Brown Stink Bug, Euschistus servus (Say) Damage Cotton Gossypium hirsutum (L. ) in California's Southern Desert Fields
Enrique G Medrano1*, Vonny M Barlow2, Jinggao Liu1, Louis K Prom1
1Department of Agricultural Research, Insect Control and Cotton Disease Research Plant Unit, F&B Road College Station, USA
2Department of Entomology and Nematology, University of California, Blythe, USA
- *Corresponding Author:
- Enrique G Medrano
Department of Agricultural Research,
Insect Control and Cotton Disease Research Plant Unit, F&B Road College Station,
USA,
E-mail: gino.medrano@usda.gov
Received date: April 10, 2023, Manuscript No. IPRJPP-23-16332; Editor assigned date: April 12, 2023, PreQC No. IPRJPP-23-16332 (PQ); Reviewed date: April 24, 2023, QC No. IPRJPP-23-16332; Revised date: May 01, 2023, Manuscript No. IPRJPP-23-16332 (R); Published date: May 08, 2023, DOI: 10.36648/ iprjpp.6.2.161.
Citation: Medrano EG, Barlow V M, L iu J, Prom L K ( 2023) Brown Stink Bug, Euschistus servus (Say) Damage Cotton Gossypium hirsutum (L. ) in California's Southern Desert Fields. J Res Plant Pathol Vol.6 No.2: 161.
Abstract
As plants senesce or are harvested, numbers of brown stink bug, Euschistus servus (Say) migrate from primary host crops into nearby susceptible crops like cotton, Gossypium hirsutum (L.). Host sources include shrubs, many broadleaf weeds, legumes (Leguminosae Juss.), corn (Zea mays L.), soybean (Glycine max L.), sorghum (Sorghum bicolor L.), okra (Abelmoschus esculentus L.), millet (Pennisetum glaucum (L.) R. Br.) and snap beans (Phaseolus vulgaris L.). A close proximity between the listed hosts and cotton increases the difficulty of managing Euschistus servus in the commodity. Euschistus servus is an established vector of cotton boll pathogens. In this study, E. servus feeding caused minimal visual damage to cotton bolls and boll disease was essentially absent resulting in a non-statistical affect in yield or fiber quality loss when compared to bolls from un-infested E. servus infested fields. This suggested that the population densities of cotton boll rotting pathogens is low in California’s southern desert and has minimal effects on yield losses. Low incidence of cotton boll rotting bacteria indicated that damage done by E. servus in California is limited to direct damage by the insect feeding trauma itself. Further support is reflected in the lack of significant differences of HVI color classing between the sampling locations as you move away from the stink bug infested cotton field perimeter.
Keywords
Cotton; Boll rot; Brown stink bug; Insect vector
Introduction
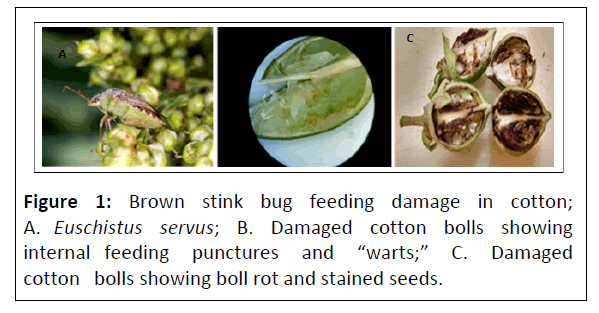
Brown Stink Bugs (BSB), Euschistus servus (Say), can be found across all of southern Canada and much of North America and often throughout the year in parts of the southern U.S. In North America there are two subspecies. E. s. servus (Say) occurs throughout the southern U.S. from Florida through Louisiana to California and E. s. euschistoides (Voltenhoven) reported across Canada and the northern U.S. South California cotton damage from E. servus (Figures 1A-1C) can result in a 25%-30% yield reduction and require repeated pesticide applications. Typical cotton insecticide applications in southern California range from 3-4 applications. Interestingly, infestations of cotton by the sweet potato whitefly biotype B, Bemisia tabaci (Gennadius) and E. servus may require up to ≈ 11 combined applications. Repeated insecticide applications, necessitated by migration from host crops, are not only costly, but increase the possibility of secondary pest outbreaks and disease resistance development [1].
In 2007, Medrano, et al. [2] showed that the southern green stink bug (Nezara viridula L.) is a capable vector of boll rot pathogens causing necrosis of lint and seed tissues. Consistent with Greene, et al. [1] neither outer boll wall damage nor “wart” like growth in the inner boll wall was consistent with disease. Conspicuously, injuries incurred by lab-reared “pathogen-free” stink bug feeding were overcome by lint, seed and no boll rot provided that the insect exposure occurred at 12-16 days postanthesis (i.e. early in the approximately 50 days for bolls to “open” or “crack”) and older resulting in normal white, billowy cotton upon complete boll maturation. In a temporal vector study, bolls at 19-23 days post-anthesis fed on by insects carrying boll pathogens were immune to infection and produced normal appearing seed and lint at natural boll opening [3]. The above studies are relevant to a report by Toews, et al. [4] which illustrated that historically the number of cotton bales lost due to stink bug activity is highly variable relative to production acres. The inconsistency suggests that increased stink bug pressure is not alone a determinant of yield losses. Further, a field study in the southeast demonstrated that plants protected with insecticides over three seasons had significantly lower boll rot than those not protected. This indicates that stink bugs are potent vectors of boll rot pathogens when they are acquired by the insects [5].
To better implement an area-wide Integrated Pest Management (IPM) program for the BSB, more information is needed concerning the transmission potential of cotton boll rot pathogens by the insect. Ashworth, et al. and Medrano, et al. showed that the BSB is a vector of boll rot pathogens. Bolls fed upon by “pathogen-free” BSB could recover from the piercingsucking wounds if infested at two weeks after bloom [6,7]. Notably, fruit infested at one or two weeks after bloom with insects harboring boll pathogens resulted in boll rot and thus, total loss of the infected fruit. Here, we present southern California field data that shows effects of BSB infested fields and supports the hypothesis that cotton yield and quality is dependent on more than insect attack. Based on the results both the non-infested and infested bolls were essentially absent of disease with negligible yield loss.
Insects are known to have directed movement towards preferred host plants [8,9]. Stink bugs will often leave a host within 24 hours after the field is harvested or senesces. Harvest of nearby crops propagates the transient condition. In order to manage BSB from an IPM perspective, it is essential to understand E. servus dispersal capability and capacity to harbor boll pathogens to manage the pest with minimal use of pesticides in cotton.
Materials and Methods
Insect and boll collections
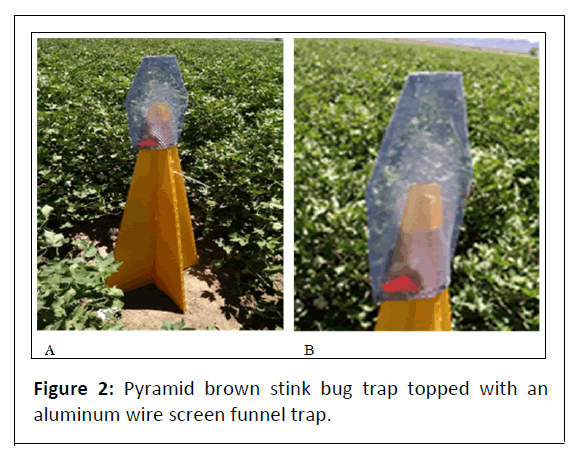
Three commercial cotton fields were planted with cultivar ‘Deltapine 1044’ round-up ready cotton (Bayer CropScience, St. Louis, Mo). Established alfalfa fields were adjacent on at least two sides of the experimental cotton plots. Field margins were mapped and then a grid was overlaid so that perimeter sampling locations could be located approximate to the edge of field (s) at 3.1, 15.2, 30.5 and 45.7 m. At each sampling location, a 1.22 m high 4 vane pyramid trap constructed of international-yellow corrugated plastic (AgBio, Westminster, CO) for strength was anchored into place with a 3 m yellow fiberglass pole (Figures 2A, 2B).
To each of the pyramid traps was affixed an aluminum screen funnel trap top which were baited with E. servus aggregation pheromone, methyl (2E,4Z)-decadienoate (1 mg/d release rate; Sigma-Aldrich, St. Louis, MO). The pheromone was replaced every 14 days and a 25% portion of insecticide impregnated animal ear tag (containing 10% lambda-cyhalothrin and 13% piperonyl butoxide) was used for improved stink bug retention.
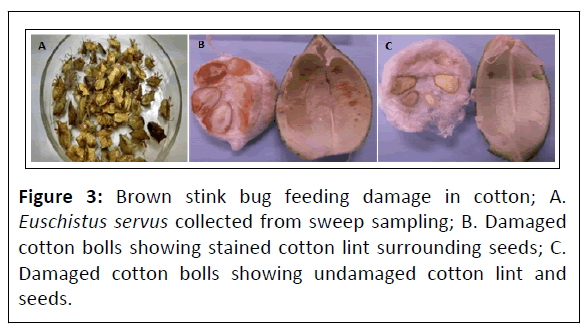
Insect sampling consisted of tallying of E. servus taken per trap location every 7 days to minimize stink bug escape. Individual trap samples were immediately transferred into individual 3.8 L plastic bags and their contents hand sorted in the laboratory to identify stink bug to species using standard entomological taxonomic analysis. Contents from a single 180° arc sampling using a sweep net were immediately transferred into individual 3.8 L plastic bags and placed in an ice cooled chest. Insect samples were processed in the laboratory by first freezing the bags and their contents, hand sorting and identifying stink bugs to species. Additionally, an adjacent cotton field was divided into three replicated 0.65 m areas which were sampled every 7 days with 50 sweeps per area for E. servus for comparison. A set of identified BSB were shipped frozen to the USDA-ARS- Insect Control & Cotton Disease Research Unit (ICCDRU) in College Station, Texas for analysis. Damage by E. servus done to cotton bolls was assessed every 7 days by sampling 10 cotton bolls (22.9 mm-27.9 mm in size) per trap location. Cotton bolls from each trap location were immediately transferred into individual 3.8 L plastic bags and placed in an ice cooled chest that was shipped overnight to the USDA-ARSICCDRU in College Station, Texas for analysis (Figures 3A-3C).
Laboratory survey of microbes in stink bugs and boll analysis
Bolls were washed in a 0.5% sodium hypochlorite solution for eight minutes and then rinsed in sterile water three times. Carpel walls were resected with a sterile scalpel, visually scored for internal boll wall feeding punctures and discarded. Each locule (3-5 boll compartments) was visually inspected for the presence/absence of disease symptoms. Approximately 1 g of seed and lint tissue from bolls with BSB inner boll wall feeding punctures was transferred into a 1.1 ml microtube (SPEX SamplePrep; Metuchen, NJ) that contained 0.5 ml of PO4 buffer (0.1 mol/l, pH 7.1) and a sterile 4 mm stainless steel ball (SPEX Sample Prep; Metuchen, NJ). A second sterile 4 mm stainless steel ball was inserted and the capped 1.1 ml microtubes were placed in a rack of 96 tubes for pulverization. The material was ground using a 2000 Geno/Grinder (SPEX SamplePrep) for 5 min at 1500 strokes/min and diluted with 0.5 ml of PO4 buffer. The resulting homogenate was 10 fold diluted and then plated on Luria-Bertani Agar (LBA; Difco Laboratories, Detroit, MI). Controls consisted of seed tissue from bolls with no evidence of insect feeding and were processed as described above. All plates were incubated for up to two weeks at 27°C until colonies were visible. Solitary colonies were purified by dilution-plating on LBA and stored at -80°C in a 40% glycerol solution diluted with 1% LB. The same pulverization procedure for the insects was followed except that they were first surface sterilized in 5% ethanol and then rinsed in sterile distilled water before being ground.
A representative set of purified isolates from both bolls and insects was identified at the Texas Plant Disease Diagnostic Laboratory (TPDDL) at Texas A&M University (https://plantclinic.tamu.edu/), College Station, on a contract basis. At the TPDDL, species identification and degree of similarity of the isolates to standard control species was performed by comparing their Fatty Acid (FA) profiles to the MIDI library TSBA version 3.9. Species identification was considered significant for those isolates with FA profiles that had similarity indices of 80% or greater.
Analysis of effects of stink bug infested and noninfested fields on boll tissues
Assessment of cotton lint quality/trap location was done by ginning 90 g-100 g of cotton lint for High Volume Instrument (HVI) color classification and fabric measurement (https://www.cottoninc.com/cotton-production/quality/productevaluation- lab/).
Data was analyzed using a mixed model with P<0.05 level of significance to analyze potential differences in mean abundance of E. servus at the various trap location along field perimeter(s) using the JMP 13.2 program (jmp.com; SAS company, Cary, NC).
Results
Insect and boll collections
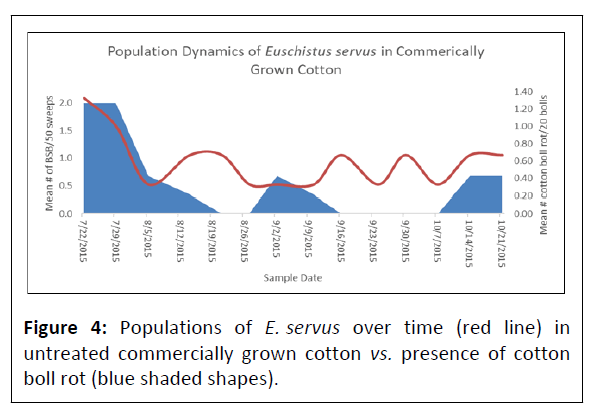
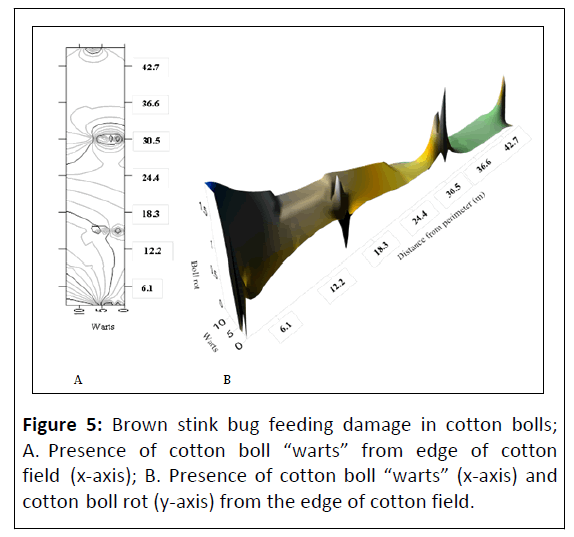
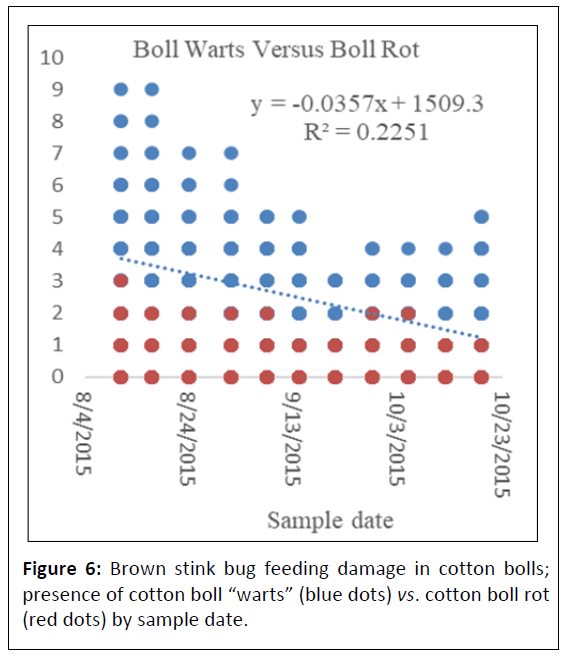
Euschistus servus collected at individual sampling sites using sweep sampling ranged from 0.33 ± 0.33-2.0 ± 0.58/50 sweeps and pheromone trapping totaled 89 insects over the course of the sampling period. Positioning of the pheromone traps as they penetrated deeper into cotton fields from the field perimeter revealed a general trend of reducing populations of E. servus but was not found significant among sampling locations (df=3, P=0.81). In untreated fields no statistical difference was detected between BSB populations and boll rot incidence (Figure 4). Cotton boll damage used to indirectly assess for presence of E. servus revealed that the presence of cotton boll warts from feeding by E. servus was not significantly different as you move deeper into cotton fields from the field perimeter (df=9, P=0.21) (Figures 5A, 5B). The correlation coefficient between the number of cotton with boll rot and the number of cotton boll warts (warts X boll rot) was not significant (df=14, P=0.89) (Figure 6). Cotton boll rot was not found to be significantly different among the sample locations deeper into cotton fields (df=3, P=0.51). However, there is a general trend of greater presence of cotton bolls with rot nearest the field perimeter. Pearson correlation analysis was done to further elucidate the relationship between presence of cotton boll rot and presence of cotton boll warts. No significant relationship between presence of cotton boll warts and boll rot by date (R2=0.23) or when the sampling date variable was removed (R2=0.02) was found. No significant relationship was found also between presence of cotton boll warts and cotton lint staining by date (R2=0.02).
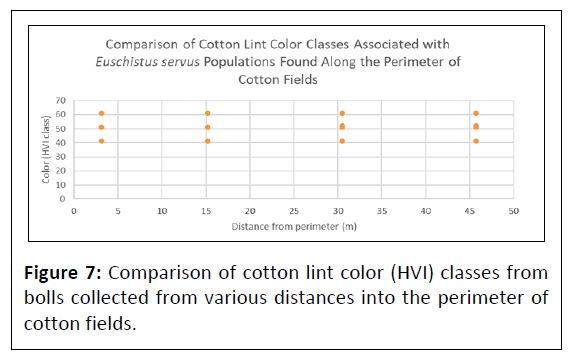
Preliminary evidence of E. servus carrying cotton boll rot bacteria was investigated and will be elaborated upon in a separate report. All of the E. servus collected during this project along with 100 cotton bolls exhibiting E. servus external feeding punctures were analyzed at the Insect Control and Cotton Disease Research Unit (USDA-ARS). Little to no presence of cotton boll rot was found (11% infected; n=100 bolls). The impact of cotton boll rot and cotton lint staining on cotton quality was assessed. No significant difference was found among the HVI color classes among the sample locations deeper into cotton fields (df=7, P=0.20) (Figure 7).
Laboratory survey of microbes in stink bugs and boll analysis
Over 100,000 Colony Forming Units (CFU) were recovered from bolls with disease and microbes. Bolls with evidence of insect feeding and no disease contained from 103-106 cfu. Bolls with no insect feeding evident possessed up to 102 cfu. Due to the vast number of bacteria the identification survey continues and will be reported on a separate manuscript.
Discussion
The determination of a need for pesticide application by economic entomologists, pest managers and consultants is dependent largely on threshold detection of pests. Notably, a correlation with crop yield-associated losses from season to season with piercing-sucking insect detection has not always been evident [4-10]. An explanation for correlation inconsistencies could be that during years when a greater percentage of bales are lost to stink bugs, the insects may have acquired and transmitted boll rot pathogens. In years with equivalent stink bug pressure and a low percentage of bales lost, the insects may have not been harboring boll pathogens.
The work presented here demonstrated that the use of pheromone trapping yielded greater numbers of E. servus compared to sweep sampling for monitoring populations of E. servus over a shorter period of time (n=112 vs. 89, respectively). Using pheromone trapping revealed that there does not appear to be a significant aggregation of E. servus along cotton field perimeters, although there is a general trend of reducing populations as you penetrate deeper into cotton fields from the field perimeter. This is consistent with population’s distribution of E. servus infesting cotton fields in the southeastern US [1].
Conclusion
Associated cotton boll warts caused by E. servus feeding and the resultant cotton boll rot, which is prevalent in the southeastern US was not significantly damaging in this study in southern California. Regardless, there is the same general trend of reducing incidence of boll rot as the trajectory moved into the cotton field perimeter. That along with the preliminary evidence of low incidence of cotton boll rotting bacteria suggested that damage done by E. servus in southern California is limited to direct damage inflicted by the insect feeding itself by which the cotton boll can withstand and heal. Generally, this suggests that California’s desert fields are not a conducive environment for boll rot pathogens to thrive when compared to the typically moist southeastern cotton belt states. This is similarly reflected in the lack of significant differences of HVI color classing between the sampling locations as you move away from the cotton field perimeter (Figure 7). Currently, we are exploring the microbiomes of stink bugs collected from California cotton fields.
Acknowledgment
Thanks to Grant Chaffin of Chaffin Farms Inc. and Joe Van Dyke of Joe Van Dyke Farms for material support. Richard Hernandez (USDA/ARS/SPA) was critical to boll evaluations. Thanks also to the California Cotton Ginners and Growers Association for funding to support this project. Thanks to the University of California summer technicians: Alonzo Mack, Damian Ramirez and Samuel Taylor for their technical assistance.
References
- Greene JK, Turnipseed SG, Sullivan MJ, May OL (2001) Treatment thresholds for stink bugs (Hemiptera: Pentatomidae) in cotton. J Econ Entomol 94: 403-409.
[Crossref], [Google Scholar], [Indexed]
- Medrano EG, Esquivel JF, Bell AA (2007) Transmission of cotton seed and boll rotting bacteria by the southern green stink bug (Nezara viridula L.). J Appl Microbiol 103: 436-444.
[Crossref], [Google Scholar], [Indexed]
- Medrano EG, Esquivel JF, Bell AA (2009) Temporal analysis of cotton boll symptoms resulting from southern green stink bug feeding and transmission of a bacterial pathogen. J Econ Entomol 102: 36-42.
[Crossref], [Google Scholar], [Indexed]
- Toews M, Roberts P, Medrano EG (2010) Interrelationships among stink bug management, cotton fiber quality and boll rot.
- Medrano EG, Bell AA, Greene JK, Roberts PM, Bacheler JS (2015) Relationship between piercing-sucking insect control and internal lint and seed rot in Southeastern cotton (Gossypium hirsutum L.). J Econ Entomol 108: 1540-1544.
[Crossref], [Google Scholar], [Indexed]
- Ashworth LJ, Hunter RE, Leigh TF (1969) Bacterial boll rot of cotton: Transmission of an Erwinia sp. by the brown stink bug. Phytopathology 59: 25. [Crossref], [Google Scholar], [Indexed]
- Medrano EG, Bell AA, Duke SE (2016) Cotton (Gossypium hirsutum L.) boll rotting bacteria vectored by the brown stink bug, Euschistus servus (Say) (Hemiptera: Pentatomidae). J Appl Microbiol 121: 757-766.
[Crossref], [Google Scholar], [Indexed]
- Bundy CS, McPherson RM (2000) Dynamics and seasonal abundance of stink bugs (Heteroptera: Pentatomidae) in a cottonâ?Âsoybean ecosystem. J Econ Entomol 93: 697-706.
[Crossref], [Google Scholar], [Indexed]
- Panizzi AR, McPherson JE, James DG, Javahery M, McPherson RM (2000) Stink bugs (Pentatomidae). CRC Press LLC, Boca Raton, Florida, USA.
- Barbour KS, Bradley JR, Bachelor JS (1990) Reduction in yield and quality of cotton damaged by green stink bug (Hemiptera: Pentatomidae). J Econ Entomol 83: 842-845.
[Crossref], [Google Scholar]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences