine NH protons) 11.93(s,1H)( amide NH).5.1(s,1H) (C-5- OCH2) ppm. MS (ESI): (M+H), 525.
(8e): (3, 4 Dimethyl benzaldehyde): Yield 92%, Melting Point, 187-189°C. Molecular formula, C26H29N304, Molecular weight. 447:IR (KBr) (Vmax, cm−1): 3331 is due to secondary amide NH, 1685 and 1633 (C-5 ester C=O), (C-3 ester C=O),; 1H NMR (400 MHz, CDCl3/TMS) VH:1.12-2.08 (m 4H) (cyclopropyl methylene protons), 1.35 (t, 3H), (C-3- ester CH3), 2.7 6(s, 3H), (C-2-CH3), 3.2 (m, 1H), (N-CH). All aromatic protons appear in the range of 6.8-8.2 δ. 4.26 (q, 2H, C-3-O-CH2) 3.7(s, 6H), 8.5(s, 1H)( NH), 5.12(s.2H)( C-5-0-CH2). 2.24 &2.51(s, 3H & 3H) (two phenyl CH3 groups) ppm. MS (ESI): (M+H), 448. (M-OCH2CH3) 402.
(8f):3-Nitrobenzaldehyde: Yield 98%, Melting Point, 179-182°C. Molecular formula, C24H24N4O6 ,Molecular weight. 464:IR (KBr) (Vmax, cm−1): 3221 is due to secondary amide NH, 1685 and 1633 (C-5 ester C=O), (C-3 ester C=O),; 1H NMR (400 MHz, CDCl3/TMS) VH:1.2-1.35 (m 4H) (cyclopropyl methylene protons), 1.34 (t, 3H), (C-3-ester CH3), 2.75(s, 3H), (C-2-CH3), 3.3 (m, 1H), (N-CH). All aromatic protons appear in the range of 6.8-8.2 δ. 4.26 (q, 2H, C-3-O-CH2), 8.5(s, 1H) (NH), 5.12(s.2H) (C-5-0-CH2). 11.8(s, 1H) (amide NH) ppm. MS (ESI): (M+H), 465. (M-OCH2CH3) 419.
(8g): 3, 4, 5 Trimethoxy benzaldehyde: Yield 97%, Melting Point, 168-170°C. Molecular formula, C27H31N3O7. Molecular weight. 509 IR (KBr) (Vmax, cm−1): 3190 is due to secondary amide NH, 1685 and 1679 (C-5 ester C=O), (C-3 ester C=O),; 1H NMR (400 MHz, CDCl3/TMS) VH:1.2-1.35 (m 4H) (cyclopropyl methylene protons), 1.34 (t, 3H), (C-3-ester CH3), 2.76(s, 3H), (C-2-CH3), 3.3 (m, 1H),(N-CH). All aromatic protons appear in the range of 6.8-8.2 δ. 4.26 (q, 2H, C-3-O-CH2), 8.5(s, 1H) (NH), 5.15(s.2H) (C-5-0-CH2). 11.68(s, 1H) (amide NH), 3.69-3, 82 (s, 9H) (3 phenylOCH3) ppm.
(8h): 4-hydroxybenzaldehyde: Yield 95%, Melting Point, 215-216°C. Molecular formula, C24H25N3O5, Molecular weight. 435 IR (KBr) (Vmax, cm−1): 3190 is due to secondary amide NH, 1683 and 1679 (C-5 ester C=O), (C-3 ester C=O),; 1H NMR (400 MHz, CDCl3/TMS) VH:1.3-2.08 (m 4H) (cyclopropyl methylene protons), 1.34 (t, 3H), (C-3-ester CH3), 2.75(s, 3H), (C-2-CH3), 3.24 (m, 1H), (N-CH). All aromatic protons appear in the range of 6.8-7.9δ.
4. 26 (q, 2H, C-3-O-CH2), 9.95(s, 1H) (NH), 5.09(s.2H) (C-5-0-CH2). 11.4(s, 1H) (amide NH), 7.9(s, 1H) (OH) ppm. (M+H), 436. (M-OCH2CH3) 390.
(8i) :(p-Dimethyl amino benzaldehyde): Yield 90%, Melting Point, 184-189°C. Molecular formula, C26H30N4O4, Molecular weight. 438 :IR (KBr) (Vmax, cm−1): 3190 is due to secondary amide NH, 1683 and 1679 (C-5 ester C=O), (C-3 ester C=O),; 1H NMR (400 MHz, CDCl3/TMS) VH:1.3-1.38 (m 4H) (cyclopropyl methylene protons), 1.34 (t, 3H),(C-3-ester CH3), 2.7(s, 3H),(C-2-CH3), 3.3 (m, 1H),(N-CH). All aromatic protons appear in the range of 6.8-7.9δ. 4.26 (q, 2H, C-3-O-CH2), 8.18(s, 1H) (NH), 5.09(s. 2H) (C-5-0-CH2). 11.32(s, 1H) (amide NH), 2.96(s, 6H) (dimethyl amino group) ppm. (M+H), 463.
(8j) :4-Methylbenzaldehyde: Yield 96%, Melting Point, 180-181°C. Molecular formula, C25H27N3O4, Molecular weight. 433 :IR (KBr) (Vmax, cm−1): 3190 is due to secondary amide NH, 1683 and 1679 (C-5 ester C=O), (C-3 ester C=O),; 1 H NMR (400 MHz, CDCl3/TMS) VH:1.3-1.38 (m 4H) (cyclopropyl methylene protons), 1.34 (t, 3H),(C- 3-ester CH3), 2.7(s, 3H),(C-2-CH3), 3.3 (m, 1H), (N-CH). All aromatic protons appear in the range of 6.8-7.9δ. 4.26 (q, 2H, C-3-O-CH2), 8.18(s, 1H) (NH), 5.09(s. 2H) (C-5-0-CH2). 11.32(s, 1H) (amide NH), 2.3(s, 3H) (phenyl p-Methyl group) ppm. (M+H), 434. (M-OCH2CH3) 388.
(8k): Thiophene 2- aldehyde: Yield 93%, Melting Point, 159-162°C. Molecular formula, C22H23N3O4S, Molecular weight. 425 :IR (KBr) (Vmax, cm−1): 3198 is due to secondary amide NH, 1683 and 1673 (C-5 ester C=O), (C-3 ester C=O),; 1 H NMR (400 MHz, CDCl3/TMS) VH:1.3-1.38 (m 4H) (cyclopropyl methylene protons), 1.34 (t, 3H), (C-3- ester CH3), 2.75(s, 3H), (C-2-CH3), 3.3 (m, 1H), (N-CH). All aromatic protons appear in the range of 6.8-7.9δ. 4.28 (q, 2H, C-3-O-CH2), 8.5(s, 1H) (NH), 5.09(s.2H) (C-5-0-CH2). 11.68(s, 1H) (amide NH). (M+H), 426. (M-OCH2CH3) 380.
(8l): o-Chlorobenzaldehyde): Yield 89%, Melting Point, 153-156°C. Molecular formula, C24H24ClN3O4 , Molecular weight. 453 :IR (KBr) (Vmax, cm−1): 3205 is due to secondary amide NH, 1695 and 1683 (C-5 ester C=O), (C-3 ester C=O),; 1 H NMR (400 MHz, CDCl3/TMS) VH:1.3-1.38 (m 4H) (cyclopropyl methylene protons), 1.34 (t, 3H),(C-3- ester CH3), 2.75(s, 3H), (C-2-CH3), 3.3 (m, 1H), (N-CH). All aromatic protons appear in the range of 6.8-7.9δ. 4.26 (q, 2H, C-3-O-CH2), 8.5(s, 1H) (NH), 5.09(s. 2H) (C-5-0-CH2). 11.8(s, 1H) (amide NH).MS (ESI): (M+H), 454. (M-OCH2CH3), 408. (M-Cl-Ph.), 300.
Results and Discussion
Chemistry
Title compounds were obtained as described in Schemes 1 and 2. In order to investigate effect of substituents of Schiff base on antimicrobial activity ,for which, starting material 1-Cyclopropyl-3-ethoxycarbonyl-5-hydroxy-2-methyl Indole synthesized by using Nenitzescu reaction, in which, p-benzquinone react with aminocrotnate under reflux condition affording moderate yield (1) Which underwent refluxed with chloroethylacetate in presence of potassium carbonate and potassium iodide yielding mixture and easily separated by chromatography on silica-gel and obtained required 1-cyclopropyl-3-ethoxycarbonyl-5-(1-ethoxycarbonyl-1-methoxy)2-methylindole (2) the said product react with hydrazine hydrate give C-5 monosubstituted chemo selective 1-cyclopropyl -3-ethoxycarbonyl-2-methylindole- 5-(1-methylethoxyaceticacidhydrazide.but not react with C-3 ester group, this is due to nonbonding electrons on nitrogen involve with double bond in pyrrole ring, as a result of this double bond character of carbonyl group at C-3 ester group decreases , therefore nucleophilic substitution reaction does not taking place at C-3 ester group. (3) Finally monocarbohydrazide reflux with different aldehyde such as(8a)–p-chlobenzaldehyde,(8b)Benzaldehyde,(8c) Vetarldehyde,(8d)Nitrovetaraldehyde,(8e)3-4-dimethylbenzaldehyde,(8f)3-Nitrobenzaldehyde,(8g)3,4,5trimeth oxybenzaldehyde,(8h)4-hydroxybenzaldehyde,(8i)p-dimethylaminobenzaldehyde,(8j)4-methylbenzaldehyde(8l) Thiophene-2-aldehyde,(8m) o-Chlorobenzaldehyde and obtained different Schiff bases .(8a-l). The spectral data given in experimental section and supporting data can be accessed on the publisher’s website (Tables 1 and 2).
| Product Code | Product | Molecular formula | Molecular weight | Annl % found (cal) | ||
|---|---|---|---|---|---|---|
| C | H | N | ||||
| 1 | 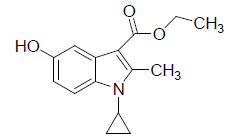 |
C15H17NO3 | 259.3 | 69.2(69.48) | 6.58(6.61) | 5.31(5.40) |
| 2 | 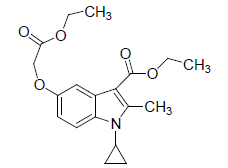 |
C19H23NO5 | 345.39 | 65.76(66.07) | 6.42(6.71) | 4.01(4.06) |
| 3 | 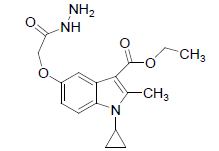 |
C17H21N3O4 | 331.37 | 61.21(61.62) | 6.28(6.39) | 12.57(12.68) |
Table 1: Selective synthesis of 5-hydroxyindole derivatives (Scheme 1).
| Product Code | Product | Molecular formula | Molecular weight | Annl % found (cal) | ||
|---|---|---|---|---|---|---|
| C | H | N | ||||
| 8a | 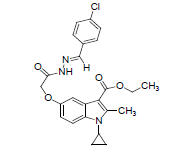 |
C24H24ClN3O4 | 453.92 | 63.32(63.50) | 5.21(5.33) | 9.14(9.26) |
| 8b | 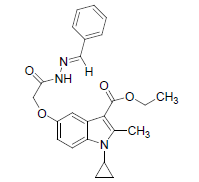 |
C24H25O4N3 | 419 | 68.31(68.72) | 5.99(6.01) | 9.9(10.02) |
| 8c | 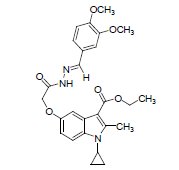 |
C26H29N3O6 | 479.21 | 64.81(65.12) | 6.04(6.10) | 8.54(8.76) |
| 8d | 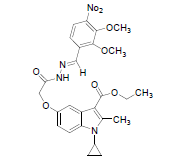 |
C25H28O6N4 | 524 | 59.13(59.54) | 5.34(5.38) | 10.67(10.68) |
| 8e | 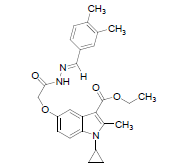 |
C26H29O4N3 | 447 | 69.46(69.78) | 6.51(6.53) | 9.27(9.39) |
| 8f | 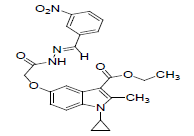 |
C24H24O6N4 | 464 | 61.88(62.06) | 5.19(5.21) | 12.03(12.06) |
| 8g | 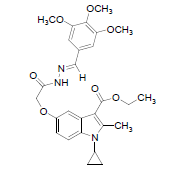 |
C27H31N3O7 | 509 | 63.26(63.64) | 6.10(6.13) | 8.21(8.25) |
| 8h | 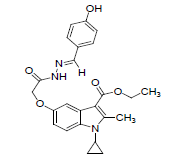 |
C24H25O5N3 | 435 | 65.79(66.19) | 5.77(5.79) | 9.60(9.65) |
| 8i | 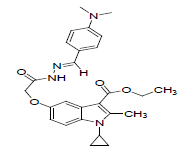 |
C26H30N4O4 | 462.23 | 67.47(67.51) | 6.52(6.54) | 12.05(12.11) |
| 8j | 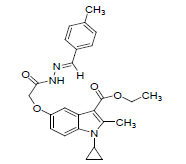 |
C25H27O4N3 | 433 | 69.20(69.27) | 6.26(6.28) | 9.63(9.69) |
| 8k | 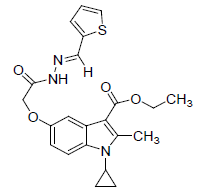 |
C22H23N3O4S | 425.5 | 62.03(62.10) | 5.38(5.45) | 9.82(9.88) |
| 8l | 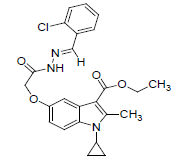 |
C24H24O4N3Cl | 453 | 63.46(63.50) | 5.27(5.33) | 9.20(9.26) |
Table 2: R-Different aromatic aldehyde (Scheme 2)
Evaluation of effect of substituents on antimicrobial activity of Schiff base and structure activity relationship
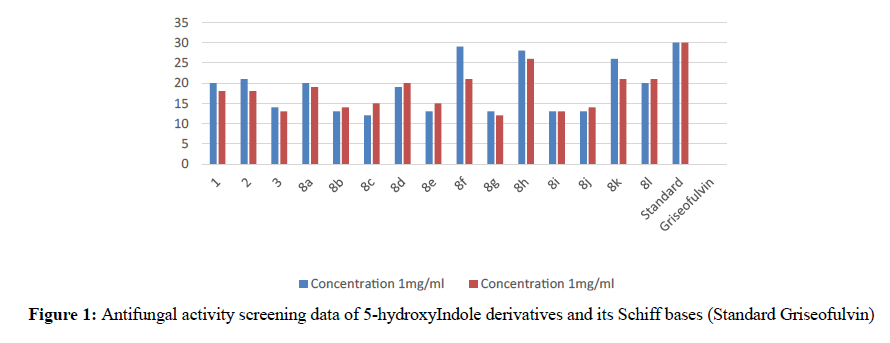
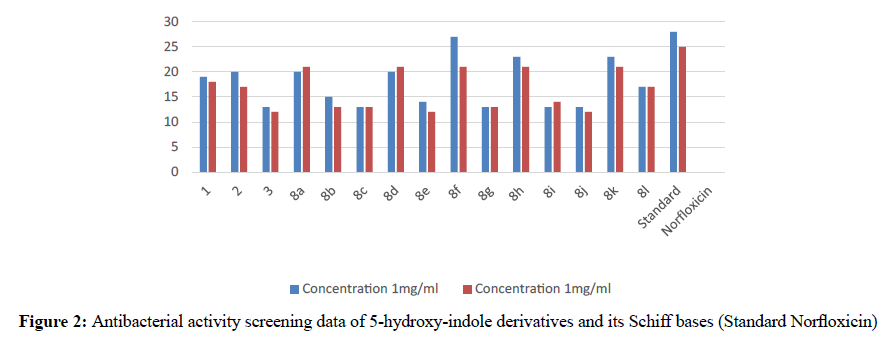
The activity and potency are very susceptible to small changes in chemical structure. The novel 1-cyclopropyl-2- methyl-3-ethoxycarbonyl-5-hydroxyindole derivatives and its Schiff base were tested for antimicrobial activity in vitro at doses 100 microgram in 0.1 ml dimethyl formamide against the gram negative bacterium Escherichia coli and gram positive bacterium B. cirroflagellosus using Norfloxcin as a standard and their antifungal activity in vitro against the fungi A. niger and C. albicans using Griseofluvin as a standard (Figure 1). While evaluation Symbols used for Zone diameter of growth inhibition (-): Inactive, Less than 12 mm (-) inactive, 12-16mm, Week active, 16- 21mm moderately active 22-28mm highly active (Table 3). The antibacterial activity of test compounds were assessed against B. cirroflagellosus and E. Coli by cup plate method [20]. Dimethyl used a solvent control, nutrient agent was used as a culture medium and the method employed was cup plate method. Norfloxcine showed a zone inhibition of 28mm against E-coli and 25mm against B cirrflagellosus (Figure 2). Grisofulvin showed the inhibition of 30 mm for both C. albcans and A. Niger. A total 12 Schiff base compounds and 3 hydroxy indole derivatives were available for this study. The compounds 8f, 8h and 8k showing highest Antibacterial activity against C. albicans and A. niger also antibacterial activity against E. Coli and B. cirroflagellosus, this is due to the effect of electron withdrawing such as NO2 and electron donating group OH, thiazole moiety and also this variation is due to change in inductive effect of substituents. Electron withdrawing donating groups could binds specific proteins located within bacterial cytoplasmic membrane through the nonbonding electrons in its outermost orbital, binding to these proteins causes inhibit the growth of bacteria. 8a and 8d displaying moderate activity antifungal activity against C. albicans and A. niger, also antibacterial activity against E. Coli and B. cirroflagellosus. The compounds 8b, 8c, 8g, 8i and 8j were showing very week activity antifungal activity against C. albicans and A. niger also antibacterial activity against E. Coli and B. cirroflagellosus. This may be due to electron donating groups such as methyl and dimethyl. As part of structure activity relationship study, compared antimicrobial activity inhibition values against 1-cyclopropyl-2- methyl-3-ethoxycarbonyl 5-hydroxyindole derivatives. Compound 1, 2 displaying very good against C. albicans and A. niger also antibacterial activity against E. Coli and B. cirroflagellosus. Schiff base compounds such as 8f, 8h and 8k showing highest antifungal activity against C. albicans and A. niger also antibacterial activity against E. Coli and B as compared to compound 1, 2 and 3. This is due to the effect of electron donating and withdrawing groups, which interference with enzymes through nonbonding electron in the outermost orbital of electron donating and withdrawing groups (Table 4).
| Compound Code | Concentration 1 mg/ml Zone of inhibition in mm after 48 hr. | |
|---|---|---|
| Candida albicans (mm) | Aspergillus niger (mm) | |
| 1 | 20 | 18 |
| 2 | 21 | 18 |
| 3 | 14 | 13 |
| 8a | 20 | 19 |
| 8b | 13 | 14 |
| 8c | 12 | 15 |
| 8d | 19 | 20 |
| 8e | 13 | 15 |
| 8f | 29 | 21 |
| 8g | 13 | 12 |
| 8h | 28 | 26 |
| 8i | 13 | 13 |
| 8j | 13 | 14 |
| 8k | 26 | 21 |
| 8l | 20 | 21 |
| Standard Griseofulvin | 30 | 30 |
Table 3: Antifungal activity screening data of 5-hydroxyIndole derivatives & its Schiff bases. Zone diameter of growth inhibition (-): Inactive, Less than 12 mm (-) inactive, 12-16mm, Week active, 16-21 mm moderately active 22-28 mm highly active
| Compound code | Concentration 1 mg/ml Zone of inhibition in mm after 48 hr. | |
|---|---|---|
| Escherichia coli (mm) | Bacillus cirroflagellosus (mm) | |
| 1 | 19 | 18 |
| 2 | 20 | 17 |
| 3 | 13 | 12 |
| 8a | 20 | 21 |
| 8b | 15 | 13 |
| 8c | 13 | 13 |
| 8d | 20 | 21 |
| 8e | 14 | 12 |
| 8f | 27 | 21 |
| 8g | 13 | 13 |
| 8h | 23 | 21 |
| 8i | 13 | 14 |
| 8j | 13 | 12 |
| 8k | 23 | 21 |
| 8l | 17 | 17 |
| Standard Norfloxicin | 28 | 25 |
Table 4: Antibacterial activity screening data of 5-hydroxyIndole derivatives & its Schiff bases. Zone diameter of growth inhibition (-): Inactive, Less than 12 mm (-) inactive, 12-16 mm, Week active, 16-21 mm moderately active 22-28 mm highly active
Conclusion
A novel chemoselctive 1-cyclopropyl-2-methyl-3-ethoxycarbonyl carbonyl-5-hydroxy Indole and its derivatives, Schiff base were synthesized, their chemical structures were confirmed by using spectroscopic tools including IR, 1HNMR, and mass spectroscopy. Antifungal and antibacterial activity of the prepared compounds was evaluated. Among all Schiff base compounds such as 8f, 8h and 8k showing highest antifungal activity against C. albicans and A. niger, also antibacterial activity against E. Coli and B. cirroflagellosus. This due to the effect of electron withdrawing such as NO2 and electron donating group such as OH and thiazole moiety group,also this variation is due to change in inductive effect of substituents. Electron withdrawing groups could binds specific proteins located within bacterial cytoplasmic membrane through the nonbonding electrons in its outermost orbital, binding to these proteins causes inhibit the growth of bacteria. The compounds 8b, 8c, 8g, 8i and 8j were showing very week activity antifungal activity against C. albicans and A. niger, also antibacterial activity against E. Coli and B. cirroflagellosus. This may be due to electron donating groups such as methyl, dimethyl etc and 5-hydroxy indole derivatives like compound 1, 2 showing moderate antibacterial activity against E. Coli and B. cirroflagellosus and antifungal activity against C. albicans and A. niger.
Acknowledgment
Authors are thankful to Manipal Institute of Technology, India for providing spectral data. We are grateful to Dr. Ravi Malabadi visiting scientist and Dr. Raju Challanavar Associate Professor, Department of Applied Botany Mangalore University Mangalore-574199, India for support of this research work.
Supporting Information
Supplementary data for this article can be accessed on the publisher’s web site.
References
- Malhotra, L., Kumar, S., Dhindsa, K.S., Indian Journal of Chemistry, 1993. 32(A): p. 457.
- Mishra, L., Singh, VK., Indian Journal of Chemistry, 1993. 32(A): p. 446.
- Meenachi, S., International Journal of Scientific Research and Reviews, 2014. 3(1): p. 08.
- Iqbal, A., et al., Molecules, 2007. 12(1): p. 245.
- Malladi, S., et al., Arabian Journal of Chemistry, 2013. 6(1): p. 335.
- Bharti, S.K., et al., European Journal of Medicinal Chemistry, 2010. 45(1): p. 651-660.
- Makawana, J.A., et al., Bioorganic & Medicinal Chemistry Letters, 2014. 24(1): p. 1734.
- Mahmood, S.U., et al., European Journal of Medicinal Chemistry, 2011. 46(1): p. 5473.
- Taha, M., et al., Molecules, 2013. 18(1): p. 10912.
- Anouar, E.H., et al., Journal of Computer Aided Molecular Design, 2013. 27(3): p. 951.
- Khan, K.M., et al., Journal of Medicinal Chemistry, 2012. 8(1): p. 452.
- Khan, K.M., et al., Journal of Medicinal Chemistry, 2012. 8(1): p. 705.
- Aziz, A.N., et al., Molecules, 2014. 19(1): p. 8414.
- Taha, M., et al., Molecules, 2014. 19(1): p. 1286.
- Kaishap, P.P., Dohutia, C., International Journal Pharmaceutical Science & Research, 2013. 4(1): p. 1312.
- Mahendra RK., Vivekanand B., Mruthyunjayaswamy B.H., Bioinorganic Chemistry and Applications, 2013. 2013(315972): p. 1-16.
- Ankri, S., et al., Antimicrobial Agents and Chemotherapy, 1997. 10(1), 2286-2288.
- Mohammed, F.A.A., Mahammad, Y., Oriental Journal of Chemistry, 2014. 30(3), 111.
- Kavannag, F., Academic press- New York, 1963. 1(1), p. 125.
- Duff, J.C., Bills, E.J.J., Journal of the Chemical Society, 1932. 5(1), p. 1987.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences