ISSN : 2576-3938
Journal of Emergency and Internal Medicine
Bacteriologic Profile and Drug Sensitivity Pattern of Urinary Tract Infection in Children with Renal Diseases in a Tertiary Care Hospital, Dhaka, Bangladesh
Afroza Begum*, Md. Firoz Anjum, Saimul Huq S, Uddin GM, Rahman MH, Roy RR, Abdullalh Al-Mamun and Tahmina Jesmin
Department of Pediatric Nephrology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
- *Corresponding Author:
- Afroza Begum
Associate Professor, Department of Pediatric Nephrology
Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
Tel: +880 2-55165760
E-mail: begumafrozabegum66@yahoo.com; firoz.anjum2013@gmail.com
Received Date: August 26, 2017; Accepted Date: August 30, 2017; Published Date: August 31, 2017
Citation: Begum A, Anjum MF, Huq SS, Uddin GM, Rahman MH, et al. (2017) Bacteriologic Profile and Drug Sensitivity Pattern of Urinary Tract Infection in Children with Renal Diseases in A Tertiary Care Hospital, Dhaka, Bangladesh. J Emerg Intern Med Vol.1:No.1:11 .
Abstract
Background: Development of regional bacteriologic profile and drug sensitivity pattern of urinary tract infection (UTI) is very important to treat early and appropriately. Our aim was to evaluate the bacteriological profile and antibiotic sensitivity patterns in children with UTI in different glomerular diseases.
Methodology: This prospective study was carried out in the Department of Pediatric Nephrology, Bangabandhu Sheikh Mujib Medical University, Dhaka from January 2015 to May 2017. All the admitted children up to 18 years of age with UTI were enrolled. Urine specimens were obtained by clean-catch method following careful preparation of the perineal area. Specimens were inoculated immediately in the Mackonky’s media.
Results: Among 1483 admitted children only 102 (6.87%) had significant UTI. Escherichia coli (51.6%) were the most common aetiological agent, followed by Pseudomonas (17.7%), Klebsiella spp. (14.5%), Enterococcus (8%), Streptococcus spp. (4.8%) and proteus (3.2%). The most sensitive drugs were imepenem, meropenem, ceftriaxone, ceftazidime and gentamicin.
Conclusion: The low growth of microorganisms in this study may be due to some patients were already getting antibiotics while collecting the specimen. The isolated organisms showed resistance to a large number of oral and per enteral antibiotics. Gentamicin may be the first option of empiric therapy while waiting for culture reports. Meropenem can be reserved.
Keywords
Urinary tract infection; Renal disease; Bacteriological profile; Drug sensitivity
Introduction
Urinary tract infections remain one of the most common infections and a leading cause of morbidity in human population [1]. The rate of UTI usually depends on age and sex. The incidence of UTI is greater in girls as compared to boys, which may be either due to anatomical structure or physiologic mechanisms [2].
Worldwide, an estimated 8% of girls and 2% of boys experience at least one episode of UTI by the age of seven years and recurrence occurs in 12% to 30% of them within a year [3]. Renal disease is a major cause of morbidity and mortality. Pediatric patients especially younger ones with renal disease may present with nonspecific signs and symptoms unrelated to the urinary tract. Therefore, should be familiar with the modes of presentation of different renal conditions and should have a high index of suspicion of renal disease [4]. Accurate diagnosis and appropriate use of antimicrobials for treatment and prevention of urinary tract infections (UTIs) is vital to reduce the burden and also to prevent the possible long-term consequences like renal scarring, hypertension and eventually end-stage renal disease [5].
Any organism present in the urinary tract can be a cause of UTI but most common organisms belong to enterobacteriaceae family containing facultative anaerobic, gram negative organisms [6].
Escherichia coli have been recognized as the most common pathogen accounting for majority of urinary tract infections in children [7].
Quite often, child receives antibiotics empirically, without adequate evaluation for UTI. The role of antibiotics is very crucial in UTI like any treatment of infection. The use of antibiotics has increased extensively these days and this intense expansion in use of antibiotics has significantly intensified the antibiotic resistance. The resistant patterns have greater variation in frequency with respect to localities and age groups. The organisms which cause UTI have shown multidrug resistance in many researches [8].
Due to the indiscriminate use of antibiotics by healthcare providers, resistance has been gradually increasing in uropathogens. Therefore, it is of utmost concern to formulate local antibiotic guidelines for the judicious treatment of UTI. The urinary antibiotics patterns help clinicians a lot in deciding the empirical therapy of UTIs so that the incidence of antimicrobial resistance may decrease. Hence, we undertook this study on the isolation of urinary pathogens and their antibiotics pattern at our institute in renal disease.
Methods
This prospective study was conducted at the Department of Paediatric Nephrology of Bangabandhu Seikh Mujib Medical University, Dhaka, Bangladesh over a period of January 2015 to May 2017.
During the study period, children up to 18 years of age of both sex presented to the pediatric Nephrology inpatient ward with a clinical diagnosis of UTI were included. Neonate were excluded from this study. The clinical diagnosis of UTI was made by the presence of fever and/or any of the symptoms such as painful micturition, increased frequency, burning micturition, or suprapubic pain/flank pain.
Study population
Present study included 1483 urine samples collected from the suspected cases of UTI. It included all inpatients irrespective of their age groups or genders presenting with symptoms of UTI (burning micturition, fever, hematuria, dysuria etc.).
Collection of urine samples
Patients were provided with sterile, wide mouthed screw capped containers and they were asked to give early morning mid-stream clean catch urine samples. The collected urine samples were properly labeled and all the patient particulars (name, age, sex, time of collection etc.) were indicated on the urine samples. Then the samples along with the requisition forms were sent to the microbiology laboratory. The samples were analyzed and processed according to the standard protocol within 2 hours of collection.
Sample processing
Culture- Culture of urine samples was done using a sterile calibrated bacteriological loop of 4 mm diameter designed to deliver 0.01 ml. A loopful of the well mixed urine sample was inoculated onto CLED Agar plate. The plate was then inverted and incubated in the incubator at 37°C for 18-24 hours. After the required incubation period, the plates were examined for bacterial growth. The colony count was done using semiquantitative method & multiplied by 100 to give an estimate of the number of bacteria present per ml of urine.
A count equal to or in excess of 105 bacteria per ml was taken as a significant bacterial count. If CFU was less than 105 bacteria per ml, it was considered as insignificant and not processed further. The patients were asked to submit repeat samples with early morning fresh midstream urine specimens in cases of mixed growth.
Identification of uropathogens- Identification of the isolated bacterial pathogens was done on the basis of gram staining, morphological characteristics and biochemical reactions by standard methods.
Antimicrobial susceptibility testing- Antimicrobial sensitivity of the isolated pathogens was determined by using a panel of 10-12 antibiotics by Kirby-Bauer disc diffusion method according to Clinical and Laboratory Standards Institute guidelines. The Mueller Hinton agar (MHA) plates were incubated at 37°C for 18-24 hours and results were read on the next day. Negative cultures were incubated further for another 24 hours and report was given as no growth at the end of 48 hours of incubation.
Statistical analysis
The information regarding patient’s profile and the results were entered into a computer program. Data analysis was carried out using the Statistical Package for Social Sciences [SPSSTM] version 20.0 [IBM, Armonk, NY, USA] and presented in percentage base distribution.
Ethical considerations
Written approval was taken from Institutional Review Committee of Bangabandhu Seikh Mujib Medical University (BSMMU) after submitting and presenting research proposal. Written informed consent was taken from every patient or their guardians before enrollment into the study.
Results
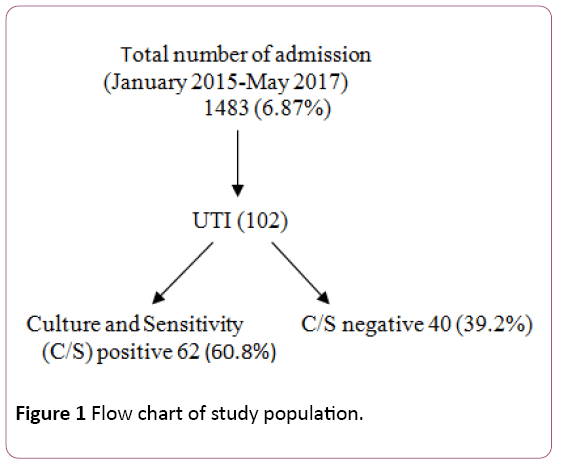
Out of the 1483 urine samples that were processed in this study, 1381 samples showed no UTI while 102 patients showed significant UTI (significant bacterial growth 62; and pyuria 40), giving rise to the total incidence of 6.87% (Figure 1). Insignificant growth and polymicrobial growth were not included in the study group. From 102 children 66 (64.7%) were males and 36 (35.3%) were females. Maximum number of cases was found in the children of age above 6 years group (Table 1).
| Age (years) | Male (%) | Female (%) | Total |
|---|---|---|---|
| ˂2 | 22 (84.6) | 4 (15.4) | 26 |
| 2-6 | 24 (72.7) | 9 (27.2) | 33 |
| ˃6 | 20 (46.5) | 23 (53.5) | 43 |
| Total | 66 (64.7) | 36 (35.3) | 102 |
Table 1: Distribution pattern of UTI according to age and sex.
Out of 62 urine samples that yielded significant bacterial growth, E. coli was the predominant etiological agent isolated (51.6%) followed by Pseudomonas (17.7%), Klebsiella spp. (14.5%), Enterococcus spp. (8%), Streptococcus (4.8%) and proteus (3.2%) (Table 2).
| Organisms | Culture positive (62) | |
|---|---|---|
| Frequency | Percentage (%) | |
| E. coli | 32 | 51.6 |
| Pseudomonas | 11 | 17.7 |
| Klebsiella | 9 | 14.5 |
| Enterococcus | 5 | 8 |
| Proteus | 2 | 3.2 |
| Streptococcus spp. | 3 | 4.8 |
Table 2: Number and percentage of culture positive UTI.
Out of 102 UTI, 40 urine samples had culture negative UTI (˃5 pus cell/HPF in the urine along with clinical features) (Table 3). The most common associated disease with UTI in children was the nephrotic syndrome 38 (37.25%) out of 985 NS, followed by obstructive uropathy 29 (28.43%) out of 120 obstructive uropathy (OU), glomerulonephritis 18 (17.64%) out of 238 GN, chronic kidney disease 17 (16.66%) out of 140 CKD (Table 4). The most common organism was E. coli in NS and also in GN and CKD; however, Pseudomonas was predominant in OU (Table 5).
| Pus cell | Total (40) | |
|---|---|---|
| Frequency | Percentage (%) | |
| ˃ 5/HPF | 40 | 100 |
Table 3: Number and percentage of culture negative (Pyuria positive) UTI.
| Associated disease (n) |
Culture positive UTI n (%) | Culture negative UTI n (%) | Total |
|---|---|---|---|
| Nephrotic syndrome (NS) (985) | 24 (38.7%) | 14 (35%) | 38 (37.25) |
| Glomerulonephritis (GN) (238) | 09 (14.5%) | 09 (22.5%) | 18 (17.64) |
| Obstructive uropathy (OU) (120) | 18 (29%) | 11 (27.5%) | 29 (28.43) |
| Chronic kidney disease (CKD) (140) | 11 (17.7%) | 6 (15%) | 17 (16.66) |
| Total | 62 (100%) | 40 (100%) | 102 |
Table 4: Different associated disease in study group.
| Disease | E. coli (n=32) |
Pseudomonas (n=11) |
Klebsiella Spp. (n=9) |
Enterococcus Spp. (n=5) |
Proteus (n=2) |
Streptococcus spp. (n=3) |
|---|---|---|---|---|---|---|
| NS | 19 (59.3) | 1 (9) | 2 (22.2) | 1 (20) | 0 | 2 (66.6) |
| GN | 7 (21.9) | 1 (9) | 0 (0) | 0 | 0 | 1 (33.4) |
| OU | 2 (6.25) | 7 (63.8) | 4 (44.5) | 2 (40) | 2 (100) | 0 |
| CKD | 4 (12.5) | 2 (18.2) | 3 (33.3) | 2 (40) | 0 | 0 |
Table 5: Organisms causing UTI in different renal diseases.
In this study, E. coli showed overall resistant to mecillinum, ceftazidime and ciprofloxacillin but was mostly sensitive to amikacin, nitrofurantoin, amoxicillin, cotrimoxazole and nalidixic acid. Pseudomonas spp. was most sensitive to amikacin, gentamicin and ceftazidime. Klebsiella spp. was resistant to most of the drugs. Other species showed sensitivity mostly to meropenem and imepenem. Most of the organisms showed resistance to nitrofurantoin (Table 6).
| Antibiotics | Escherichiae coli | Pseudomonas (n=11) | Klebsiella spp. (n=9) | Enterococcus spp. (n=5) | Proteus (n=2) | Streptococcus spp. (n=3) | |
|---|---|---|---|---|---|---|---|
| (n=32) | |||||||
| Amikacin | S | 28 (87.5) | 07 (63.6) | 05 (55.5) | 03 (60) | 02 (100) | 01 (33.3) |
| Amikacin | R | 04 (12.5) | 04 (36.4) | 04 (44.5) | 02 (40) | 00 (00) | 02 (66.7) |
| Gentamycin | S | 16 (50) | 08 (72.7) | 04 (44.5) | 03 (60) | 02 (100) | 01 (66.7) |
| Gentamycin | R | 16 (50) | 03 (27.3)) | 05 (55.5) | 02 (40) | 00 (00) | 02 (33.3) |
| Imepenem | S | 20 (62.5) | 05 (45.5) | 05 (55.5) | 05 (100) | 02 (100) | 03 (100) |
| Imepenem | R | 12 (37.5) | 06 (54.5) | 04 (44.5) | 00 (0) | 00 (00) | 00 (00) |
| Meropenem | S | 19 (60) | 06 (54.5) | 06 (66.6) | 03 (60) | 02 (100) | 03 (100) |
| Meropenem | R | 13 (40) | 05 (45.5) | 03 (33.4) | 02 (40) | 00 (00) | 00 (00) |
| Mecillinum | S | 10 (31) | 04 (36.4) | 01 (11.1) | 01 (20) | 00 (00) | 02 (66.7) |
| Mecillinum | R | 22 (69) | 07 (63.6) | 04 (88.9) | 04 (80) | 02 (100) | 01 (33.3) |
| Netilmycin | S | 23 (72) | 06 (54.5) | 03 (33.4) | 03 (60) | 02 (100) | 02 (66.7) |
| Netilmycin | R | 09 (28) | 05 (45.5) | 06 (66.6) | 02 (40) | 00 (00) | 01 (33.3) |
| Nitrofurantoin | S | 24 (75) | 03 (27.3) | 05 (55.5) | 03 (60) | 00 (00) | 02 (66.7) |
| Nitrofurantoin | R | 08 (25) | 08 (72.7) | 04 (44.5) | 02 (40) | 02 (100) | 01 (33.3) |
| Amoxicillin | S | 28 (87.5) | 06 (54.5) | 01 (11.1) | 04 (80) | 01 (50) | 01 (33.3) |
| Amoxicillin | R | 04 (12.5) | 05 (45.5) | 08 (88.9) | 01 (20) | 01 (50) | 02 (66.7) |
| Ceftriaxone | S | 26 (81) | 05 (45.5) | 05 (55.5) | 05 (100) | 02 (100) | 03 (100) |
| Ceftriaxone | R | 06 (19) | 06 (54.5) | 04 (44.5) | 00 (00) | 00 (00) | 00 (00) |
| Ceftazidime | S | 12 (37.5) | 05 (45.5) | 03 (33.4) | 05 (100) | 02 (100) | 03 (100) |
| Ceftazidime | R | 20 (62.5) | 06 (54.5) | 06 (66.6) | 00 (0) | 00 (00) | 00 (00) |
| Cefuroxime | S | 16 (50) | 06 (54.5) | 05 (55.5) | 02 (40) | 01 (50) | 03 (100) |
| Cefuroxime | R | 16 (50) | 05 (45.5) | 04 (44.5) | 03 (60) | 01 (50) | 00 (00) |
| Cephradine | S | 25 (78) | 06 (54.5) | 02 (22.2) | 02 (40) | 01 (50) | 02 (66.7) |
| Cephradine | R | 07 (22) | 05 (45.5) | 07 (77.8) | 03 (60) | 01 (50) | 01 (33.3) |
| Ciprofloxacillin | S | 07 (22) | 08 (72.7) | 05 (55.5) | 00 (20) | 01 (50) | 01 (33.3) |
| Ciprofloxacillin | R | 25 (78) | 03 (27.3) | 04 (44.5) | 05 (100) | 01 (50) | 02 (66.7) |
| Cotrim | S | 28 (87.5) | 05 (45.5) | 04 (44.5) | 01 (20) | 01 (50) | 01 (33.3) |
| Cotrim | R | 04 (12.5) | 06 (54.5) | 05 (55.5) | 04 (80) | 01 (50) | 02 (66.7) |
| Nalidixic acid | S | 27 (84) | 04 (36.4) | 05 (55.5) | 01 (20) | 01 (50) | 01 (33.3) |
| Nalidixic acid | R | 05 (16) | 07 (63.6) | 04 (44.5) | 04 (80) | 01 (50) | 02 (66.7) |
| Note:S= Sensitivity, R= Resistant | |||||||
Table 6: Antibiotics susceptibility patterns of bacterial agents causing UTI (n=62).
Discussion
Any part of the urinary tract can be infected during UTI including kidneys (pyelonephritis), bladder (cystitis) and urethra. Usually UTI in children occurs due to ascending infection but in the first year of life hematogenous spread may be more common. It is one of the common infections in children but difficult to diagnose because symptoms are nonspecific [9,10]. Particularly children with renal diseases are more susceptible to UTI due to the disease process and drugs causing immunosuppression and also some congenital abnormalities causing obstruction.
The pediatric age group is very sensitive to different types of infections and UTI is considered an important cause of childhood morbidity [11]. The prevalence of UTI observed in this study was 6.87% which was lower to the finding of 34.5% by Dash et al. 36.6% by Mehta et al. and 14.7% by Mohanty et al. [12-14], this could be due to large sample size in our study.
In this study, total number of UTI found 102 but culture positive UTI were 62 (60.8%), this is in full agreement with other studies [12-14]; however, microscopic examination of urine samples revealed that culture negative UTI (significant pyuria) occurred in (39.2%). Many possible explanations can be given for this; antibiotic administration prior to bacterial cultures could lead to suppression of bacterial growth [15].
Previous studies showed females predominance in pediatric age with respect to UTI. The results of this present study showed, there were 66 (64.7%) male while remaining 36 (35.3%) were female. Males outnumbered females during the 1st year of life, this is in full agreement with other studies [14]. With increase in age, the positivity gap between male and female gradually reduced to give high culture positivity in female than male in more than 6 years of age group. During the first few months of life, uncircumcised male infants are at increased risk for UTIs than their female counterparts. The periurethral area was found to be more frequently and more heavily colonized with uropathogens.
On the incidence and identity of the various aetiological agents it was observed that, in this study, E. coli (51.6%), Pseudomonas (17.7%), Klebsiella spp. (14.5%), Enterococcus (8%), Streptococcus spp. (4.8%), and proteus spp. (3.2%) were the most frequently isolated causal agents of UTI. In agreement with this finding, several previous works have also reported these same organisms [16-18].
Highest number of UTI occurred in this study in nephrotic syndrome patients 24 (38.7%) followed by obstructive uropathy 18 (29%), chronic kidney disease 11 (17.7%) and glomerulonephritis 09 (14.5%). These results are not in agreement with Shakya et al. who reported that highest number of UTI detected in CKD 58 (56.68%) followed by acute kidney injury 14 (13.73%), renal stone 14 (13.73%) and nephrotic syndrome 12 (11.76%) [17].
Naushadi et al. found that E. coli was detected 63.3% cases of UTI with nephrotic syndrome followed by proteus 18.3% and klebsiella 9.2%; [19] however in present study showed E. coli was 59.3% followed by Streptococcus spp. 66.6%, Klebsiella 22.2%, Enterococcus spp 20%, and Pseudomonas 9%. Rahman et al. found in obstructive uropathy that E. coli 84.37%, followed by klebsiella 12.5% and Streptococcus 3.1% [20]. In our study proteus was found 100%, Pseudomonas 63.8%, klebsiella 44.5%, Enterococcus spp. 40%, and E. coli 6.25%. Jadav et al. showed that gram negative bacteria were more frequent isolated in CKD than gram positive bacteria [21]. Similar finding was found in this study.
On antimicrobial susceptibility testing in this study, it was noted that E. coil spp. was mostly sensitive to imepenem, meropenem, ceftriaxone, ceftazidime, gentamicin and nitrofurantoin. Nupur et al. also found that E. coli isolates were highly sensitive to nitrofurantoin as compared to Klebsiella (81.39% and 62.5%, respectively). This drug also exhibited low resistance rate in the major part of the world (0% to 5.4%) [22].
In this study, for the Klebsiella, amoxicillin and cephradine were resistant and other drugs had more or less 50% sensitivity. For Pseudomonas, amikacin, gentamicin was more sensitive in comparision to mecillinum, nitrofurantoin and nalidixc acid. This finding is not similar to other reports where nitrofurantoin and fluoroquinolones have been reported to be most effective drug for empirical therapy in UTI [23,24]. In Soltani study, all Enterococcus spp. were sensitive to linezolid, imepenem, meropenem, cephalosporin [25]. Similar finding was observed in our study.
Conclusion
The low growth of microorganisms in this study may be due to some patients were already getting antibiotics while collecting the specimen. The isolated organisms showed resistance to a large number of oral and parenteral antibiotics. Gentamicin may be the first option of empiric therapy while waiting for culture reports. Meropenem can be a reserved drug.
References
- Prais D, Straussberg R, Avitzur Y, Nussinovitch M, Harel L, et al. (2003) Bacterial susceptibility to oral antibiotics in community acquired urinary tract infection. Arch Dis Child 88: 215-218.
- Muoneke V, Ibekwe M, Ibekwe R (2012) Childhood urinary tract infection in abakaliki: Etiological organisms and antibiotic sensitivity pattern. Ann Med Health Sci Res 2: 29-32.
- Desai DJ, Gilbert B, McBride CA (2016) Paediatric urinary tract infections: Diagnosis and treatment. Aust Fam Physician. 45: 558-563.
- Barakat AJ (2012) Robinson presentation of the child with renal disease and guidelines for referral to the pediatric nephrologist. Int J Pediatr.
- Hay AD, Sterne JA, Hood K, Little P, Delaney B, et al. (2016) Improving the diagnosis and treatment of urinary tract infection in young children in primary care: Results from the dutyprospective diagnostic cohort study. Ann Fam Med 14: 325-336.
- Brad GF, Sabau I, Marcovici T, Mariş I, Daescu C, et al. (2010) Antibiotic resistance in urinary tract infections in children. J Pediatr 13: 51-52.
- Hanna-Wakim RH, Ghanem ST, El-Helou MW, Khafaja SA, Shaker RA, et al. (2015) Epidemiology and characteristics of urinary tract infections in children and adolescents. Front Cell Infect Microbiol 5: 45.
- Paryani JP, Memon SR, Rajpar ZH, Shah SA (2012) Pattern and sensitivity of microorganisms causing urinary tract infection at teaching hospital. J Liaquat Uni Med Health Sci 11: 97-100.
- Marild S, Jodal U (1998) Incidence rate of first-time symptomatic urinary tract infection in children under 6 years of age. Acta Paediatr 87: 549-553.
- American Academy of Pediatrics Task Force on Circumcision (2012) Male circumcision. Pediatrics 130: e756.
- Sheinfeld J, Schaeffer AJ, Cordon-Cardo C (1989) Association of the Lewis blood-group phenotype with recurrent urinary tract infections in women. N Engl J Med 320: 773-780.
- Dash M, Padhi S, Mohanty I, Panda P, Parida B (2013) Antimicrobial resistance in pathogens causing urinary tract infections in a rural community of Odisha, India. J Family Community Med 20: 20‑26.
- Mehta M, Bhardwaj S, Sharma J (2013) Screening of urinary isolates for the prevalence and antimicrobial susceptibility of enterobacteria other than Escherichia coli. Int J Life Sci Pharma Res 3: 100‑104.
- Mohanty S, Kapil A, Das BK, Dhawan B (2002) Antimicrobial resistance profile of nosocomial uropathogens in a tertiary care hospital. Indian J Med Sci 57: 148‑154.
- MacFaddin JF (2000) Biochemical tests for identification of medical bacteria. Lippincott Williams and Wilkins, Philadelphia, USA.
- Nerurkar A, Solanky P, Naik SS (2012) Bacterial pathogens in urinary tract infection and antibiotic susceptibility pattern. J Pharm Biomed Sci 21: 1-3.
- Shakya R, Amatya R, Karki BM, Mandal PK, Shrestha KK (2014) Spectrum of bacterial pathogens and their antibiogram from cases of urinary tract infection among renal disorder patients. Nepal Med Coll J 16: 75-79.
- Akinbami AA, Ajibola S, Bode-Shojobi I, Oshinaike O, Adediran A, et al. (2014) Prevalence of significant bacteriuria among symptomatic and asymptomatic homozygous sickle cell disease patients in a tertiary hospital in Lagos, Nigeria. Niger J Clin Pract 17: 163-167.
- Naushadi AK, Ahammed S (2016) Incidence and clinico-etiological study of UTI in nephrotic syndrome in pediatric age group. IOSR-JDM 15: 28-31.
- Rahman MH, Laila K, Muinuddin G (2014) Pattern of infection in children presented with obstructive uropathy-Ahospital based study. BSMMU 7: 44-52.
- Jada SK, Sant SM, Acharya V (1977) Bacteriological of urinary tract infection in patients of renal failure undergoing dialysis. J Postgrad Med 23: 10-18.
- Nupur P, Kalidas, Somnath N, Simit K, Rajyasri G (2016) A study of bacteriological and antibiotic susceptibility profile of pediatric urinary tract infection with special emphasis on extended spectrum beta‑lactamase production in a tertiary care hospital of Eastern India. Int J Health & Allied Scie 5: 257-262.
- Patel SK, Taviad PP, Sinha M, Javadekar TB, Chaudhari VP (2012) Urinary tract infections among patients at G.G Hospital and Medical College, Jamnagar. Nat J Comm Med 3: 138-141.
- Joshi SMC, Rashid MK, Joshi HS (2011) Study of antibiotic sensitivity pattern in urinary tract infection at a tertiary hospital. NJIRM 2: 43-46.
- Soltani R (2009) Evaluation of antimicrobial resistance pattern of gram‑positive microorganisms isolated from biologic samples of patients hospitalized at imam khomeini hospital using disk diffusion and E‑test methods. Clinical pharmacy residency thesis. Tehran University of Medical Sciences, Iran.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences