ISSN : 2573-0320
Journal of Transmitted Diseases and Immunity
Association of Chediak-Higashi Syndrome and Human Immunodeficiency Virus Type 1 Acute Infection
Ariani V Bernarchi1, Gabriela M Gehring1, Uheyna G Ruzon1, Marco Aurélio C Rodrigues1, Susana L Wiechmann1,2, Zuleica N Tano1,2 and Edna Maria V Reiche3*
1Department of Infectious Diseases, Londrina University Hospital, Londrina, Paraná, Brazil
2Department of Internal Medicine, Health Sciences Center, Londrina University Hospital, Londrina, Paraná, Brazil
3Department of Pathology, Clinical Analysis and Toxicology, Health Sciences Center, State University of Londrina, Londrina, Paraná, Brazil
- *Corresponding Author:
- Edna Maria V Reiche
Department of Pathology
Clinical Analysis and Toxicology
Health Sciences Center
Londrina State University
Av. Robert Koch, 60
CEP 86.038-440
Londrina, Paraná, Brazil
Tel: + 55-43-3371-2619
E-mail: reiche@sercomtel.com.br
Received Date: August 01, 2017; Accepted Date: August 10, 2017; Published Date: August 17, 2017
Citation: Bernarchi AV, Gehring GM, Ruzon UG, Rodrigues MAC, Wiechmann SL, et al. (2017) Association of Chediak-Higashi Syndrome and Human Immunodeficiency Virus Type 1 Acute Infection. J Transm Dis Immun. Vol. 1 No. 2:12
Abstract
Context: The Chediak- Higashi syndrome (CHS) is a rare autosomal and recessive disease associated with a genetic alteration of the lysosomal trafficking regulator (LYST) gene (CHS1/LYST). The LYST protein interacts with some cytoplasmic proteins that play an important role in vesicular transport regulation signal transduction that leads to a decreased phagocytosis, which results in recurrent pyogenic infections, partial albinism and peripheral neuropathy. The association between CHS and infection by human immunodeficiency virus type 1 (HIV-1) is rare. The aim of this study is to report the case of a patient with CHS and had incidental diagnosis of acute HIV-1 infection during hospitalization for treatment of a urological disorder
Keywords
Chediak-higashi syndrome; Immunodeficiency; CHS1/LYST gene; Genetic disorder; Human immunodeficiency virus; Viral load; Intracitoplasmatic inclusion
Introduction
The Chediak- Higashi syndrome (CHS) is a rare autosomal and recessive disease characterized by severe congenital immunodeficiency, moderate coagulation defects, cutaneous hypopigmentation and progressive neurological disorders, which might lead to recurrent skin infections, muscle weakness, ataxia, sensory loss, and nystagmus [1]. Classic CHS presents in early childhood with severe infectious or hematologic complications unless treated with bone marrow transplantation. Atypical CHD has less severe hematologic and infectious manifestations. Both classical and atypical CHD develop neurological problems [2]. First described over 50 years ago, less than 500 cases of CHS were reported in the past 20 years [1,3]. The central morphological characteristic of the CHS is the presence of giant inclusion bodies in all granulated cells including granulocytes, histiocytes, mast cells, platelets, melanocytes, Schwann cells, neurons, renal tubular epithelial cells and fibroblasts [4]. CHS is associated with a genetic alteration of the lysosomal trafficking regulator (LYST) gene (CHS1/LYST) localized in chromosome 1q42-44, which results in 51 coding exons and encodes for a 429kD protein.
The LYST gene produces a cytoplasmic protein that modulates lysosomal exocytosis and is involved either in the structure or functioning of a variety of intracellular organelles, including melanosomes, secretory granules, and intracellular lysosomes [3]. The LYST protein interacts with some cytoplasmic proteins that play an important role in vesicular transport regulation signal transduction [3] that leads to a decreased phagocytosis, which results in recurrent pyogenic infections, partial albinism and peripheral neuropathy. The idea that toll-like receptor (TLR) signaling and function can be controlled by the regulation of the endosomal trafficking network has been explored [5] and the results demonstrated that LYST protein selectively controls TLR3- and TLR4-mediated proinflammatory responses. Mechanistic in vitro studies revealed that Lyst regulates phagosomal trafficking/ maturation upon microbial encounter and specifically affects TRIF/IRF3 (IFN regulatory factor 3)-mediated endosomal TLR signaling. In vivo, loss of functional Lyst results in impaired immune responses against infection and prevents uncontrolled inflammation during septic shock. The identification of Lyst as a physiological regulator of TLR function reveals how the regulation of the cellular membrane trafficking network can affect specific immune receptor signaling pathways and inflammatory responses [5]. The association between CHS and infection by human immunodeficiency virus type 1 (HIV-1) is rare. The aim of this study is to report the case of a patient with previous diagnosis of CHS and had incidental diagnosis of acute HIV-1 infection during hospitalization for treatment of a urological disorder.
Case Description
An 18-year-old male, single and unemployed, residing in North Parana State, Brazil, had a penile injury evaluated in the emergency room of the University Hospital of State University of Londrina. During the interview, the patient could not describe the evolution or the history of his lesion, and showed limited mental capacity. He was addicted to marijuana, cocaine, injectable drugs, alcohol and reported unprotected sexual activity in the past six months. Further investigation revealed that the patient and his brothers had CHS. Since childhood, the patient presented learning disabilities, attention deficit, recurrent skin infections and several ophthalmologic disorders, such as hyperopia carrier, astigmatism, strabismus and photophobia.
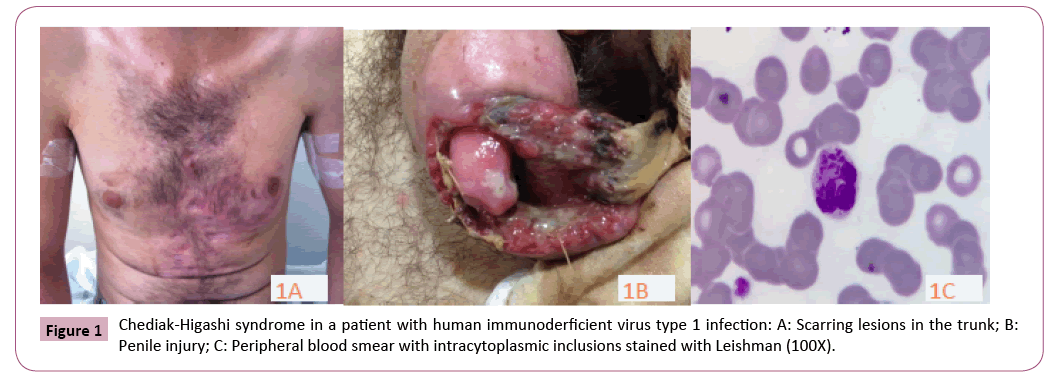
On physical examination, the patient was febrile and complained of important penile pain. His eyes presented no opacity signal. His anterior chest region and distal lower limb presented several scar lesions (Figure 1A). No palpable Lymph nodes. The penis presented a large foreskin lesion, and areas of necrosis, bleeding, and fetid smelling (Figure 1B). The diagnosis of CHS was confirmed by peripheral blood smears that showed giant inclusion bodies formed by large lysosome vesicles in neutrophils (Figure 1C). The patient was treated with ciprofloxacin and clindamycin, as well as surgical debridement. Firstly, HIV infection and other sexual transmitted diseases were investigated in blood samples using serological tests. The rapid test for anti-HIV (immunoassay VIKIA HIV1/2, bioMerieux, Brazil) was negative. New blood sample was collected the next day and the results were positive in two screening tests: test 1 (Chemiluminescence, HIV Ag/Ab Combo Architect, and Abbott Laboratories) and test 2 (enzyme linked immunoassay, ELISA, Bio-Rad-Genscreen ultra HIV), with results of 4.06 (cut-off 1.0) and 0.950 (cut-off 0.286), respectively. A complimentary immunoblot anti-HIV test was performed (Bio Manguinhos, Rio de Janeiro, Brazil), but the result was inconclusive. The following day, another peripheral blood sample was collected and the same results were obtained for anti-HIV antibodies. In order to confirm the diagnosis of HIV-1 infection, the polymerase chain reaction (PCR) for quantitative HIV-RNA was performed using the branched DNA method (Versant – HIV-RNA 3.0, bDNA, Siemens Medical Solutions, Mississauga, ON, Canada), and the viral load was greater than five hundred thousand copies/mL (Table 1).
Table 1: Laboratory results obtained during the two-year follow-up period monitoring human immunodeficiency virus type 1 (HIV-1) infection in a patient with Chediak-Higashi syndrome without the use of antiretroviral therapy.
| July, 2013 | October, 2013 | May, 2014 | October, 2014 | April, 2015 | |
|---|---|---|---|---|---|
| CD4+ T lymphocyte (cell/μL) | 608 | 711 | 624 | 696 | 689 |
| CD8+ T lymphocyte (cell/μL) | 848 | 782 | 686 | 710 | 958 |
| CD4+ T/CD8+T ratio | 0.72 | 0.91 | 0.91 | 0.98 | 0.72 |
| HIV- RNA viral load Copies/mL Log10/mL |
258,970 5.413 |
26,266 4.419 |
7,541 3.877 |
9,706 3.987 |
17,229 4.24 |
| Leukocytes (cell/μL) | 1800 | 3500 | 4600 | ||
| Metamyelocyte n(%) | 36 (2.0) | 0 (0.0) | 0 (0.0) | ||
| Band neutrophil n(%) | 360 (20.0) | 0 (0.0) | 0 (0.0) | ||
| Neutrophil n(%) | 792 (44.0) | 1295 (37.0) | 1748 (38.0) | ||
| Eosinophil n(%) | 18 (1.0) | 70 (2.0) | 138 (3.0) | ||
| Lymphocyte n(%) | 288 (16.0) | 1645 (47.0) | 1932 (42.0) | ||
| Atypical lymphocyte n(%) | 54 (3.0) | 0 (0) | 0 (0.0) | ||
| Monocyte n(%) | 252 (14.0) | 490 (14.0) | 782 (17.0) | ||
| Presence of intracytoplasmic inclusions in neutrophils characteristics of CHS | Positive | Positive | Positive | ||
| Platelets (cell//μL) | 46,000 | 193,000 | 193,000 |
CHS: Chediak-Higashi Syndrome; HIV-RNA: quantification of the RNA of HIV (viral load) in peripheral blood sample, using the branched DNA method.
The clinical and laboratory data allowed the diagnosis of acute HIV-1 infection, according to the criteria previously established [6,7]. According to the Clinical Protocol and Therapeutic Guidelines for Infection Management HIV in Adults of the Brazilian Ministry of Health [8], the combined antiretroviral therapy (cART) was immediately initiated, with zidovudine and lamivudine, associated with lopinavir/ritonavir.
The patient abandoned cART just after hospital discharge and was attended at the outpatient clinic irregularly, but remained asymptomatic with no complaints related to HIV-1 infection. The results of laboratory tests performed for monitoring the HIV-1 infection without cART during the study period are also shown in Table 1. The CD4+ T cell counts, evaluated using flow cytometry (BD FACSCalibur, Becton Dickinson Biosciences, San Jose, CA, USA), showed no significant decline. Similarly, the RNA-HIV viral load, using branched DNA (Versant - HIV-RNA 3.0, bDNA, Siemens Medical Solutions, Mississauga, ON, Canada), showed no significant changes except for the first viral load performed after the diagnosis of HIV-1 acute infection.
Discussion
This study reports a rare association between CHS and the HIV-1 infection, indicating the possible effect of this genetic dysfunction in the progression of HIV-1 infection. Even in the absence of cART during the two-year follow-up, there was no deterioration of the immune response or viral replication growth. In CHS, the mutation of CHS1/LYST affects many processes, mainly the regulation and sizing of vesicles in the lysosomal exocytosis. These changes might modify cells, such as melanosomes where increased pigment granules are not properly transferred to the keratinocytes, causing hypopigmentation; moreover, neutrophils and cytotoxic T cells, which develop larger and inefficient vesicles, present diminished secretion and compromised bactericidal action; and the repair of the plasma membrane, since it’s also mediated by a vesicle secreting process [1]. CHS patients have a profound defect in the function of cytotoxic and NK cells [9,10]. Moreover, defects of neutrophils include ineffective granulopoiesis, moderate neutropenia, and delayed and incomplete degranulation associated with phagocytic, chemotactic, and bacterial killing defects [11]. Platelets are also functionally defective with reduced dense granules and impaired functions. Immunoglobulin levels and complement are generally normal [12]. The natural history and pathogenic processes of HIV- 1 infection are complex. The control of the infection progression is regulated by the balance between host and viral factors. Human allelic variants do not only interfere in the susceptibility to HIV-1 infection, but also in the subsequent rates of disease progression towards AIDS [13-19]. Regarding the association of the CHS with HIV-1, studies have reported that the secretory pathway in CD4+ T cells supports HIV-1 cell-to-cell spread at the virological sinapse [20,21]. Defects in this mechanism may interfere with viral replication, particularly avoiding the formation of the virological synapse, a decisive level in viral spreading [20,21]. The mutated CHS1/LYST protein present in the CHS causes changes in lysosomal secretory vesicle making the cell-to-cell interaction demanding in the CD4+ T cells, the very process used by HIV-1 to disseminate infection [22]. These authors also found that cells from controlled healthy donors and cells from CHS patients infected with HIV- 1 showed equivalent viral infectivity, demonstrating that the cells from patients with CHS are equally susceptible to HIV-1. However, the quantification of the viral p24 antigen release was significantly lower in the cell culture supernatant from patients with CHS when compared to the healthy individuals. Moreover, these authors demonstrated a significant lower number of HIV-1- infected cells in the CHS compared to controls, noticing that the virus needs cell secretion apparatus to spread [22]. These results suggest that the progression of HIV-1 between adjacent cells is diminished, which might contribute to a slower clinical course of HIV-1 infection in patients with CHS.
The diagnosis of CHS was made by examination of peripheral blood smear after specific staining to view the classic finding of increased granules in neutrophils. The diagnosis can be confirmed by genetic testing of mutations in CHS1/LYST gene. The hematological alterations observed in the first laboratory evaluation are in agreement with the most white blood cells defects reported in CHS [11] including neutropenia and thrombocytopenia, consistent with the initial clinical symptoms, such as fever and a penile infectious and bleeding lesion. Moreover, the production of anti-HIV antibodies was not impaired in this patient, as reported previously [12]. The HIV-1 infection reported in this study fits in stage IV [6,7], which is characterized by positive detection tests of RNA-HIV, p24 antigen, and anti-HIV antibodies using rapid and serological tests. Rapid HIV test carried out in this patient was negative. This test was based on immunochromatography, which requires the use of a higher concentration of antigen and the antigen/antibody complex for detection. The immunoblot method presented unspecified pattern, with the presence of some specific antigenic bands recognized by anti-HIV-1 antibodies that, however, did not meet the criteria of the interpretation as positive immunoblot [23]. This may be explained by the sixth day length of the immunological window, as described previously [6,7].
Conclusion
The present case report emphasizes that patients with CHS are equally susceptible to HIV-1 infection; nonetheless, they may show slower viral replication and immune deterioration when compared with individuals without this genetic disorder, which results in a slower clinical course of HIV-1 infection. Therefore, CHS can be considered one of the host factors involved in slow progression to AIDS. Moreover, identifying the pathway of cellto- cell spread of HIV-1 and the immunological consequences may suggest novel targets for rational drug-design that can specifically limit HIV-1 spreading.
Conflicts of Interest: The authors declare no conflict of interest.
References
- Kaplan J, De Domenico I, Ward DM (2008) Chediak-Higashi Syndrome. Current Opinion in Hematology 15:22-29.
- Lehky TJ, Groden C, Lear B, Toro C, Introne WJ (2017) Peripheral nervous system manifestations of Chediak-Higashi disease.Muscle Nerve 55: 359-365.
- Nagai K, Ochi F, Terui K, Maeda M, Ohga S, et al. (2013) Clinical Characteristics and Outcomes of Chediak-Higashi Syndrome: A Nationwide Survey of Japan. Pediatric Blood & Cancer 60: 1582-1586.
- Tchernev VT, Mansfield TA, Giot L, Kumar AM, Nandabalanm K, et al.(2002) The Chediak–Higashi Protein Interacts with SNARE Complex and Signal Transduction Proteins. Molecular Medicine 8:56-64.
- Westphal A, Cheng W, Yu J, Grassi G, Krautkrämer M,et al.(2017)Lysosomal trafficking regulator Lyst links membrane trafficking to toll-like receptor-mediated inflammatory responses.Journal of Experimental Medicine 214:227-244.
- Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, et al.(2003) Dynamics of HIV Viremia and Antibody Seroconversion in Plasma Donors: Implications for Diagnosis and Staging of Primary HIV Infection. AIDS 17: 1871-1879.
- Cohen MS, Gay CL, Busch MP, Hecht FM (2010) The Detection of Acute HIV infection. The Journal of Infectious Diseases 202 Suppl 2: S270-S277.
- Brazil Ministry of Health (2013) Secretariat of Surleivance in Health, Department of Sexual Transmitted Diseases, AIDS and Viral Hepatitis. Protocolo Clínico e Diretrizes Terapêuticas para Manejo da Infecção pelo HIV em Adultos. Brasília.
- Kaya Z, Ehl S, Albayrak M, Maul-Pavicic A, Schwartz K, et al.(2011) A Novel Single Point Mutation of the LYST Gene in Two Siblings with Different Phenotypic Features of Chediak Higashi Syndrome. Pediatric Blood & Cancer 56:1136-1139.
- Bryceson YT, Pende D, Maul-Pavicic A, Gilmour KC, Ufheil H,et al. (2012) A prospective evaluation of degranulation assays in the rapid diagnosis of familial hemophagocytic syndromes. Blood 119:2754-2763.
- Dinauer MC (2007) Disorders of neutrophil function: an overview. Methods Molecular Biology 412:489-504.
- Lozano ML, Rivera J, Sánchez-Guiu I, Vicente V (2014) Towards the targeted management of Chediak-Higashi syndrome. Orphanet Journal Rare Disease 9:132.
- Bates I, Fenton C, Gruber J, Lalloo D, Lara M, et al. (2004) Vulnerability to malaria, tuberculosis, and HIV/AIDS infection and disease. Part 1: determinants operating at individual and household level. Lancet 4: 267-277.
- Pantaleo G, Graziosi C, Demarest JF, Butini L, Montroni M, et al. (1993) HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature 362: 355-358.
- Hogan C, Hammer S (2001) Host determinants in HIV infection and disease: Part 1: cellular and humoral responses. Annals of Internal Medicine 134: 761-776.
- Hogan C, Hammer S (2001) Host determinants in HIV infection and disease: Part 2: genetic factors and implications for an antiretroviral therapeutics. Annals of Internal Medicine 134: 978-996.
- Reiche EM, Bonametti AM, Voltarelli JC, Morimoto HK, Watanabe MA (2007) Genetic polymorphisms in the chemokine and chemokine receptors: impact on clinical course and therapy of the human immunodeficiency virus type 1 infection (HIV-1). Current Medical Chemistry 14:1325-1334.
- Reiche EM, Ehara Watanabe MA, Bonametti AM, Kaminami Morimoto H, Akira Morimoto A, et al.(2006) The effect of stromal cell-derived factor 1 (SDF1/CXCL12) genetic polymorphism on HIV-1 disease progression. International Journal of Molecular Medicine 18:785-793.
- Reiche EM, Ehara Watanabe MA, Bonametti AM, Morimoto HK, Akira Morimoto A, et al. (2008) Frequency of CCR5-Delta32 deletion in human immunodeficiency virus type 1 (HIV-1) in healthy blood donors, HIV-1-exposed seronegative and HIV-1-seropositive individuals of southern Brazilian population. International Journal of Molecular Medicine 22:669-675.
- Sattentau Q (2008) Avoiding the Void: Cell-to-cell Spread of Human Viruses. Nature Reviews Microbiology 6: 815-826.
- Jolly C, Kashefi K, Hollinshead M, Sattentau QJ (2004) HIV-1 Cell to Cell Transfer Across an Env-induced, Actin-dependent Synapse. The Journal of Experimental Medicine 199:283-293.
- Jolly C, Welsch S, Michor S, Sattentau QJ (2011) The Regulated Secretory Pathway in CD4(+) T cells Contributes to Human Immunodeficiency Virus Type-1 Cell-to-Cell Spread at the Virological Synapse. PLOS Pathogens 7:e1002226.
- Brazil Ministry of Health (2014) Secretariat of Surleivance in Health, Department of Sexual Transmitted Diseases, AIDS and Viral Hepatitis. Technical Guideline for the diagnosis of HIV infection. Brasília.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences